Abstract
Laboratory and field investigations aimed to characterize the chemical communication system of the date palm pest Oryctes agamemnon. Live males or extracts of male effluvia attracted conspecifics in an olfactometer, whereas female effluvia attracted only males. Volatile emissions from adults feeding on sugarcane were sampled and analysed by gas chromatography (GC) and GC/mass spectrometry (GC/MS). Males emitted a blend of 1) ethyl 4-methyloctanoate, 2) 4-methyloctanoic acid, 3) 4-methyloctanyl acetate, and 4) 4-methyloctanol in variable ratio. Single sensillum recordings demonstrated that compounds 1, 2, and 3 are detected by specific olfactory receptor neurons. Olfactometric experiments showed that compounds 1 and 3 attract both sexes of O. agamemnon, but females are more attracted by compound 1 and males by compound 3. Compound 2 was more attractive for females, especially virgin ones. Field experiments confirmed that compound 1 and compound 2 attracted O. agamemnon of both sexes and showed synergy with palm odors. No clear activity of compound 3 was observed. A mix of compounds 1 and 2 with date palm core odor was significantly the most attractive, and captured more females than males. The male aggregation pheromone of O. agamemnon appears therefore to be based on a mixture in contrast to previously identified Oryctes pheromones. Our results provide the basis for developing mass trapping to control this pest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Date palm, Phoenix dactylifera L., is one of the most economically important fruit trees in the Arab world. In southern Tunisia, oases occupy an area of 40.000 ha, which produced 200,000 tons of dates in 2013, half of which were exported. Tunisia has the first and the third world positions in terms of export value and tonnage of dates, respectively (GIF 2014). The palm trees and their fruits are exposed to depredation by several insect pests (Zaid and de Wet 2002). In Tunisia, the date palm sector experiences many difficulties, especially phytosanitary problems that have increased because of the introduction of several new pests during recent decades (Khoualdia et al. 1997; Rhouma 1996).
The genus Oryctes (Coleoptera: Scarabaeidae) is represented by several species in the date palm orchards of many countries of North Africa, and in the Near and Middle East (Endrödi and Petrovitz 1974). The main species are O. elegans, O. agamemnon, and O. rhinoceros (Bedford 1980), known as rhinoceros beetles. Oryctes agamemnon is the only species of the genus Oryctes which has been reported in Tunisian oases (Khoualdia et al. 1997; Soltani et al. 2008a). It was introduced accidentally from the United Arab Emirates by means of an exchange of date palm varieties between the two countries, and first appeared at the governmental Mrah Lahouar oasis in the late 1970s to the early 1980’s (Khoualdia et al. 1997). Later, the insect was transported to the new oasis of Rjim Maâtoug (Kebili area) in infested offshoots, and it is now widespread in all plantations and causes serious damage, especially on young date palm trees (Ehsine et al. 2009; Soltani et al. 2008a; Soltani 2010). In the Arabian Peninsula, O. agamemnon is widespread in the Gulf countries including the Kingdom of Saudi Arabia, the United Arab Emirates, and the Sultanate of Oman (Balachowsky 1962).
The larvae mine galleries to feed, but attacks on the stem do not induce notable danger to the palm tree, as all the bored parts are dead and are not important for any of the plant’s vital functions. In contrast, larvae cause real danger when they attack the aerial roots, as this can lead to sudden collapse of the palm tree (Soltani et al. 2008a; Soltani 2010). Oversight of Oryctes damage, misidentification of the species due to confusion of its larvae with white grubs of other species, and the absence of efficient chemical and biological control, has allowed continuous increase of O. agamemnon populations. There currently is no cheap and easy control of O. agamemnon by insecticides because this insect spends most of its life in galleries bored in the palms. The only control method currently used is the manual extraction of adults and larvae from feeding galleries. This is highly labor intensive and is impossible for tall standing palms. Furthermore, adult beetles are excellent flyers, capable of rapidly colonizing new feeding and breeding sites.
Male-produced aggregation pheromones have been described for five rhinoceros beetles attacking palm trees: Oryctes monoceros (Olivier) in Africa (Gries et al. 1994), Oryctes rhinoceros L. in the Asia-Pacific area (Hallett et al. 1995; Morin et al. 1996), Scapanes australis Boisduval in the Papua New Guinea region, Strategus aloeus L. in the New World (Rochat et al. 2000b, 2002), and Oryctes elegans (Prell) in Iran (Rochat et al. 2004). Ethyl 4-methyloctanoate was identified as the major compound in O. monoceros (Gries et al. 1994) and O. rhinoceros (Hallett et al. 1995) pheromones and is present in the O. elegans pheromone (Rochat et al. 2004). 4-Methyloctanoic acid is a major component of O. elegans aggregation pheromone (Rochat et al. 2004) and also is produced by O. monoceros (Gries et al. 1994). 4-Methyloctanyl acetate and 4-methyloctanol have been identified in very small amounts in O. elegans male effluvia (Rochat et al. 2004). These pheromones have been synthesized and used to capture these species in traps. Mass trapping has been implemented on a large scale to control O. rhinoceros, O. elegans, and S. australis (Chung 1997; Hallett et al. 1995; Ho 1996; Purba et al. 2000; Rochat et al. 2004; Witzgall et al. 2010).
Attraction of O. rhinoceros, O. elegans, and S. australis to the synthetic pheromone is enhanced by plant odors from decaying wood for O. rhinoceros (Alfiler 1999; Hallett et al. 1995; Sudharto et al. 2001), or from fresh palm tissues for O. elegans and S. australis (Rochat et al. 2002, 2004). Mass trapping with aggregation pheromone and a plant synergist has been reported for O. monoceros (Allou et al. 2002, 2006), O. elegans (Rochat et al. 2004), and O. rhinoceros (Chung 1997; Ho 1996).
Here, we report identification and field activity of the male aggregation pheromone of O. agamemnon and provide data to develop an efficient trapping system. The results of a four-year population monitoring study are presented, and the differences between the chemical communication systems of the rhinoceros beetles are discussed.
Methods and Materials
Insects
The insects used for experimentation were collected from the Rjim Maâtoug oasis in Tunisia (33 43′N, 8 40′E, 43 m above sea level), 120 km South-West of Kebili and near the Algerian border. The area is characterized by a Saharan continental climate, with precipitation below 100 mm/year and an average yearly temperature of 21 °C with extremes of 55 °C in the summer and 7 °C in the winter.
Larvae of various stages and pupae were transported to the laboratory and kept in a room under a controlled photoperiod (13 L:11D; darkness from 8:00 h to 19:00 h) and temperature (26–28 °C). The larvae were reared on tender stem tissue and aerial roots of date palms complemented with apples (Soltani et al. 2008b). Wild adults also were collected in the field from light traps during April. Virgin and wild insects were kept separately in the laboratory and fed on apple.
Collection of Pheromone
The volatile emissions from live O. agamemnon males and females were collected by passing an air stream (purified over activated charcoal; 100 ml/min) into four glass flasks containing six males or six females on 20 cm halved sugarcane. Volatile compounds were trapped with a cartridge loaded with 350 mg Supelpak™-2 (Supelco, USA) for 4 to 7 d. Effluvia from only sugarcane were trapped as a control. Either wild specimens with unknown mating status or reared and virgin individuals were used. Five series of collections were undertaken. The adsorbent cartridges were eluted with 5 ml 99.5 % hexane (Sigma-Aldrich, USA). Extracts were stored at −20 °C and used for GC and/or GC/MS analyses.
Gas Chromatography (GC) and Gas Chromatography coupled to Mass Spectrometry (GC/MS) Analyses
GC/MS analyses were carried out using a Bruker Scion 436 gas chromatograph coupled to a Bruker SQ mass spectrometer. The chromatograph was equipped with an apolar Rxi-5 Sil-MS column (30 m × 0.32 mm i.d. × 0.25-μm; Restek, France) with oven temperature held at 50 °C for 1 min then to 80 °C at 50 °C/min and held for 2 min and then to 300 °C at 10 °C/min. Helium was used as carrier gas at 2 ml/min. Samples were injected using a SPI injector at 250 °C.
Additional GC analyses were carried out using a Varian GC-430 gas chromatograph equipped with a FID detector heated to 250 °C. Two types of column were used: an apolar CP-Sil-5-CB column (30 m × 0.25 mm i.d. × 0.25 μm df, Varian, USA) operated from 50 °C for 1 min then heated to 80 °C at 50 °C/min, held for 1 min, then to 220 °C at 10 °C/min; a polar Rtx-Wax fused silica column (30 m × 0.32 mm i.d. × 0.5-μm crossbond PEG phase, Restek, USA) operated at 50 °C for 0.75 min then heated to 75 °C at 20 °C/min, then to 200 °C at 6 °C/min, and finally from 200 °C to 240 °C at 15 °C/min. Helium was used as carrier gas at 3 ml/min. Samples were injected with an auto-sampler in split-less mode for 0.75 min at 240 °C. The volume injected for each analysis was 0.5 μl.
Quantification of male specific compounds was done using GC calibration curves of each component. Three standard solutions (1 ng to 100 ng/μl) of the four synthetic compounds were injected as data points, and analyzed in three replicates. The calibration curve was obtained by plotting the analyzed peak area vs. the analyte concentration.
Synthetic Chemicals
Six pheromone components of other Oryctes species or related molecules identified from Oryctes males were used as analytical standards: ethyl 4-methyloctanoate (1), 4-methyloctanoic acid (2), 4-methyloctanyl acetate (3), 4-methyloctanol (4), methyl 4-methyloctanoate (5), and nonanyl acetate (6). Compounds 1 to 4 also were used for bioassays. Chiral molecules were used as racemic mixes. Compound 1 was purchased from E.G.N.O.-Chimie (Tancarville, France). Compound 2 was purchased from the same source or synthesized in the laboratory. Compounds 2, 3, and 4 were synthesized as described in Rochat et al. (2004).
Single Sensillum Recordings
Male and female O. agamemnon were anesthetized with CO2. An antenna was removed by cutting its scape. It was fixed on a cover glass and the lamellae of the antenna were kept open by using tiny pins. The antenna preparations were preferred over in situ recordings because the movement of the living insects impeded stable recordings of ORN activity (Renou et al. 1998). Single sensillum recordings were obtained from the inner lamella with electrically sharpened tungsten wires. The reference electrode was inserted into the scape. The recording electrode was brought into contact with the surface of the lamella until spikes were recorded.
Both electrodes were connected to a preamplifier NL 102 (Digitimer, UK). The signal was amplified (×1000) and filtered (0.2–10 kHz). It was digitized at 10 kHz and 12 bits with a Data conversion board (DT3001, Data Translation, USA). Spike firing was analyzed using Awave software (Marion-Poll 1995) to detect and sort spikes and to calculate the time of occurrence of individual spikes. Recordings started less than 1 min after connecting the recording electrode to a sensillum, and the recording session lasted less than 10 min for each sensillum. Recordings were repeated on at least five different insects with a maximum of five sensilla from the same antenna. Recording sessions from two sensilla of the same insect were separated by 5 min.
Stimulation of Olfactory Sensilla
Olfactory stimuli were delivered by a programmable stimulator that used distinct sources for pheromone and plant compounds. Air coming from the building supply was charcoal filtered and humidified. To create eight equal flows, the main flow of pure air was first divided into two in a Y connector (model 1/8 inch P-514, Upchurch Scientific, USA). The resulting flows were each divided into four equal flows by a 5-port manifold (model P-115, Upchurch Scientific). Each of the eight flows was connected to a miniature electro-valve (model LHDA1233115H, The Lee Company, USA). The output of each valve was connected to a stimulus source through a polytetrafluorethylene (PTFE) tubing (1.32 mm i.d. × 15 cm long).
Stimuli were deposited on filter paper and conserved in 4-ml glass vials, closed by septum caps. The inlet and outlet of the sources were made of two hypodermic needles (18-G size) inserted through each septum and connected to PTFE tubing. The tube was inserted into the input needle of the vial after evaporation of the solvent. Each sensillum was stimulated by effluvia of 4 male or female beetle day equivalents, fed on sugarcane and of four synthetic male specific compounds at 500 ng. Responses of O. agamemnon olfactory neurons to hexane, used as solvent for all stimulation solutions, were evaluated.
An 8-channel stimulation pencil was made by sealing eight PTFE tubes (1.32 mm i.d. × 35 cm long) with epoxy glue into a stainless steel cylinder (10 log cm × 7 mm i.d.). Each PTFE tube ended exactly at the opening of the metal cylinder, and its other end was connected to a stimulus source. A 1-ml plastic pipette tip, with its tip cut to make a 3- to 4-mm diam opening, was inserted into the output end of the cylinder. Thus, the odorized channels remained separate, and odorants were mixed only in the plastic cone. The stimulation pencil was mounted on a micromanipulator, and the outlet of the cone was positioned 6 ± 2 mm from an insect antenna.
Behavioural Tests
General
Behavioral tests were carried out both in Tunisia and in France. One day before a test, insects were isolated in small boxes without food. Half-an-hour before a test, these insects were transferred to the bioassay room and allowed to acclimatize for at least 30 min. All tests were carried out during the first 3 h of darkness under red light and at a temperature of 29–30 °C. After each olfactometric test, we cleaned the olfactometer with ethanol then dried it to eliminate any source of contamination. After each test series, the beakers were washed with detergent and oven-dried at 100 °C for 30 min.
Pitfall Olfactometer
The first experiments used a two-choice pitfall olfactometer comprised of an arena (45 cm diam × 20 cm high) and two beakers, adapted from Rochat et al. (1991). A beetle was released in the center of the arena and its behavior recorded until it fell into one of the beakers, or for 30 min, based on previous research on other Oryctes spp. (Rochat et al. 2004). A test was discarded when the beetle remained inactive for 5 min at the center of the olfactometer. For each series, we tested approximately 30 adults (15 males and 15 females). Eleven series were carried out. Distributions of choices were compared using one-tailed binomial tests at α = 0.05.
Four-Way Olfactometer
Responses by both sexes were tested in a four-armed olfactometer adapted from Saïd et al. (2006). A beetle was placed in the center of the olfactometer and its locomotor behavior was monitored for 10 min to determine the time spent in each arm. A test was discarded when the beetle remained inactive for more than 5 min. One-way ANOVAs (insects as blocks) followed by Tukey tests (multiple comparisons, α = 0.05) were used to compare the mean times spent in each arm.
Tested Odors
We used natural odors emitted by live males and hexane extracts of effluvia of 4 male or female day equivalents. The three major components identified in male effluvia, compounds 1, 2, and 3 were tested at 10 μg of standards, each alone, and mixed as 1 + 3 (80:20) or 1 + 2 + 3 (60:25:15). The ratios of synthetic compounds were determined according to their relative amounts in the chromatographic profiles.
Field Trapping Trials
Chemical Dispensers
Synthetic chemicals were emitted from heat-sealed polyethylene sachets (ALPLAST, France). Their characteristics and dimensions were adapted to the chemicals and the targeted release rates by laboratory calibration. Emission rates depended on the compound and were estimated following Rochat et al. (2004). Due to the high air temperatures, dispensers were enclosed in a second sachet to decrease evaporation. Dispensers were stored at −20 °C. The release rates of the synthetic compounds from the dispensers were estimated under conditions similar to those in the field and were 13, 6, and 3 mg/day, respectively for compounds 1 and the mixes 1 + 3 (80:20) and 1 + 2 + 3 (60:25:15).
Location and General Features
All field tests took place at Rjim Maâtoug oasis in Tunisia. The trials were carried out in a 2-ha plot containing 308 palm trees aged 23 yr, 2–5 m high. The maximum temperatures during the trapping period were between 40–55 °C. Traps were transparent plastic boxes (26 × 18 × 15.5 cm high; volume = 6 L) equipped with a lid with four (6 × 3 cm) openings at the four corners allowing insects to enter the box. An additional circular (diam 5 cm) central opening covered with a mosquito net facilitated aeration of the pheromone dispensers and of the palm pieces suspended in the box. Palm fiber was put on the sides of the trap to facilitate the access to the openings by the walking beetles. Plant odors were emitted by either 3 cm3 of freshly cut aerial roots or stem pieces of young offshoots. The palm material was wrapped in a punctured plastic bag to limit dehydration and was suspended from a hole in the lid. Plant tissues were replaced every 4 d. Water (0.5 L) was poured into the bottom to retain the captured insects, and to create moisture in the traps to ensure the longevity of palm tissues.
The traps were installed for 3 wk from July 25th to August 23rd, 2010 (Trial 1), and from July 5th to August 4th, 2013 (Trial 2), which corresponded to the periods of reproduction and maximum flight of O. agamemnon (Ehsine et al. 2009; Soltani et al. 2008a). Preliminary tests in the field with live insects to establish the best position for the traps showed that insects entered the traps only when they were set on the ground (Abdallah 2009). So in trials 1 and 2, traps were buried in the sandy ground at the base of a date palm tree up to the lid. Traps were visited every day to collect trapped beetles, and replace dead bait insects and empty chemical dispensers as needed. Traps were moved round every 3 d, such that each treatment was tested at each location.
Traps in Trial 1 were spaced only 18 m apart and regardless of the type of palms in their vicinity, which locally strongly influenced the captures. Thus, in Trial 2 they were set 27 m apart and the palm trees were categorized. Each treatment was equally distributed beside older male or female, or younger palm trees in order to reduce the impact of the environment heterogeneity: O. agamemnon populations indeed proved to be higher in areas with young or male palm trees than in areas planted with older or female palm trees (Hasni, personal observations).
Statistical Analyses
ANOVAs (glm procedure) evaluated the total captures in the traps per plant bait cycle (3 d). Data were transformed to homogenize the variances. Trial 1 was analyzed in a first step using a 2-way model: plant bait and synthetic beetle bait with the interaction using Ln(x + 1) transformation. Since the captures with the palm core were much greater than with the aerial roots (ca. 80 %) and the age of the palm core appeared to affect the captures, we performed a second ANOVA on the subset of the daily captures with palm core after (x + 0.5)1/2 transformation. The model tested the effect of the synthetic beetle bait with a co-variable: the mid-age of the plant bait. Mean total captures per synthetic bait were compared to only palm bait using either Tukey multiple comparison tests.
Trial 2 was analyzed with a model testing only for a global bait effect. Mean total captures per treatment were compared using Tukey multiple comparison tests.
The heterogeneity of the sex-ratio of the captures according to the baits was evaluated by χ 2 tests on the tables of the total captures per sex and treatments or groups of treatments. All analyses were carried out using MINITAB (v. 17, USA).
Results
Chemical Analysis
Comparisons of the odors emitted by sugarcane and of males or females feeding on sugarcane showed that the presence of males was correlated with four volatile compounds in variable proportions. In contrast, no female-produced chemicals were detected. The four major compounds were identified by their mass spectra as ethyl 4-methyloctanoate (1), 4-methyloctanoic acid (2), 4-methyloctanyl acetate (3), and 4-methyloctanol (4) (Fig. 1). The structural identifications were confirmed by comparisons of their GC retention times with those of reference compounds.
GC analysis on a polar Rtx-Wax column of Supelpak-2-trapped volatiles emitted by Oryctes agamemnon males (upper trace) or females (lower trace) feeding on sugarcane (SC) showing the four specific compounds emitted by males: ethyl 4-methyloctanoate (1), 4-methyloctanoic acid (2), 4-methyloctanyl acetate (3), and 4-methyloctanol (4). The others pics corresponded to volatiles emitted by sugarcane. No volatile compounds specific to the females were detected in these analyses in spite of significant attraction of males
The absolute and relative amounts of these compounds emitted in the five collections differed (Table 1). The acid 2 was by far the major compound in the three collections made from lab-reared males where the volatiles were 103 to 104 more abundant than in the others. In the other two collections made from field-collected insects of uncertain mated status, 2 was apparently absent, and ethyl ester 1 was the main component. Compound 1 was found in all five collections. Acetate 3 was present in four collections but as a minor component (approx. 4 %). Alcohol 4 was found in three out of five series as a minor component (approx. 3 %).
Pitfall Olfactometer
When stimulated, some insects moved directly towards the odor source, and others made several turns in the arena before reaching it. When males and females were stimulated by live males, all choices expressed were for the male side (Table 2). Seventy-six percent of the active males and females that made a choice stimulated by natural extracts preferred the natural male + plant odors to the plant odor. The natural female + plant odors attracted only males. In binary choice between natural male and female + plant odors, 94 % of active insects chose the natural male odors.
Males and females responded differently to synthetic potential pheromone compounds. Females were significantly more attracted to ethyl 4-methyloctanoate (1) than to hexane. Responses to this compound were less clear with males. On the other hand, males were significantly more attracted to 4-methyloctanoyl acetate (3) than to hexane while females were not. The responses of both sexes to the blend of 1 + 3 were significant, but responses by each sex were weak and not significant. Females responded significantly to 4-methyloctanoic acid (2), particularly the virgin ones, contrary to males, which did not react at all. The same behavior was observed in tests with the 1 + 2 + 3 blend: females responded significantly to this combination whereas males did not react.
Four-Way Olfactometer
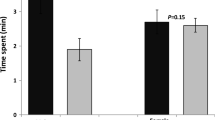
Times spent in each arm of the 4-way olfactometer by males and females differed significantly (Fig. 2). Males tended to respond more to the 1+3 blend (46 %) than to compound 1 alone (31 %). In contrast, females were significantly more attracted by compound 1 alone (60 %) than by the blend (7 %). The addition of compound 2 to the blend changed completely the responses by males and females. Binary choice tests between the 1+3 and 1+2+3 blends showed that females were significantly attracted by this combination (62 %) but not males (34 %).
Relative proportions of time spent by Oryctes agamemnon in the arms of the four-way olfactometer in response to ethyl 4-methyloctanoate (1) and blends with 4-methyloctanoic acid (2) and 4-methyloctanyl acetate (3) (10 μg total; 1 + 3 (80:20), 1 + 2 + 3 (60:25:15); percentages of time spent (total 10 min) in each of the four fields with the same letters do not differ significantly by Tukey tests, P < 0.05)
Electrophysiological Tests
Recordings from males and female pore plate sensilla with tungsten microelectrodes showed high firing even before stimulation leading in most cases to complete silence after a few minutes. Thus, the large number of rejected recordings and the short longevity of the antennal preparations limited the number of useful recordings. However, increased firing observed in response to volatile compounds confirmed the olfactory function of the pore plate sensilla, which provided consistent firing over 30 min or more. Generally, only one or two olfactory neurons from the same sensillum were recorded. One hundred sensilla were connected, but only 13 sensilla gave stable recordings. Olfactory neurons were specific and responded only to one compound. Five responded to compound 2 (Fig. 3), one to compound 1, and one to compound 3. The other neurones did not respond to our stimuli. The sex specificity of responses was not evaluated because of the limited number of active olfactory neurons.
Electrophysiological responses of one sensillum of Oryctes agamemnon to a hexane; b male + sugarcane (SC) volatile collection; c 1 μg of 4-methyloctanoic acid (2); d 1 μg ethyl 4-methyloctanoate, (1); e 1 μg 4-methyloctanyl acetate (3) showing firing of an olfactory receptor neuron tuned to compound 2 but not to compounds 1 and 3
Field Tests
Plant Effect
Trial 1 showed that the date palm core was more attractive than the aerial roots (F 1,232 = 56.03; P < 0.001). No interaction was found between plant bait and synthetic bait (F 3,232 = 3.44; P > 0.05). Irrespective of the synthetic beetle bait date palm core captured 3 to 5 times more beetles than did aerial roots (Table 3). Whatever the synthetic bait, trap catches decreased as date palm core tissue aged, and its renewal increased significantly the insect captures. In the subset of data with palm core, the daily captures significantly decreased with aging (a = −0.0675 ± 0.0220; t = −3,07; P = 0.002). The captures were about 4 times lower at the fourth day of trapping than at first reading (Fig. 4). A similar trend was observed with the aerial roots, but the difference was less clear due to lower and more variable effects than with palm core.
Pheromone Effect
In trial 1, the addition of male compounds to palm tissue (roots and palm core) increased the trap captures (F 3,232 = 4.45; P < 0.05; Tukey test; P < 0.05). The mixture of compounds 1 + 3 was as attractive as 1 alone when combined with palm core (Table 3). The addition of compound 2 led to more insects captured but not significantly more than with 1 or the blend of 1 + 3 (Dunett test, P > 0.05).
The baits tested in Trial 2 (Table 4) led to very different levels of captures (F 6,210 = 14.20; P < 0.001). The combinations of palm core and male compounds were more attractive than palm core or pheromone compounds alone (Dunett test, P < 0.05). Here, the 1 + 2 mixture combined with date palm core captured significantly more insects then all the other baits (Tukey test; P < 0.05), about 8 times more than only palm core and 5 times more than only the 1 + 2 mixture. Large variability of the captures obscured any possible differences in attractiveness of the other baits.
Sexual Dimorphism in the Response
Overall, the traps attracted as many males as females in Trial 1 (Table 3; χ 2 = 1.37, 1 df = 1, P > 0.10). Some heterogeneity in the sex-ratio of the captures according to the bait was observed but could not be consistently correlated to a type of bait. In turn, Trial 2 caught more females than males (Table 4; χ 2 = 52.65, df = 1, P < 0.001). The predominance of females was greater with what was by far the most attractive bait of palm core plus 1 + 2 (71.5 %) than with all the other baits (59.7 %) (Table 4; χ 2 = 8.74, df = 1, P = 0.003).
Discussion
Our data demonstrate that male O. agamemnon produce an aggregation pheromone as do other dynastid species. Live males and isolated male effluvia mixed with plant odors attracted males and females, which suggested the emission of an aggregation, and possibly sexual, signal. Female effluvia mixed with plant odors moderately attracted only males, suggesting that females might also emit a sex pheromone. The analysis of the insect effluvia revealed four male-specific compounds, while no female specific compounds were detected. Two of them (1 and 2) have been previously reported as aggregation pheromones of other Oryctes species. Compound 2, 4-methyloctanoic acid, is the major component of the aggregation pheromone of O. elegans (Rochat et al. 2004), and 1, ethyl 4-methyloctanoate, is the major compound of the aggregation pheromones of O. rhinoceros (Hallett et al. 1995) and of O. monoceros (Gries et al. 1994). The quantities of 1 and 2 varied greatly from one effluvia collection to another. Compounds 1 and 3, 4-methyloctanyl acetate, were detected in most collections and compound 2 was the most abundant male volatile by far overall. Compound 4, 4-methyloctanol, was detected in three of the five collections in small quantities. This quantitative variability could be explained by the different and physiological states of the emitters. Indeed the insects of the two collections with relatively low amounts of material were collected early in April. They probably emerged the previous year, and had mated and over-wintered (Ehsine et al. 2009; Soltani et al. 2008a). The males used in the three other collections emerged in the laboratory and were isolated from females. These virgin individuals yielded a high quantity of compounds 1 and 2. Compounds 3 and 4 have been reported from O. elegans male effluvia by Rochat et al. (2004), who did not evidence a pheromone role.
Electrophysiological screening of the peripheral olfactory system of O. agamemnon in response to the four male-specific compounds was limited but highlighted the presence of olfactory neurons that independently detected compounds 1, 2, and 3. This result strongly supported that these molecules were involved in the chemical communication of O. agamemnon. Electroantennography recordings in O. elegans showed that the response to the pheromone was significantly greater in females than males (Rochat et al. 2004) contrary to O. rhinoceros (Renou et al. 1998) and O. monoceros (Gries et al. 1994) in which no sex dimorphism was observed at the peripheral olfactory system. Thus, a more comprehensive study is necessary to characterize fully the antennal olfactory receptors tuned to pheromone detection in O. agamemnon and assess its functional properties and sex dimorphism.
Sex dimorphism was recorded in the behavioral responses in the olfactometers and in the field. Compound 1, either alone or with palm odor, attracted both males and females, which suggested that this compound plays a pheromone role as in O. rhinoceros and O. monoceros (Gries et al. 1994; Hallett et al. 1995). Compound 2 was attractive to females, particularly virgin, irrespective of the additional odors proposed, either from palm or conspecifics. Males, especially virgin, did not respond to, or even seemed inhibited by 2, particularly when presented alone in the laboratory assays. This sex-specific response was confirmed during the field assays where more females were captured in the traps baited with 2 either alone or in mixture. We hypothesize that compound 2 primarily ensures a sexual attraction of virgin females towards the date palms where males emit the pheromone. In O. monoceros, this acid antagonized the responses to compound 1, the attractive pheromone, in a dose dependant manner, in traps complemented with a palm co-attractant (Allou et al. 2006). The effect was not sex specific. Oryctes monoceros males also produce both 1 and 2 in a variable ratio, but the connection between the composition of the male effluvia, the mating status of the emitters, and the behavioral responsiveness has not been studied.
Compound 3 likely is contributing to pheromone communication in O. agamemnon at least between males since it was detected by specific olfactory receptor neurons in both sexes and was active in olfactometers, especially on males. However we did not observe an effect in the field in our trial. Further studies obviously are required to clarify its role under natural conditions possibly with 4, to which no olfactory receptor neurones showed a response in our study and which behavioral activity has not been investigated after this negative result. Other mixtures of the male specific compounds covering a broader range of ratios should be evaluated as well as dose–response relationships under field conditions.
We did not investigate the enantiomeric composition of the molecules produced by O. agamemnon. Insects responded clearly to racemic compounds 1 and 2, indicating that chirality was not a critical point in the species or suggesting that it uses a racemic pheromone as assumed for O. elegans (Rochat et al. 2004). Oryctes elegans could produce (4S)-ethyl 4-methyloctanoate as shown by Hallett et al. (1995) for O. rhinoceros. The authors reported in field trapping trials that 4S-1, the natural enantiomer, and the racemic mixture were equally attractive (Hallett et al. 1995).
At the dose evaluated, the mixture of 1 with 2 and/or 3 triggered better responses than compound 1 alone in most cases on one or the other sex or both. The male aggregation pheromone of O. agamemnon then is a mixture of compounds previously reported from other Oryctes species: at least of the two pheromone compounds previously identified for O. elegans, O. monoceros, and O. rhinoceros, which all responded to either only 4-methyloctanoic acid or only ethyl 4-methyloctanoate.
In the field, both the pheromone components and the plant odors attracted O. agamemnon to the traps. The position of the trap was important: no beetle was captured 2 m above the ground even when live males were used as stimulus (Abdallah 2009). Night observations of O. agamemnon, which were approaching a date palm tree, indicated that the arriving beetles were walking and not flying (Hasni, personal observation). Therefore, traps were embedded in the ground for the trials.
Weather conditions also influenced the capture rates. More insects were captured when moonlight intensity was low, or the wind was moderate, or temperature relatively high (Hasni, personal observation). However, temperatures higher than 45 °C (mean daily value) for several days were lethal to the adults, which were then found dead around the palm trees in numbers in correlation with lower captures than during the cooler periods.
The palm material, especially core, led to low but regular captures. It enhanced the attraction of the synthetic pheromone as earlier reported for O. elegans (Rochat et al. 2004). Host plant odors seem, therefore, important for O. agamemnon, which has been reported as a borer feeding on aerial roots, with possible subsequent severe damage (Ehsine et al. 2009; Soltani et al. 2008a; Soltani 2010). Khalaf et al. (2013) indicated that larvae and pupae also were present in other parts of the palm tree, and showed that mated females laid eggs between the aerial roots, as well as all along the stem, particularly at the base of cut fronds. Oryctes agamemnon and O. elegans are adapted to an arid climate where the dead woody material necessary to larval growth is available only on standing palm trees in contact with living tissues that bring water. In contrast, larval substrate is essentially available on the ground (fallen stems) or in the litter in moist tropical climates where O. monoceros and O. rhinoceros live. In the latter species, attraction to the pheromone is increased by odors emanating from decaying plant material (e.g., empty fruit bunches), which is a larval substrate where females lay eggs (Alfiler 1999; Allou et al. 2006; Hallett et al. 1995; Sudharto et al. 2001).
The aging of the palm substrate was correlated with a loss of attraction and synergy with the pheromone in O. agamemnon as observed for O. elegans (Rochat et al. 2004). Part of this loss was likely due to strong dehydration under the summer desert climate. However, water was regularly added to the traps, and the change in attraction suggests rather that the species is not interested in an odor of decayed tissues. In contrast, aging of the empty fruit bunches led to increased synergy with aggregation pheromone in O. monoceros (Allou et al. 2006) stressing the very different ecology of the tropical species seeking substrates with quite an advanced degradation of the woody material, likely more suitable for larval development than fresh ones. Variable attractiveness of cut palm tissue is well-known in the Rhynchophorus palm weevils, but the latter preferably orient towards wounded and fermenting tissues and are known to respond to some fermentation volatiles (Giblin-Davis et al. 1996; Guarino et al., 2011; Rochat et al. 2000a; Vacas et al. 2014).
As mentioned earlier, O. agamemnon and O. elegans are sympatric in several regions of Near and Middle East and can be observed at the same time, for instance attracted to light (Rochat, personal observation). Both species develop in the same date palm orchards with an apparent important overlap in the ecological niches. The composition of the male aggregation pheromone should play a decisive role in reproductive isolation.
In O. elegans, the aggregation pheromone is only compound 2 (Rochat et al. 2004), while it is a mixture of 1 and 2, and possibly 3 in O. agamemnon. In the field, better attraction was achieved into traps attached at the stem for O. elegans while O. agamemnon was only caught in traps embedded in the soil, in agreement with reports of egg laying at the base of the palms, which is less reported for O. elegans. Thus, the two species would not be accessing the palm tree the same way and prefer different areas for meeting in addition to responding to different pheromones, although they share common components. Finally the synergistic effect of palm odors with the pheromone seems lesser in O. agamemnon than in O. elegans where both sources of semiochemicals were weakly attractive alone (Rochat et al. 2004). The difference may reflect additional sensory or behavioral features specific to each Oryctes species.
Specific experimental studies should be undertaken to understand how the two Oryctes species share the palm resource and maintain reproductive isolation. This will be particularly important in view of developing efficient monitoring or control tools based on semiochemicals.
Our results offer the opportunity to develop semiochemical-based monitoring and possibly mass trapping of O. agamemnon. The high proportion of females caught is favorable to the latter option, which should positively contribute to reducing the use of conventional insecticide application and thus provide a more efficient and safer elimination of these beetles, as reported for other dynastids (Rochat et al. 2002). This method is particularly interesting considering the human health risks of using insecticide in the fragile environment of the oases and the major economic importance of date palm for farmers in south Tunisia. In addition, the farmers have little economic means. Reducing the cost of production with an alternative cheaper than insecticides also would be beneficial. One critical point of this trapping strategy is the necessity to use fresh date palm tissue to synergize the pheromone. Further research is underway to find cheap substitutes for date palm core, either natural or synthetic, to cope with this limitation.
References
Abdallah Z (2009) Investigation de l’écologie chimique du ravageur du palmier dattier Oryctes agamemnon Burmeister. Mémoire présenté pour l’obtention du diplôme de mastère en chimie industrielle et environnement à la faculté des sciences de Gafsa, Tunisia, pp. 14–44
Alfiler ARR (1999) Increased attraction of Oryctes rhinoceros aggregation pheromone, ethyl 4-methyloctanoate, with coconut wood. Coconut Res Dev 15:131–149
Allou K, Morin, J-P, Rochat D (2002) Amélioration du piégeage olfactif de Oryctes monoceros (Olivier), Coleoptera, Dynastidae, ravageur du cocotier et du palmier à huile en Côte d’Ivoire. Proceedings of the AFPP – Sixième Conférence Internationale sur les Ravageurs en Agriculture, Montpellier, France, pp. 295–302
Allou K, Morin J-P, Kouassi P, Hala N’klo F, Rochat D (2006) Oryctes monoceros Trapping with Synthetic Pheromone and Palm Material in Ivory Coast. J Chem Ecol 32:1743–1754
Balachowsky AS (1962) Entomologie appliquée à l’agriculture. Tome I Coléoptères. Masson, Paris, Vol. I, p.564
Bedford GO (1980) Biology, ecology and control of palm rhinoceros beetles. Annu Rev Entomol 25:309–339
Chung GF (1997) The bioefficacy of the aggregation pheromone in mass trapping of rhinoceros beetles (Oryctes rhinoceros L.) in Malaysia. Planter 73:119–127
Ehsine M, Belkadhi MS, Chaeib M (2009) Bio-ecologic Observations on Rhinoceros Beetle Oryctes agamemnon (Burmeister 1847) on the Palm Dates Oasis of Rjim Maatoug in South-western Tunisia. J Arid Land Stud 19:379–382
Endrödi S, Petrovitz R (1974) Die Arten und Rassen der Gattung Oryctes Illiger in Iran. Entomol Phytopathol Appl (Iran) 36:4–19
Giblin-Davis RM, Oehlschlager AC, Perez A, Gries G, Gries R, Weissling TJ, Chinchila CM, Pena JE, Hallett RH, Pierce HD Jr, Gonzalez LM (1996) Chemical and behavioral ecology of palm weevils (Curculionidae: Rhynchophorinae). Fla Entomol 79:153–167
Groupement interprofessionnel des fruits (2014) Rapport annuel des activités du Ministère de l’agriculture, des Ressources Hydrauliques et de la Pêche. Janvier 2014, p. 30
Gries G, Gries R, Perez AL, Oehlshlager AC, Gonzalez LM, Pierce HD Jr, Zebeyou M, Kouame B (1994) Aggregation pheromone of the African rhinoceros beetles, Oryctes monoceros (Olivier) (Coleoptera: Scarabaeidae). Z Naturforsch 49c:363–366
Guarino S, Bue PL, Peri E, Colazza S (2011) Responses of Rhynchophorus ferrugineus adults to selected synthetic palm esters: electroantennographic studies and trap catches in an urban environment. Pest Manag Sci 67:77–81
Hallett RH, Perez AL, Gries G, Gries R, Pierce HD Jr, Yue J, Oehlschlager AC, Gonzalez LM, Borden JH (1995) Aggregation pheromone of the coconut rhinoceros beetle, Oryctes rhinoceros (L.) (Coleoptera: Scarabaeidae). J Chem Ecol 21:1549–1570
Ho CT (1996) The integrated management of Oryctes rhinoceros (L.) populations in the zero burning environment, in Proceedings of the PORIM International Oil Palm Congress. Kuala Lumpur, Malaysia, pp 336–368
Khalaf MZ, Al Rubeae HF, Al-Taweel AA, Naher FH (2013) First record of Arabian Rhinoceros Bettle, Oryctes agamemnon arabicus Fairmaire on date palm trees in Iraq. Agric Biol J N Am 4:349–351
Khoualdia O, Rhouma A, Marro J-P, Brun J (1997) Première observation sur Oryctes agamemnon, ravageur de palmier dattier en Tunisie. Fruits 52:111–115
Marion-Poll F (1995) Object-oriented approach to fast display of electrophysiological data under MS-Windows. J Neurosci Methods 63:197–204
Morin J-P, Rochat D, Malosse C, Lettere M, Desmier De Chenon R, Wibowo H (1996) Ethyl 4-methyloctanoate, major component of Oryctes rhinoceros (L.) (Coleoptera, Dynastidae) pheromone. CR Acad Sci Paris, Sciences de la vie 319:595–602
Purba R, Prawirosukarto S, Desmier de Chenon R, Morin J-P, Rochat D (2000) Effect of Oryctes rhinoceros pheromone (ethyl 4-methyloctanoate) diffusion rate on the size of pest catches, in Proceedings of the XXIth International Congress of Entomology. Foz do Iguaçu, Brasil, August 20–26, 2000, Vol. I, p. 181
Renou M, Tauban D, Morin J-P (1998) Structure and function of antennal pore plate sensilla of Oryctes rhinoceros L (Coleoptera-Dynastidae). Int J Insect Morphol Embryol 27:227–233
Rhouma A (1996) Le palmier dattier en Tunisie : Un secteur en pleine expansion. Options Méditerranéennes Série A 85–103
Rochat D, González AV, Mariau D, Villanueva AG, Zagatti P (1991) Evidence for male-produced aggregation pheromone in American palm weevil, Rhynchophorus palmarum (L.) (Coleoptera: Curculionidae). J Chem Ecol 17:1221–1230
Rochat D, Nagnan-LeMeillour P, Esteban-Duran JR, Malosse C, Perthuis B, Morin J-P, Descoins C (2000a) Identification of pheromone synergists in American palm weevil, Rhynchophorus palmarum, and attraction of related Dynamis borassi (Coleoptera, Curculionidae). J Chem Ecol 26:155–187
Rochat D, Ramirez-Lucas P, Malosse C, Aldana R, Kakul T, Morin JP (2000b) Role of solid-phase microextraction in the identification of highly volatile pheromones of two Rhinoceros beetles Scapanes australis and Strategus aloeus (Coleoptera, Scarabaeidae, Dynastinae). J Chromatogr A 885:433–444
Rochat D, Morin JP, Kakul T, Beaudoin-Ollivier L, Prior R, Renou M, Malosse I, Stathers T, Embupa S, Laup S (2002) Activity of male pheromone of Melanesian rhinoceros beetle Scapanes australis. J Chem Ecol 28:479–500
Rochat D, Mohammadpoor K, Malosse C, Avand-Faghih A, Lettere M, Beauhaire J, Morin JP, Pezier A, Renou M, Abdollahi GA (2004) Male aggregation pheromone of date palm fruit stalks borer Oryctes elegans. J Chem Ecol 30:387–407
Saïd I, Aldana de la Torre R, Morin JP, Rochat D (2006) Adaptation of a four arm olfactometer for behavioural bioassays of large insects. Chemoecology 16:9–15
Soltani R (2010) The rhinoceros beetle Oryctes agamemnon arabicus in Tunisia: Current challenge and future management perspectives. Tunis J Plant Prot 5:179–193
Soltani R, Chaieb I, Ben Hamouda MH (2008a) Descriptive study of damage caused by the rhinoceros beetle, Oryctes agamemnon, and its influence on date palm oases of Rjim Maâtoug, Tunisia. J Insect Sci 8, Article 57
Soltani R, Chaieb I, Ben Hamouda MH (2008b) The life cycle of the root borer, Oryctes agamemnon, under laboratory conditions. J Insect Sci 8:1–6
Sudharto PS, Purba RY, Rochat D, Morin JP (2001) Synergy between empty oil palm fruit bunch and synthetic aggregation pheromone (ethyl 4-methyloctanoate) for mass trapping of Oryctes rhinoceros beetles in the oil palm plantation in Indonesia, in Proceedings of the PORIM International Oil Palm Congress. Kuala Lumpur, Malaysia, pp 661–664
Vacas S, Abad-Payá M, Primo J, Navarro-Llopis V (2014) Identification of pheromone synergists for Rhynchophorus ferrugineus trapping systems from Phoenix canariensis palm volatiles. J Agric Food Chem 62:6053–6064
Witzgall P, Kirsch P, Cork A (2010) Sex Pheromones and their impact on pest management. J Chem Ecol 36:80–100
Zaid A, de Wet PF (2002) Date palm cultivation. Chapter I: Botanical and systematic description of the date palm. FAO Plant Production and Protection Paper 156 Rev 1
Acknowledgments
This work received financial support from The French-Tunisian University Cooperation Joint Committee (CMCU PHC Utique project no. 13G0927). We thank Josiane Beauhaire for synthesis of compound 2, Centina Pinier and Brigitte Frérot for additional GC/MS analysis, the Office of Development of Rjim Maâtoug that allowed the realization of field tests in their oasis, all the technicians from the Office, without whom the field work could not have been possible, Ann Porter Cloarec for English revision, and two anonymous reviewers for helpful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saïd, I., Hasni, N., Abdallah, Z. et al. Identification of the Aggregation Pheromone of the Date Palm Root Borer Oryctes agamemnon . J Chem Ecol 41, 446–457 (2015). https://doi.org/10.1007/s10886-015-0577-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-015-0577-7