Abstract
Hyperaccumulation is the phenomenon whereby plants take up and sequester in high concentrations elements that generally are excluded from above-ground tissues. It largely is unknown whether the metals taken up by these plants are transferred to floral rewards (i.e., nectar and pollen) and, if so, whether floral visitation is affected. We grew Streptanthus polygaloides, a nickel (Ni) hyperaccumulator, in short-term Ni supplemented soils and control soils to determine whether Ni is accumulated in floral rewards and whether floral visitation is affected by growth in Ni-rich soils. We found that while supplementation of soils with Ni did not alter floral morphology or reward quantity (i.e., anther size or nectar volume), Ni did accumulate in the nectar and pollen-filled anthers—providing the first demonstration that Ni is accumulated in pollinator rewards. Further, S. polygaloides grown in Ni-supplemented soils received fewer visits per flower per hour from both bees and flies (both naïve to Ni-rich floral resources in the study area) relative to plants grown in control soils, although the probability a plant was visited initially was unaffected by Ni treatment. Our findings show that while Ni-rich floral rewards decrease floral visitation, floral visitors are not completely deterred, so some floral visitors may collect and ingest potentially toxic resources from metal-hyperaccumulating plants. In addition to broadening our understanding of the effects of metal accumulation on ecological interactions in natural populations, these results have implications for the use of insect-pollinated plants in phytoremediation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metal hyperaccumulation has been described in over 500 plant species, representing 101 families (Sarma 2011), and refers to the uptake and sequestration of soil metals, e.g., copper, nickel, and zinc, into above ground tissues (reviewed in van der Ent et al. 2013). Concentrations that define hyperaccumulators vary by metal, although thresholds generally are at least one order of magnitude higher than average metal concentrations in plant tissues. For example, Nickel [Ni] hyperaccumulators have at least 1,000 ppm Ni dry weight in tissues, while average Ni concentrations in plant tissues are <10 ppm (van der Ent et al. 2013). Roughly three quarters of metal hyperaccumulating plants are accumulators of the metal Ni and occur on serpentine soils (Reeves 2006), which are derived from metal-rich ultramafic rocks (Alexander et al. 2007). Despite the documented abundance of natural populations of Ni hyperaccumulating plants, the adaptive value and ecological significance of hyperaccumulation is still uncertain (Boyd 2004; Boyd and Martens 1992).
Several hypotheses regarding the adaptive value of metal hyperaccumulation have been proposed, including its role in interference (i.e., elemental allelopathy), drought resistance, and defense against antagonists (reviewed in Boyd and Martens 1992). However, special attention has been paid to plant-herbivore interactions. Specifically, Boyd and Martens (1992) posit that metal hyperaccumulation in vegetative tissues provides defense against insect herbivores and bacterial/fungal pathogens, since moderate to high concentrations of metals in tissues can be toxic to many organisms (Coleman et al. 2005). Metals in vegetative tissues can act as feeding deterrents (Boyd and Jhee 2005), or, when ingested, can decrease growth and survival of insect herbivores (Boyd and Martens 1994). Beyond impacts on herbivory, however, little is known about how metal hyperaccumulation in plant tissues may alter ecological interactions, particularly mutualistic interactions. Of the limited studies in terrestrial systems, evidence suggests that prolonged metal exposure has led to divergent microbial communities in the guts of soil-dwelling isopods (Lapanje et al. 2010) and decreased mycorrhizal abundance and diversity in plants growing in metal-polluted environments (Leyval et al. 1997; Vogel-Mikus et al. 2005). However, effects of metal accumulation in flowers on plant-pollinator interactions remain largely unstudied, despite the fact that toxic plant secondary chemicals, e.g., alkaloids, in nectar are well known to alter pollinator foraging (reviewed in Adler 2001).
Metals accumulate in flowers of metal hyperaccumulators (Baker and Brooks 1989), but floral rewards (i.e., nectar and pollen) rarely have been separately evaluated. Recent experiments have shown that Ni in artificial nectar solutions decreases visitation by bumblebees (Meindl and Ashman 2013). In contrast, recent studies with selenium (Se) hyperaccumulator and non-hyperaccumulator taxa suggest that Se accumulation does not influence pollinator visitation (Hladun et al. 2013; Quinn et al. 2011). Considering that Se is toxic to honeybees (Hladun et al. 2012), generalist naïve pollinators (i.e., those not adapted to Se-rich floral rewards) foraging on Se-rich flowers may suffer negative fitness consequences. Similar effects may be seen for metal hyperaccumulating plants, since some bees cannot detect Ni in flowers prior to visitation (Meindl and Ashman 2013) although Ni is toxic to insect herbivores (Boyd and Jhee 2005). Our study was designed to determine whether (1) Ni hyperaccumulating plants accumulate Ni into floral rewards (e.g., nectar and pollen), and (2) generalist floral visitors forage indiscriminately on Ni-rich flowers. These determinations will inform pollination ecology of metal hyperaccumulating plants in natural populations, as well as provide insight into ecological consequences of using these plants to remediate metal contaminated soils (i.e., phytoremediation; Pilon-Smits 2005).
While metals present in flowers may influence pollinator visitation directly (e.g., Meindl and Ashman 2013), there also may be indirect effects via modification of floral traits important for pollinator attraction, such as flower production and floral reward quantity. For example, heavy metals such as copper and Ni can delay flowering (Brun et al. 2003) and decrease total production of flowers per plant (Saikkonen et al. 1998), which in turn can reduce pollinator visitation (Mitchell et al. 2004). Furthermore, soil metals can decrease viable pollen production/flower (Slomka et al. 2012) and thereby lower the quantity and quality of rewards offered to pollinators, though effects of metals on nectar production and chemistry are unknown. Thus, soil metals may alter pollinator visitation to plants growing in metal-rich soils either directly by altering reward amount (i.e., reward quantity) or chemical composition (i.e., reward quality), or indirectly by altering floral display.
Here, we provide an initial test of whether Ni accumulation by a serpentine-endemic Ni hyperaccumulator (Streptanthus polygaloides Gray [Brassicaceae]) alters floral display, floral reward quantity and quality, and visitation by naïve floral visitors. Specifically, we address the following questions: (1) Does short-term exposure to Ni-supplemented soil alter floral display or quantity of nectar and pollen? (2) Is soil Ni absorbed and incorporated into floral nectar and pollen? (3) Does soil Ni alter the likelihood a plant is visited by flower-visiting insects or its overall visitation rate per flower?
Methods and Materials
Study System
Streptanthus polygaloides is an annual serpentine endemic and a Ni hyperaccumulator (Baldwin et al. 2012; Reeves et al. 1981).
Its zygomorphic flowers attract bees, flies and beetles (e.g., Dianthidium spp., Ceratina spp., Apis mellifera (Hymenoptera), Syrphidae (Diptera), Bupresitidae (Coleoptera); Wall and Boyd 2002; unpublished data) that feed on pollen from exerted anthers (Preston 1991) and nectar produced at the base of stamens. Individual flowers remain open for at least 4 d (unpublished data).
Experimental Design
Seeds collected from a population (37°36′ 48.71″ N, 120°08′ 22.08″ W) in Mariposa County, CA, USA were germinated on a thin layer of perlite (~6 mm) in 27 cm3 ‘rocket’ pots (Deepots, Stuewe and Sons, Inc.) filled with potting soil (Fafard® #4) in a greenhouse at the University of Pittsburgh. After twelve weeks of growth, 48 plants were moved to a site in an open field at the Powdermill Nature Reserve in western PA (40°10′N, 79°16′W). This site provided abundant generalist pollinators (unpublished data). At onset of flowering during June 2012, plants were divided into two soil treatments: + Ni and control (no Ni added to soil). Nickel was applied to Ni-treated plants by top-watering once per day with 40 ml of solution containing 200 ppm Ni for 14 d prior to floral visitor observation experiments. Because metal hyperaccumulating plants have a high affinity for metals and thus the ability to rapidly acquire them from soils (Li et al. 2003), we applied solution treatments during flowering to ensure that Ni was available to plants during flower production. While this may not simulate natural conditions, it allows us to focus on Ni accumulation rather than other ontogenetic changes that might occur over long periods of exposure. Nickel treatment solutions were prepared using Ni nitrate [Ni(NO3)2], a Ni salt commonly used for studies of Ni hyperaccumulation (e.g., Kramer et al. 1997). The treatment solution was slowly and carefully applied to the soil surface using a plastic syringe, such that solution did not excessively contact shoots. Serpentine soils contain phytoavailable fractions of Ni that generally range from 50 to 500 ppm (Chardot et al. 2005; L’Huillier and Edighoffer 1996), thus the Ni concentration used here to treat soils is conservative. Nitrates do not directly affect pollinator visitation generally (Burkle and Irwin 2010), so it is assumed that any differences in visitation between treatments here are due to differences in Ni concentrations. Furthermore, the short duration of treatment application makes it unlikely that nitrates significantly altered floral visitation, as even long-term nitrogen supplementation experiments have failed to find strong effects of nitrogen addition on floral visitation (Burkle and Irwin 2010). Control plants similarly were top-watered with 40 ml of pure water for 14 d prior to floral visitor observation experiments. When plants were not being observed (see below), they were kept under an awning to protect them from occasional rainfall.
Floral Measurements
Prior to pollinator observations, we measured flower size with digital calipers (the product of flower [i.e., perianth] length and width, in mm) for two randomly selected flowers per plant. We also counted the number of open flowers per plant. Open flowers and flower size were enumerated once at the beginning of each week of observation. Following floral visitor observations, plants were moved indoors, and we collected anthers and nectar. For each individual plant, all six anthers were collected from ten mature but unopened buds (i.e., 60 anthers collected per plant), air dried for 48 hr, and then weighed on a AE200 Mettler® analytical balance to the nearest 0.0001 g. Nectar was allowed to accumulate within flowers for 12 hr, and then it was collected on filter paper wicks (Whatman®, Grade 1) from four flowers per plant (generally 3–4 μl collected per plant). By folding a circular piece of filter paper in half, and then touching the folded edge to the floral nectaries, we collected nectar consistently in a circular pattern. Nectar volume was determined via Baker’s (1979) spot-staining method, as described in Kearns and Inouye (1993), by comparing the measured diameter (mm) of each circular nectar spot on filter paper to a table of nectar spot diameters corresponding to nectar volumes (μl). This technique is valid for nectars with sugar concentrations ranging from 10–50 %, which is in the range of many Brassicaceae species (Masierowska 2003), and provided that nectar spot diameters are ≤12 mm, which was the case in our study. Average anther mass and nectar volume per flower were calculated as estimates of reward quantity. Anther samples and nectar samples each were pooled within individual plants for chemical analysis; thus each individual plant was treated as a separate replicate providing one nectar and one anther sample. We assume that anther mass and number of pollen grains per anther are positively correlated, as has been shown for other species (Bhowmik and Datta 2013), and hereafter consider anther mass as a measure of pollen quantity.
Floral Reward Analysis
Anther and nectar samples from each plant were microwave digested in two ml of trace metal grade HNO3 and brought to a final volume of 12 ml with MilliQ H2O (Millipore, Bedford, MA, USA). A five ml aliquot of diluted digest was further diluted with five ml of 2 % HNO3 solution and mixed with a small volume (80 μl) of known concentrations of three internal standards (Beryllium, Germanium, Thallium). Concentration of Ni in anthers (mg kg−1) and nectar (μl L−1) were determined via Inductively Coupled Plasma Mass Spectrometry (ICP-MS, Perkin/Elmer NEXION 300X) at the University of Pittsburgh. A series of five samples with known Ni concentrations were used to construct standard calibration curves prior to each run of samples on the ICP-MS. Duplicate samples and blanks, each containing internal standards, were analyzed at regular intervals as a measure of quality control during sample processing on the ICP-MS, and were within 10 % of each other. Control filter paper wicks also were processed to verify the absence of Ni in the filter paper itself. Previous work has shown that elevated Ni concentrations in anthers are positively correlated with elevated Ni concentrations in pollen grains for S. polygaloides (ρ = 0.61; unpublished data), thus here we use anther Ni concentrations as a surrogate for pollen Ni concentrations and as a measure of pollen quality. Nickel concentrations of pollen or nectar for each plant were used in analyses of reward quality.
Floral Visitor Observations
We arranged plants on trays for floral visitor observations, placing four Ni-treated plants and four control plants at random locations along the perimeter of a circle with a diameter of 52 cm on each tray. Two trays were placed side-by-side for observation outside at the study location. Observations were made for ten 10-min intervals per day for 2–3 consecutive days, with the positions of the trays switched after each observation to avoid spatial bias. Two new trays of plants were observed each week for three consecutive weeks (N = 48 plants; 800 min of observation). Soil treatments were applied to each group of 16 plants for the 2 wk immediately prior to observation of those plants. To determine whether Ni exposure in soil affects the likelihood of flower visitation, we calculated the probability that individual plants were visited (the number of observation intervals with at least one visit divided by the total number of observation intervals). To determine whether exposure to Ni in soil alters visitation rate, we recorded the number of flowers visited per plant per observation interval, and calculated flower visitation rates as the total number of visits to each plant / number of flowers per plant / hour. Visitation rates for each plant were averaged across all observation intervals of each week, and these average values were used in analysis of visitation rates (N = 24 per soil treatment). We recorded identity of flower visitors and kept records of visitation by bees and flies separately.
Statistical Analysis
All statistical analyses were conducted using SAS version 9.3 (SAS 2012). Flower size, display size, anther mass, and nectar volume were analyzed using MANOVA (PROC GLM), with soil treatment as a fixed effect. We used mixed-model ANOVA (PROC GLM) to determine whether plants grown in high-Ni soil accumulated more Ni into floral rewards than control plants, with soil treatment, reward type (i.e., nectar or pollen) and their interaction as fixed effects, and week as a random factor. We also used mixed-model ANCOVAs (PROC GLM) to determine whether Ni-treatment altered the probability of visitation or visitation rate, with floral visitor type (bee or fly), treatment (Ni or control) and their interaction as fixed effects, and week as a random factor. Flower size and the number of open flowers per plant were included as covariates in the ANCOVAs on visitation to account for effects of morphological variation. For all mixed-models, F-values were calculated by dividing the mean square of each fixed effect by the mean square of the interaction between that fixed effect and the random factor (i.e., week). Post-hoc Tukey tests were used to compare Ni concentrations in each tissue type (i.e., anther or pollen) across treatments and weeks, as well as to compare floral visitation for each floral visitor type (i.e., fly or bee) across treatments and weeks. To improve normality of residuals, visitation rate was square-root transformed and Ni concentration was natural-log transformed prior to analysis.
Results
Floral Measurements
MANOVA revealed no significant effect of Ni-treatment on any component of floral morphology or reward quantity (Wilks’ λ = 0.89; F 4,43 = 1.3; P = 0.28). Plants produced similar: (1) numbers of open flowers (control: 18.71 [± 2.53]; Ni-treated: 18.25 [± 2.29]), (2) sized flowers (control: 44.28 [± 5.01] mm; Ni-treated: 44.85 [± 5.93] mm), (3) nectar volume (control: 0.95 [± 0.1] ul; Ni-treated: 0.90 [± 0.13] ul) and (4) anther mass (control: 2.2 [± 0.7] mg; Ni-treated: 2.5 [± 1.1] mg).
Floral Reward Analysis
Plants grown in Ni-supplemented soil accumulated more Ni into floral rewards than control plants, with the difference in Ni concentration between Ni-treated plants and controls greater in pollen (400 %) than nectar (100 %) (Fig. 1; Table 1). As a result, Ni concentrations in treated plants were approximately three times greater in pollen than in nectar (Fig. 1; Table 1). While mean Ni concentrations were similar for Ni-treated plants in the first two weeks of the experiment, plants in the third week had significantly (27 %) higher Ni concentrations overall (i.e., in pollen and nectar).
Nickel concentrations (ppm) in pollen and nectar samples collected from Streptanthus polygaloides plants (N = 24 samples per reward type per treatment). Black symbols represent plants grown in control soil, while white symbols represent plants grown in Ni-supplemented soil. Within a floral reward type, asterisks indicate a significant difference between treatments (P < 0.001)
Floral Visitor Observations
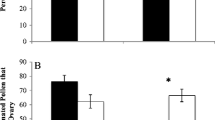
Experimental plants were visited by two groups of floral visitors- small bees in the genus Lasioglossum (Halictidae) and flies in the Syrphidae family. Overall, soil treatment did not affect the probability of visitation by either insect group (Fig. 2a). However, the probability of visitation to plants varied nearly two fold among weeks (0.16–0.25), and a significant pollinator type by week interaction was found (Table 2), indicating a temporal component to variation. Specifically, the overall probability of visitation by bees was 90 % lower in week one relative to weeks two and three, and the overall probability of visitation by flies was 50 % higher in week one relative to weeks two and three. There was no effect of flower number or flower size on the probability of visitation (Table 2). Per flower visitation rate to Ni-treated plants was ~50 % lower than control plants (Fig. 2b; Table 3), and this effect was similar for bees and flies (Table 3). There was no effect of week or floral display on visitation rate, though a significant pollinator type by week interaction was found, again indicating a temporal component to visitation (Table 3). Specifically, visitation rate by bees was 60 % lower in week one relative to weeks two and three.
a Proportion of observation intervals in which visitation to Streptanthus polygaloides was observed and b mean visitation rates by floral visitor type (fly, bee) to S. polygaloides plants in experimental arrays (N = 48 plants observed over 80 10-min observation intervals). Black symbols represent plants grown in control soil, while white symbols represent plants grown in Ni-supplemented soil. Within a floral visitor type, bars with asterisks are significantly different between treatments (P < 0.05)
Discussion
Short-term exposure to elevated soil Ni did not alter floral display or reward quantity, but it did lead to elevated Ni concentrations in nectar and pollen of the Ni hyperaccumulator S. polygaloides. Our results also suggest that naïve floral visitors are unable to discriminate between Ni-treated and control flowers prior to flower visitation but instead respond to differences in floral reward chemistry following arrival at plants by visiting fewer flowers containing Ni.
The effects of soil metals on plant-animal interactions are most often considered in the context of metal hyperaccumulating plants, whose above-ground tissue metal concentrations generally are greater than 1,000 ppm dry weight (van der Ent et al. 2013). Periodic Ni nitrate solution treatments result in Ni hyperaccumulation in leaves, but lower metal levels in pollen and nectar of S. polygaloides (unpublished data). Here, we built on these results to show that Ni concentrations in pollen and nectar well below values established as hyperaccumulator thresholds alter plant-flower visitor interactions. While we directly measured Ni concentrations in anthers, rather than pollen, previous work suggests that pollen Ni accumulation would be >100 ppm in our experimental plants (unpublished data), a concentration in floral rewards already known to alter floral visitor foraging (Meindl and Ashman 2013). Our findings corroborate recent evidence that metal accumulation, defined as tissue metal concentrations >20 or >100 ppm dry weight, depending on the metal (Reeves and Baker 2000), alters plant-insect interactions (reviewed in Mogren and Trumble 2010). For example, copper and cadmium concentrations below 1,000 ppm in artificial diets have resulted in feeding deterrence (Bahadorani and Hilliker 2009). Arsenic accumulation deters herbivory by grasshoppers at concentrations as low as 46 ppm in leaf tissue (Rathinasabapathi et al. 2007). Furthermore, insects feeding on tissues with relatively low metal concentrations have displayed decreased survival, including diamondback moths (Coleman et al. 2005) and armyworms (Cheruiyot et al. 2013) when fed diets containing <1,000 ppm cobalt, copper, Ni, or zinc, among others. These studies suggest that metal concentrations below hyperaccumulator thresholds may alter plant-insect interactions. Our study is among the first to indicate plant-flower visitor interactions also are affected by metal accumulation.
The study also found a temporal component to metal accumulation and floral visitation, as Ni accumulation was highest in the third week of the experiment, and floral visitation varied throughout. However, because we did not observe significant interactions between soil treatment and week, or a three-way interaction between soil treatment, week, and floral visitor type for either the probability of visitation or visitation rate, differences in visitation across weeks likely are not related to differences in Ni accumulation over time. While it is unclear why plants in the third week accumulated higher concentrations of Ni relative to plants in weeks one and two, this pattern may be explained by increased transpiration rates associated with elevated mean temperatures in week 3 (81 °F) relative to those in week one (75 °F) and two (72 °F). Regardless of temporal variation in Ni accumulation, Ni treatment produced a similar effect on floral visitation across weeks, in which visitation rates were consistently lower in plants treated with Ni.
The decrease in floral visitation to Ni-treated plants in this study suggests floral visitors may respond to differences in floral reward chemistry, i.e., Ni concentration. However, the mechanism by which insects perceive Ni-rich floral rewards is uncertain. Deterrence effects have been observed for insects feeding on Ni-rich vegetative tissue (Boyd et al. 2002) as well as other metals (reviewed in Vesk and Reichman 2009), but it is unclear whether deterrence occurs through taste or other mechanisms. Some studies suggest that insect herbivores feeding on metal-rich leaf tissue are deterred via post-ingestional mechanisms rather than initial taste perception (Behmer et al. 2005). While honeybees possess fewer gustatory receptors than other insects, such as fruit flies (10 vs. 68, respectively; de Brito Sanchez and Giurfa 2011), they still can detect a wide variety of compounds in nectar (de Brito Sanchez et al. 2007), Future studies to establish pollinator abilities to detect metals will provide a broader understanding of the pollination ecology of metal accumulating plants.
Metal accumulation into floral rewards has implications for the use of hyperaccumulating plants in phytoremediation. Recently, researchers have brought to light the potential ecological and environmental consequences of phytoremediation, not all of which are positive (Gerhardt et al. 2009). For example, several species of selenium (Se) hyperaccumulators have been proposed for use in phytoremediation of Se-contaminated soils (Zhu et al. 2009). However, selenium recently was associated with toxicity to bees (Hladun et al. 2012), suggesting that the use of hyperaccumlator insect-pollinated flowering plants for phytoremediation may be detrimental to foraging pollinators. Selecting wind-pollinated plants, many of which show potential as phytoremediators (Chen et al. 2004), may limit risks to insect pollinators in metal-contaminated areas.
Our results also suggest that metal hyperaccumulation in natural populations alters plant-pollinator interactions. In this study, generalist floral visitors exposed to Ni-accumulating plants were naïve to Ni-rich resources, as no plants in western PA are known to accumulate Ni in high concentrations. However, recent surveys of insect communities associated with natural populations of S. polygaloides in CA suggest that this species hosts a distinct floral visitor community compared to closely related sympatric, non-accumulating plant species (unpublished data). Taken together this suggests that floral metal accumulation may promote specialization by pollinators tolerant to metal-rich resources. In an analogous system, one hypothesis proposed for the function of toxic alkaloids in nectar is to favor specialist pollinators (reviewed in Adler 2001), as not all generalist pollinators would be able to tolerate relatively high concentrations of secondary compounds present in floral resources. Considering recent findings of floral visitor deterrence in response to Ni in nectar (Meindl and Ashman 2013; this study), Ni accumulation in natural populations also may have consequences for patterns of pollen transfer and ecological specialization. For example, plant secondary compounds in nectar have been shown to alter pollinator foraging and behavior (Gegear et al. 2007), and specifically impact patterns of pollen transfer (Irwin and Adler 2008). Our study suggests that metals in floral rewards also may alter pollinator behavior, warranting further study of the pollination ecology of metal-accumulating plants.
References
Adler LS (2001) The ecological significance of toxic nectar. Oikos 91:409–420
Alexander EB, Coleman RG, Keeler-Wolf T, Harrison SP (2007) Serpentine geoecology of Western North America. Oxford University Press, New York
Bahadorani S, Hilliker AJ (2009) Biological and behavioral effects of heavy metals in Drosophila melanogaster adults and larvae. J Insect Behav 22:399–411
Baker I (1979) Methods for the determination of volumes and sugar concentrations from nectar spots on paper. Phytochem Bull 12:40–42
Baker AJM, Brooks RR (1989) Terrestrial higher plants which hyper accumulate metallic elements- review of their distribution, ecology and phytochemistry. Biorecovery 1:81–126
Baldwin BG, Goldman DH, Keil DJ, Patterson R, Rosatti TJ, Wilken DH (eds) (2012) The Jepson manual: vascular plants of California. University of California Press, Berkeley
Behmer ST, Llloyd CM, Raubenheimer D, Stewart-Clark J, Knight J, Leighton RS, Herper FA, Smith JAC (2005) Metal hyperaccumulation in plants: mechanisms of defence against insect herbivores. Funct Ecol 19:55–66
Bhowmik S, Datta BK (2013) Pollen production in relation to ecological class of some hydrophytes and marsh plants. Am J Plant Sci 4:324–332
Boyd RS (2004) Ecology of metal hyperaccumulation. New Phytol 162:563–567
Boyd RS, Jhee EM (2005) A test of elemental defense against slugs by Ni in hyperaccumulator and non-hyperaccumulator Streptanthus species. Chemoecology 15:179–185
Boyd RS, Martens SN (1992) The raison d’etre for metal hyperaccumulation by plants. In: Baker AJM, Proctor J, Reeves RD (eds) The vegetation of ultramafic (Serpentine) soils. Intercept, GB-Andover, pp 279–289
Boyd RS, Martens SN (1994) Nickel hyperaccumulated by Thlaspi montanum var. montanum is cutely toxic to an insect herbivore. Oikos 70:21–25
Boyd RS, Davis MA, Wall MA, Balkwill K (2002) Nickel defends the South African hyperaccumulator Senecio coronotus (Asteraceae) against Helix aspersa (Molluska: Pulmonidae). Chemoecology 12:91–97
Brun LA, Le Corff J, Mallet J (2003) Effects of elevated copper on phenology, growth and reproduction of five ruderal plant species. Environ Pollut 122:361–368
Burkle LA, Irwin RE (2010) Beyond biomass: measuring the effects of community-level nitrogen enrichment on floral traits, pollinator visitation and plant reproduction. J Ecol 98:705–717
Chardot V, Massoura ST, Echevarria G, Morel JL (2005) Phytoextraction of the nickel hyperaccumulators Leptoplax emarginata and Bornmuellera tymphaea. Int J Phytoremediat 7:323–335
Chen Y, Shen Z, Li X (2004) The use of vetiver grass (Vetiveria zizanioides) in the phytoremediation of soils contaminated with heavy metals. Appl Geochem 19:1553–1565
Cheruiyot DJ, Boyd RS, Moar WJ (2013) Exploring the lower limits of plant elemental defense by cobalt, copper, nickel, and zinc. J Chem Ecol 39:666–674
Coleman CM, Boyd RS, Eubanks MD (2005) Extending the elemental defense hypothesis: dietary metal concentrations below hyperaccumulator levels could harm herbivores. J Chem Ecol 31:1669–1681
de Brito Sanchez G, Giurfa M (2011) A comparative analysis of neural taste processing in animals. Philos Trans R Soc 366:2171–2180
de Brito Sanchez G, Ortigão-Farias J, Gauthier M, Liu F, Giurfa M (2007) Taste perception in honeybees: just a taste of honey? Arthropod-Plant Interactions 1:69–76
Gegear RJ, Manson JS, Thomson JD (2007) Ecological context influences pollinator deterrence by alkaloids in floral nectar. Ecol Lett 10:375–382
Gerhardt KE, Huang X-D, Glick BR, Greenberg BM (2009) Phytoremediation and rhizoremediation of organic soil contaminants: potential and challenges. Plant Sci 176:20–30
Hladun KR, Smith BH, Mustard JA, Morton RR, Trumble JT (2012) Selenium toxicity to honey bee (Apis mellifera L.) pollinators: effects on behaviors and survival. PLoS ONE 7:e34137
Hladun KR, Parker DR, Tran KD, Trumble JT (2013) Effects of selenium accumulation on phytotoxicity, herbivory, and pollination ecology in radish (Raphanus sativus L.). Environ Pollut 172:70–75
Irwin RE, Adler LS (2008) Nectar secondary compounds affect self-pollen transfer: implications for female and male reproduction. Ecology 89:2207–2217
Kearns CA, Inouye DW (1993) Techniques for pollination biologists. University Press of Colorado, Niwot
Kramer U, Smith RD, Wenzel WW, Raskin I, Salt DE (1997) The role of metal transport and tolerance in nickel hyperaccumulation by Thlaspi goesingense Halacsy. Plant Physiol 115:1641–1650
L’Huillier L, Edighoffer S (1996) Extractability of nickel and its concentration in cultivated plants in Ni rich ultramafic soils of New Caledonia. Plant Soil 186:255–264
Lapanje A, Zrimec A, Drobne D, Rupnik M (2010) Long-term Hg pollution-induced structural shifts of bacterial community in the terrestrial isopod (Porcellio scaber) gut. Environ Pollut 158:3186–3193
Leyval C, Turnau K, Haselwandter K (1997) Effect of heavy metal pollution on mycorrhizal colonisation and function, physiological, ecological and applied aspects. Mycorrhiza 7:139–153
Li Y-M, Chaney R, Brewer E, Roseberg R, Angle JS, Baker A, Reeves R, Nelkin J (2003) Development of a technology for commercial phytoextraction of nickel: economic and technical considerations. Plant Soil 249:107–115
Masierowska ML (2003) Floral nectaries and nectar production in brown mustard (Brassica juncea) and white mustard (Sinapis alba) (Brassicaceae). Plant Syst Evol 238:97–107
Meindl GA, Ashman T-L (2013) The effects of nickel and aluminum on the foraging behavior of bumblebees. Environ Pollut 177:78–81
Mitchell RJ, Karron JD, Holmquist KG, Bell JM (2004) The influence of Mimulus ringens floral display size on pollinator visitation patterns. Funct Ecol 18:116–124
Mogren CL, Trumble JT (2010) The impacts of metals and metalloids on insect behavior. Entomol Exp Appl 135:1–17
Pilon-Smits E (2005) Phytoremediation. Annu Rev Plant Biol 56:15–39
Preston RE (1991) The intrafloral phenology of Streptanthus tortuosus (Brassicaceae). Am J Bot 78:1044–1053
Quinn CF, Prins CN, Freeman JL, Gross AM, Hantzis LJ, Reynolds R, Yang S, Covey PA, Banuelos GS, Pickering IJ, Fakra SC, Marcus MA, Arathi HS, Pilon-Smits AH (2011) Selenium accumulation in flowers and its effects on pollination. New Phytol 192:727–737
Rathinasabapathi B, Rangasamy M, Froeba J, Cherry RH, McAuslane HJ, Capinera JL, Srivastava M, Ma LQ (2007) Arsenic hyperaccumulation in the Chinese brake fern (Pteris vittata) deters grasshopper (Schistocerca americana) herbivory. New Phytol 175:363–369
Reeves RD (2006) Hyperaccumulation of trace elements by plants. In: Morel JL, Echevarria G, Goncharova N (eds) Phytoremediation of metal-contaminated soils, vol 68. Springer, New York
Reeves RD, Baker AJM (2000) Metal-accumulating plants. In: Raskin I, Ensley BD (eds) Phytoremediation of toxic metals: using plants to clean up the environment. John Wiley and Sons, New York
Reeves RD, Brooks RR, Macfarlane RM (1981) Nickel uptake by Californian Streptanthus and Caulanthus with particular reference to the hyperaccumulator S. polygaloides Gray (Brassicaceae). Am J Bot 68:708–712
Saikkonen K, Koivunen S, Vuorisalo T, Mutikainen P (1998) Interactive effects of pollination and heavy metals on resource allocation in Potentilla anserina L. Ecology 79:1620–1629
Sarma H (2011) Metal hyperaccumulation in plants: a review focusing on phytoremediation technology. J Environ Sci Technol 4:118–138
Slomka A, Jerdrzejczyk-Korycinska M, Rotanski A, Karcz J, Kawalec P, Kuta E (2012) Heavy metals in sol affect reproductive processes more than morphological characters in Viola tricolor. Environ Exp Bot 75:204–211
van der Ent A, Baker AJM, Reeves RD, Pollard AJ, Schat H (2013) Hypraccumulators of metal and metalloid trace elements: facts and fiction. Plant Soil 362:319–334
Vesk PA, Reichman S (2009) Hyperaccumulators and herbivores—A Bayesian meta-analysis of feeding choice trials. J Chem Ecol 35:289–296
Vogel-Mikus K, Drobne D, Regvar M (2005) Zn, Cd, and Pb accumulation and arbuscular mycorrhizal colonization of pennycress Thlaspi praecox Wulf. (Brassicaceae) from the vicinity of a lead mine and smelter in Slovenia. Environ Pollut 133:233–242
Wall MA, Boyd RS (2002) Nickel accumulation in serpentine arthropods from the Red Hills, California. Pan Pac Entomol 78:168–176
Zhu Y-G, Pilon-Smits EAH, Zhao F-J, Williams PN, Meharg AA (2009) Selenium in higher plants: understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci 14:436–442
Acknowledgments
We thank R. Boyd, J. Wenzel, and D. Bain for technical support, E. York for greenhouse assistance, M. Koski and three anonymous reviewers for comments on a previous version of this manuscript, and members of the Ashman lab for discussion. Funding was provided by the Powdermill Nature Reserve, a Botany In Action Fellowship from the Phipps Conservatory and Botanical Gardens, an Ivy McManus Diversity Fellowship (University of Pittsburgh) and an Andrew Mellon Predoctoral Fellowship (University of Pittsburgh) to GAM, and NSF (DEB 1020523) to TLA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meindl, G.A., Ashman, TL. Nickel Accumulation by Streptanthus polygaloides (Brassicaceae) Reduces Floral Visitation Rate. J Chem Ecol 40, 128–135 (2014). https://doi.org/10.1007/s10886-014-0380-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-014-0380-x