Abstract
Mycorrhizas play a vital role in soil fertility, plant nutrition, and resistance to environmental stresses. However, mycorrhizal effects on plant resistance to herbivorous insects and the related mechanisms are poorly understood. This study evaluated effects of root colonization of tomato (Solanum lycopersicum Mill.) by arbuscular mycorrhizal fungi (AMF) Glomus mosseae on plant defense responses against a chewing caterpillar Helicoverpa arimigera. Mycorrhizal inoculation negatively affected larval performance. Real time RT-PCR analyses showed that mycorrhizal inoculation itself did not induce transcripts of most genes tested. However, insect feeding on AMF pre-inoculated plants resulted in much stronger defense response induction of four defense-related genes LOXD, AOC, PI-I, and PI-II in the leaves of tomato plants relative to non-inoculated plants. Four tomato genotypes: a wild-type (WT) plant, a jasmonic acid (JA) biosynthesis mutant (spr2), a JA-signaling perception mutant (jai1), and a JA-overexpressing 35S::PS plant were used to determine the role of the JA pathway in AMF-primed defense. Insect feeding on mycorrhizal 35S::PS plants led to higher induction of defense-related genes relative to WT plants. However, insect feeding on mycorrhizal spr2 and jai1 mutant plants did not induce transcripts of these genes. Bioassays showed that mycorrhizal inoculation on spr2 and jai1 mutants did not change plant resistance against H. arimigera. These results indicates that mycorrhizal colonization could prime systemic defense responses in tomato upon herbivore attack, and that the JA pathway is involved in defense priming by AMF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arbuscular mycorrhizal fungi (AMF) are the most important symbionts for the majority of terrestrial plant species (Douds and Millner 1999). Through their widespread extra radical mycelium networks, AMF facilitate plants to acquire nutrients and water in soils that plant roots can not reach. Mycorrhizas not only improve plant nutrition (Evelin et al. 2009; Smith et al. 2010) and soil stability, but also influence nutrient cycling (Bethlenfalvay and Schüepp 1994; Rillig and Mummey 2006) and a number of important ecosystem processes, including plant diversity, ecosystem variability, and productivity (van der Heijden et al. 1998; Vogelsang et al. 2006).
Mycorrhizas play a key role in nutrient acquisition for plant growth and reproduction, and thereby have great influences on plant physiology, primary and secondary metabolism, and hormone balance (Smith and Read 2008). The below-ground mycorrhiza may indirectly influence the performance of above-ground herbivorous arthropods through changes in the quality and quantity of plants (Gange 1996; Koricheva et al. 2009), and even affect behavior of natural enemies of herbivores (Wooley and Paine 2011) and plant-pollinators (Cahill et al. 2008). Therefore, mycorrhizas may influence food chains and webs in ecosystems.
Many studies have showed that arbuscular mycorrhizas (AM) reduce plant diseases (Elsen et al. 2008; Zeng 2006). However, effects of mycorrhizal colonization on insect herbivores are much more complicated and unpredictable. Herbivore performance can be affected either positively (Gange and West 1994; Gange et al. 1999) or negatively (Gange 2001; Gange et al. 1994; Gange and West 1994) by vesicular-arbuscular mycorrhizal (VAM) colonization, depending on both herbivore and fungal species present. This variation may result from specificity between a given plant and AMF. In general mycorrhizal colonization enhances plant resistance to root feeding insects and generalist herbivores, but it may increase plant susceptibility to sucking insects and specialist herbivores (Hartley and Gange 2009).
Defense priming is defined as enhanced readiness of defense responses (Conrath et al. 2006; Kim and Felton 2013). Primed plants display faster and/or stronger activation of various cellular defense responses following attack by either pathogens or insects, or in response to other biotic or abiotic stress (Jung et al. 2009; Slaughter et al. 2012; Ton et al. 2006). Some plants previously exposed to herbivore-inducible plant volatiles from neighboring plants under herbivore attack display faster or stronger defense activation and enhanced insect resistance following herbivory (Kessler et al. 2006). The primed state in the plant also can be provoked by various natural and synthetic compounds, such as jasmonic acid (JA), salicylic acid (SA), and β-aminobutyric acid (BABA) (Worrall et al. 2012). Priming may be initiated in response to an environmental cue that reliably indicates an increased probability of a forthcoming attack. Anti-herbivore defense response often is induced with greater efficiency in plants that have previously experienced insect attack (Karban and Baldwin 1997). Some long-lived trees can maintain a primed state across multiple growing seasons (Zvereva et al. 1997). Recent studies demonstrate that the primed state of Arabidopsis thaliana plants can be transferred to their progeny, conferring better protection from pathogen attack as compared to the descendants of unprimed plants (Slaughter et al. 2012). Tomato plants grown from JA-treated and BABA-treated seeds showed increased resistance against herbivory by spider mites, caterpillars, and aphids, and against fungal pathogens (Worrall et al. 2012). The priming responses can last for more than 8 weeks.
Although AM-mediated plant resistance against insect herbivores has been documented in several plant-AMF systems, the underlying mechanisms remain elusive. Besides mechanisms such as improved plant nutrition and quality, increasing evidences show that systematic induction of plant defense responses is involved. Recently defense priming has been suggested as a mechanism of AMF-induced plant resistance (Jung et al. 2012).
The objectives of this study were to examine effects of pre-inoculation of tomato with Glomus mosseae on resistance to cotton bollworm, as well as on defense responses in tomato leaves, and to determine whether priming is a mechanism of enhanced herbivore resistance of tomato. We also determined involvement of the jasmonate signaling pathway in AMF-induced priming in tomato by using both a defective mutant and over-expression of a transgenic plant in the signaling pathway.
Methods and Materials
Plant, Mycorrhizal Fungal and Insect Materials
Tomato seeds (Solanum lycopersicum Mill. cv. Jin Bao) were surface-sterilized with 10 % H2O2 and rinsed five times with sterile distilled water before sowing in autoclaved sand-soil mixture.
The starting inoculum of mycorrhizal fungus Glomus mosseae (Gm) (Nicol. and Gerd) Gerdemann and Trappe BEG 167 used, was kindly provided by Prof. Runjin Liu at Qingdao Agricultural University. The mycorrhizal inoculum was produced in pot culture using corn (Zea mays L.) plants and autoclaved sand media (Chellappan et al. 2002). A mixture of rhizospheric sand from trap cultures (Z. mays) containing 35 infective propagules per gram was used for mycorrhizal inoculation.
Helicoverpa arimigera (HA, Lepidoptera: Noctuidae) was used to attack tomato plants. These larvae were reared on a semisynthetic diet containing wheat germ (Waldbauer et al. 1984) and maintained in an insectary at 23–26 °C, 16 hr/8 hr (day/night) and 65–70 % relative humidity. Homogenously 12-h-molted third instar larvae were used to treat tomato plants.
Chemicals
TRIzol reagent, AMV reverse transcriptase, Taq polymerase, dNTPs, primer inhibitor, were purchased from TaKaRa (Shuzo Co. Ltd., Shiga, Japan), while 4-morpholine-propanesulfonic acid (MOPS) and diethylpyrocarbonate (DEPC) were purchased from AMRESCO (Solon, OH, USA).
Experimental Design
Glomus mosseae was used for mycorrhizal inoculation in tomato plants, and cotton bollworm Helicoverpa arimigera (Lepidoptera: Noctuidae), a generalist chewing caterpillar, was used for insect damage.
Four treatments (Control, HA, Gm, and Gm+HA) were designed to determine effects of defense priming and to exclude possible effects of sole insect attack and AMF inoculation. (1) Control: control plants without insect and mycorrhizal inoculation; (2) HA: plants inoculated with only H. arimigera; (3) Gm: plants inoculated with only G. mosseae; (4) Gm+HA: plants inoculated with both G. mosseae and H. arimigera.
Plant Growth and Mycorrhizal Inoculation
The brown loam soil was collected from the university campus in Guangzhou (China) containing 1.45 % organic matter, 0.725 g/kg total N, 0.44 g/kg total P, 1.68 g/kg total K, 34.87 mg/kg available N, 1.27 mg/kg available P, and 36.21 mg/kg available K with a pH of 4.72. The soil was autoclaved at 121 °C for 2 hr and mixed with sterilized sand (2:1). Mycorrhizal inoculation of G. mosseae in treatments Gm and Gm+HA was obtained by incorporating 120 g of sand containing mycorrhizal inocula into 2.0 kg of the soil/sand mixture before transplanting. The non-mycorrhizal control pots received only the same amount of sterilized sand/soil mixture and 120 g of sand obtained from the growth media of corn plants without mycorrhizal inoculation.
Two pre-germinated tomato seeds were transplanted into each plastic pot with the growth substrate. Ten days later, the seedlings were thinned to one plant per pot. Plants were grown in a growth chamber at 25 ± 1 °C with a 16 hr photoperiod, 150 Md/m2/s PAR, and 60 % relative humidity. Seedlings were watered daily and fertilized every 5 d with 60 ml of Hoagland nutrient solution (Song et al. 2010).
Bioassay
To test whether mycorrhizal infection can enhance host plant resistance against HA, a bioassay was conducted to compare weight gain of HA larvae feeding on the living leaves of mycorrhizal and non-mycorrhizal tomato plants.
Forty days after transplanting, leaves of mycorrhizal and non-mycorrhizal tomato were inoculated with HA third instars. The weight of larva fed on tomato plants was recorded 72 hr after insect inoculation. Before treatment all larvae had been starved for 2 hr and then weighed. Four sets of bioassays were carried out independently, and four pots per treatment were set up for each set of bioassays.
Transgenic Tomato Plants and Roles of the JA Signaling Pathway
The jasmonic acid (JA) signaling pathway plays an essential role in plant responses to chewing insects (Browse and Howe 2008; Howe and Jander 2008). A JA-overexpressing 35S::prosystemin (35S::PS) line, a JA biosynthesis defective mutant suppressor of prosystemin-mediated responses2 (spr2), and a JA-signalling defective mutant jasmonic acid–insensitive1 (jai1) tomato, which were derived from a tomato wild-type (WT, Solanum lycopersicum Castlemart) parent (Li et al. 2003), were used to determine the role of JA signaling in mycorrhiza-mediated defense priming. Seeds for the 35S::prosystemin (35S::PS) transgenic plants were collected from a 35S::prosys/35S::prosys homozygous line (Howe and Ryan 1999) that was backcrossed five times using tomato cv Castlemart as the recurrent parent.
Real-Time RT-PCR Analysis
Leaves of tomato plants were harvested 0, 6, 12, 24, and 48 hr after caterpillar inoculation for real-time RT-PCR analysis. Transcripts of selected anti-herbivore defense-related genes (LOXD, AOC, PI-I, and PI-II) were verified using the RNA samples isolated from tomato leaves obtained from differential treatments. The total RNA was extracted and isolated according to the method described by Kiefer et al. (2000) with slight modification (Song et al. 2010). Gene-specific forward/reverse primer sets used in these reactions were (5′-ATCTCCCAAGTGAAACACCACA-3′/R: 5′-TCATAAACCCTGTCCCATTCTTC-3′) for LOXD; (5′-CTCGGAGATCTTGTCCCCTTT-3′/R: 5′-CTCCTTTCTTCTCTTCTTCGTGCT-3′) for AOC; (5′-CGGTTCCTTCACTCTTTAC-3′/R: 5′-GTGCCATGAGAGTTTCAAA-3′) for PI-I; (5′-AATTATCCATCATGGCTGTTCAC-3′/R: 5′-CCTTTTTGGATCAGATTCTCCTT-3′) for PI-II; and (5′-TCCATCTCGTGCTCCGTCT-3′/R: 5′-GAACCTTTCCAGTGTCATCAACC-3′) for Ubi3 that was used as a reference. Real-time PCR reactions were carried out with 0.2 μl (0.15 μM) of each specific primers, 1 μl cDNA, 12.5 μl of the SYBR green master mix (Quanti Tech SYBR Green kit, Qiagen, Gmbh Hilden, Germany), and the final volume was made up to 25 μl with RNase-free water. In the negative control, cDNA was replaced by RNase free water. The reactions were performed on a DNA Engine Opticon 2 Continuous Fluorescence Detection System (MJ Research Inc., Waltham, MA, USA). The program used for real-time PCR was 3 min initial denaturation at 95 °C, followed by 35 cycles of denaturation for 20 sec at 95 °C, annealing for 20 sec (LOXD: 56.9 °C; AOC: 56.5 °C; PI-I: 47.6 °C; PI-II: 55 °C; Ubi3: 51.5 °C), and extension for 20 sec at 72 °C. The fluorescence signal was measured immediately after incubation for 2 sec at 75 °C following the extension step, which eliminates possible primer dimer detection. At the end of the cycles, melting temperatures of the PCR products were determined between 65 °C and 95 °C. The specificity of amplicons was verified by melting curve analysis and agarose gel electrophoresis.
Three independent biological replicates for each treatment were used for qRT-PCR analyses. The reference gene was constitutively expressed in all treatments (Song et al. 2010, 2011). Relative expression of the target genes to reference gene Ubi in the same tissue was calculated by a Double- standard Curves method.
Statistical Analysis
For each treatment, four replicates were maintained in a completely randomized design. SAS 8.0 (SAS Institute, Cary, NC, USA) package for windows was used for statistical analysis. Data were analyzed with a one-way analysis of variance with the significant differences among means identified by Tukey’s multiple range test (P < 0.05).
Results
Effects of Mycorrhizal Colonization on Tomato Resistance Against Helicoverpa arimigera
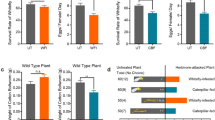
Mycorrhizal colonization significantly enhanced tomato resistance of both WT and JA-overexpressing 35S::PS plants against HA (Table 1, Fig. 1). The HA-larvae fed mycorrhizal plants of WT and 35S::PS tomato gained 62.3 and 78.2 % less weight relative to non-inoculated corresponding control, respectively. However, JA biosynthesis mutant spr2 and JA signaling mutant jai1 of tomato plants were very susceptible to HA infestation. HA larvae fed on the two mutants gained significantly more weight than those fed on WT and 35S::PS plants (Fig. 1). Furthermore, mycorrhizal colonization did not affect their resistance against HA. In addition, spr2 plants had lower mycorrhizal colonization in their roots, but jai1 plants had similar mycorrhizal colonization with WT and 35S::PS plants (Table 1).
Leaf damage (a–d) of mycorrhizal and non-mycorrhizal tomato plants by Helicoverpa arimigera (HA) and HA larval performance fed on different plants (e). The photos were taken 72 hr after insect inoculation. Glomus mosseae (Gm) was used for mycorrhizal inoculation on tomato plants of four genotypes including wild-type (WT, a), JA-overexpressing 35S::PS line (b), JA biosynthesis mutant spr2 (c), and JA-signaling mutant jai1 (d). HA larvae were fed on mycorrhizal (+Gm) and non-mycorrhizal (−Gm) tomato plants of four genotypes, e.g. WT, 35S::PS line, spr2 and jai1 mutants (e)
Induction of Defense-Related Genes in Mycorrhizal Plants Upon Insect Attack
Quantitative real time RT-PCR was used to detect the transcripts of four defense-related genes: genes that encode lipoxygenase D (LOXD) and allene oxide cyclase (AOC), which are two key enzymes of the jasmonic acid biosynthesis pathway; two wound-response genes that encode serine protease inhibitors (PI-I and PI-II), in the leaves of tomato plants. No significant difference in transcript levels of the four genes was found among four treatments (Control, HA, Gm, or Gm+HA) at the beginning time point of insect inoculation (Fig. 2). However, 6 hr after insect inoculation, transcript levels of LOXD, AOC, PI-I, and PI-II in treatment Gm+HA were significantly higher relative to those in the other three treatments, while insect feeding (treatment) alone induced transcripts of AOC. Twelve hours after the insect inoculation and thereafter, pre-inoculation of tomato plants with AMF G. mosseae and later inoculation with caterpillar HA (treatment Gm+HA) induced accumulation of LOXD, AOC, PI-I, and PI-II transcripts over basal levels present in the leaves of non-mycorrhizal and non-insect inoculated control and sole insect inoculation (HA) and G. mosseae colonization (Gm) (Fig. 2). In the treatment Gm+HA, LOXD transcript levels increased by 49.6-, 62.3- 357.3-, and 7.0-fold (Fig. 2a), and PI-II transcript levels increased by 8.5-, 16.7- 21.2-, and 10.6-fold (Fig. 2b) at 6, 12, 24, and 48 hr after the insect attack relative to those in the control, respectively. Although insect herbivory induced transcripts of the four tested genes, induction in treatment Gm+HA was stronger than that in the HA treatment. In contrast, mycorrhizal colonization alone only marginally induced PI-II transcripts and did not induce the other three genes (Fig. 2a–d).
Expression of four defense-related genes in leaves of tomato plants in response to mycorrhizal colonization by Glomus mosseae (Gm) and herbivore infestation by third instar larvae of Helicoverpa arimigera (HA). Four treatments (Control, Gm, HA, Gm+HA) included (1) Control: control plants without insect and mycorrhizal inoculation; (2) HA: plants inoculated with only H. arimigera; (3) Gm: plants inoculated with only G. mosseae; (4) Gm+HA: plants inoculated with both G. mosseae and H. arimigera. Quantitative real time RT-PCR was used to detect the transcripts of five defence-related genes encoding (a) lipoxygenase D (LOXD), (b) allene oxide cyclase (AOC), (c) proteinase inhibitor I (PI-I), and (d) proteinase inhibitor II (PI-II). Values are means + standard error from three sets of independent experiments with three pots per treatment for each set of experiments. For each time point, letters above bars indicate significant difference among treatments (P < 0.05 according to Tukey’s multiple range test)
Involvement of the JA Signaling Pathway
To determine the role of the JA pathway in AMF-induced insect resistance, four tomato genotypes: JA biosynthesis mutant (spr2), JA perception mutant (jai1), and JA-overexpressing 35S::PS line and the corresponding wild-type (WT) were used to study their differential defense responses to insect herbivory and mycorrhizal colonization.
Pre-inoculation of 35S::PS and WT tomato plants with AMF strongly induced transcripts of defense-related genes LOXD and PI-II after insect inoculation with HA (treatment Gm+HA) (Figs. 3 and 4). HA herbivory (treatment HA) induced transcript accumulation of LOXD and PI-II in 35S::PS and WT tomato plants, but mycorrhizal colonization in the roots of 35S::PS and WT plants led to stronger induction of LOXD and PI-II upon insect attack. Insect attack alone increased accumulation of LOXD transcripts 4.7-, 11.7-, and 13.3-fold in WT plants, while insect attack on mycorrhizal WT plants induced LOXD transcripts 13.2-, 35.9, and 81.1-fold at 12, 24, and 48 hr after the insect inoculation, respectively, relative to untreated control (Fig. 3). The highest transcript levels of LOXD and PI-II were found in the mycorrhizal pre-inoculated 35S::PS plants after insect inoculation. Sole insect infestation increased accumulation of LOXD transcripts 2.5-, 1.7-, 8.6-, and 11.0-fold in 35S::PS plants, while insect infestation on mycorrhizal pre-inoculated 35S::PS plants led to 7.6-, 6.4-, 30.4-, and 31.4-fold induction of LOXD transcripts at 12, 24, and 48 hr after the insect inoculation, respectively (Fig. 3b–e). Only marginal induction of LOXD transcripts was observed in 35S::PS and WT tomato plants at the beginning of insect treatment (Fig. 3a).
Expression of LOXD encoding lipoxygenase D in leaves of wild-type (WT), JA-overexpressing 35S::PS line, JA biosynthesis mutant spr2, and JA-signaling mutant jai1 of tomato plants in response to mycorrhizal colonization by Glomus mosseae (Gm) and herbivore infestation by third instar larvae of Helicoverpa arimigera (HA). Four treatments (Control, Gm, HA, Gm+HA) were set up as described in Fig. 2. Quantitative real time RT-PCR was used to detect the gene transcripts. Values are means + standard error from three sets of independent experiments with three pots per treatment for each set of experiments. For each genotype within one time point, letters above bars indicate significant difference among treatments (P < 0.05 according to Tukey’s multiple range test)
Expression of PI-II encoding proteinase inhibitor II in leaves of wild-type (WT), JA-overexpressing 35S::PS line, JA biosynthesis mutant spr2, and JA-signaling mutant jai1 of tomato plants in response to mycorrhizal colonization by Glomus mosseae (Gm) and herbivore infestation by third instar larvae of Helicoverpa arimigera (HA). Four treatments (Control, Gm, HA, Gm+HA) were set up as described in Fig. 2. Quantitative real time RT-PCR was used to detect the gene transcripts. Values are means + standard error from three sets of independent experiments with three pots per treatment for each set of experiments. For each genotype within one time point, letters above bars indicate significant difference among treatments (P < 0.05 according to Tukey’s multiple range test)
In contrast, AMF pre-inoculation of spr2 and jai1 plants did not induce transcripts of LOXD and PI-II upon HA attack (Figs. 3 and 4). Insect infestation on spr2 plants slightly induced transcripts of PI-II, but mycorrhizal colonization did not have any induced effect. For JA signaling perception mutant jai1, no induction of LOXD and PI-II was observed in either treatment HA or Gm+HA.
Discussion
Mycorrhizal fungi associate with the majority of plants in all terrestrial ecosystems. There is now increasing recognition of the influence of underground mycorrhizal fungi on plant health and aboveground plant interactions with other organisms (Hartley and Gange 2009; Vogelsang et al. 2006). However, less is understood about how soil mycorrhizal fungi influences aboveground plant interactions. This study showed that mycorrhizal colonization enhanced tomato resistance against H. arimigera, a chewing generalist herbivore causing severe damage to many important crops. This raises the possibility of use of AMF as a promising alternative for management of some agricultural pests.
The JA signaling pathway plays a crucial role in mediating anti-herbivore defense responses in plants (Howe and Jander 2008). Lipoxygenase (LOX) catalyses the initial reaction in the JA biosynthesis pathway, which inserts molecular oxygen into position 13 of α-linolenic acid (Christensen et al. 2013). Allene oxide cyclase (AOC) is another important enzyme in JA biosynthesis (Schaller et al. 2005). Numerous studies have showed that JA and its derivates play a role in the establishment of functional AM symbiosis (Hause et al. 2007; León-Morcillo et al. 2012). Hause et al. (2002) found that mycorrhizal colonization of barley roots by AMF Glomus intraradices led to elevated levels of endogenous jasmonic acid (JA) and increased expression of genes coding for an enzyme of JA biosynthesis (allene oxide synthase). Induction of LOXD and AOC by AMF indicates that mycorrhizal colonization can also activate the JA pathway, which in turn induces broad-spectrum protection against subsequent insect infestation (Christensen et al. 2013; De Vos et al. 2005).
To determine whether mycorrhizal colonization leads to systemic priming of defense upon insect attack in tomato plants, we compared the different response of mycorrhizal and non-mycorrhizal tomato plants following the inoculation of H. armigera larvae in the leaves. Expression levels of defense-related genes in the leaves were examined at 6, 12, 24, and 48 hr after insect inoculation (Fig. 2). Our study showed that mycorrhizal inoculation itself did not affect transcripts of the tested genes (Fig. 2). However, pre-inoculation with G. mosseae strongly induced defense responses of all four tested genes in tomato plants upon herbivore attack. Although HA herbivory induced defense responses, mycorrhizal pre-inoculation and late herbivore attack led to much stronger induction of defense-related genes in the leaves, suggesting that root colonization of AMF induces systemic defense and primes defense responses upon herbivore infestation, and consequently, mycorrhizal tomato plants show higher resistance against HA.
Most studies on defense priming focus on priming signals of herbivore-induced volatile compounds (Engelberth et al. 2004; Frost et al. 2007; Heil and Silvabueno 2007; Ramadan et al. 2011; Ton et al. 2006). Actually, priming of plant defense can be triggered by certain beneficial micro-organisms (Conrath et al. 2006; van Hulten et al. 2006; Van Wees et al. 2008), including AMF (Pozo and Azcon-Aguilar 2007; Pozo et al. 2009). The stronger induction of defense-related genes in mycorrhizal tomato plant upon insect herbivory indicates that priming is an important mechanism of mycorrhizal-induced herbivore resistance. Upon AMF colonization, plants modulate their defense responses. As a consequence of this modulation, a mild, but effective activation of the plant immune responses occurs (Hause et al. 2002). This activation may lead to a primed state of the plant that allows a quicker and more efficient activation of defense mechanisms in response to subsequent attack by insect herbibores (Jung et al. 2012; Pozo and Azcon-Aguilar 2007).
Use of JA biosynthesis (spr2 and jai1) mutants and JA-overexpressing 35S::PS plants made it possible to identify an essential role the JA signaling pathway in AMF-primed defense in tomato plants. Mycorrhizal 35S::PS plants showed stronger defense responses to herbivore infestation than mycorrhizal WT plants and non-mycorrhizal 35S::PS plants (Figs. 3 and 4). Induction of defense-related genes was significantly higher in mycorrhizal 35S::PS plants. The mycorrhizal 35S::PS plants were the most resistant to HA among four tested genotypes (Table 1). However, AMF pre-inoculation and herbivore infestation did not lead to induction of defense-related genes in spr2 and jai1 plants. The two mutant plants were the most susceptible to insect infestation, and mycorrhizal colonization did not affect their insect resistance (Table 1 and Fig. 1). These results indicate that the JA pathway is required for AMF-induced systemic priming of defense against HA. Rasmann et al. (2012) showed that herbivory in the previous generation primed Arabidopsis and tomato for enhanced insect resistance, and Arabidopsis mutants that are deficient in jasmonate perception (coronatine insensitive1) did not exhibit inherited resistance, demonstrating that the JA pathway is required for defense priming inheritance.
In summary our results indicate that mycorrhizal-induced herbivore resistance in tomato is associated with priming for an efficient activation of defense responses upon herbivore attack by chewing caterpillar H. armigera. The study also indicates that the JA pathway is involved in the AMF-mediated defense priming. Since the majority of land plants establish symbiotic associations with mycorrhizal fungi (Smith and Read 2008), defense priming by common symbiotic organisms may be an important evolutionary strategy for plant defense against herbivores and other biotic stresses.
References
Bethlenfalvay GJ, Schüepp H (1994) Arbuscular mycorrhizas and agrosystem stability. In: Gianinazzi S, Schüepp H (eds) Impact of arbuscular mycorrhizas on sustainable agriculture and natural ecosystems. Birkhäuser Verlag, Basel, pp 117–131
Browse J, Howe GA (2008) New weapons and a rapid response against insect attack. Plant Physiol 146:832–838
Cahill JF Jr, Elle E, Smith GR, Shore BH (2008) Disruption of a belowground mutualism alters interactions between plants and their floral visitors. Ecology 89:1791–1801
Chellappan P, Christy SAA, Mahadevan A (2002) Multiplication of arbuscular mycorrhizal fungi on roots. In: Mukerji KG, Manoharachary C, Chamola BP (eds) Techniques in mycorrhizal studies. Kluwer Academic Publishers, Dordrecht, pp 285–297
Christensen SA, Nemchenko A, Borrego E, Murray I, Sobhy IS, Bosak L, Deblasio S, Erb M, Robert CA, Vaughn KA, Herrfurth C, Tumlinson J, Feussner I, Jackson D, Turlings TC, Engelberth J, Nansen C, Meeley R, Kolomiets MV (2013) The maize lipoxygenase, ZmLOX10, mediates green leaf volatile, jasmonate and herbivore-induced plant volatile production for defense against insect attack. Plant J 74:59–73
Conrath U, Beckers GJM, Flors V, Garcia-Agustin P, Jakab G, Mauch F, Newman MA, Pieterse CMJ, Poinssot B, Pozo MJ, Pugin A, Schaffrath U, Ton J, Wendehenne D, Zimmerli L, Mauch-Mani B (2006) Priming: getting ready for battle. Mol Plant Microbe Interact 19:1062–1071
de Vos M, van Oosten VR, van Poecke RMP, van Pelt JA, Pozo MJ, Mueller MJ, Buchala AJ, Métraux JP, van Loon LC, Dicke M, Pieterse CMJ (2005) Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol Plant Microbe Interact 18:923–937
Douds DD, Millner P (1999) Biodiversity of arbuscular mycorrhizal fungi in agroecosystems. Agric Ecosyst Environ 74:77–93
Elsen A, Gervacio D, Swennen R, de Waele D (2008) AMF-induced biocontrol against plant parasitic nematodes in Musa sp.: a systemic effect. Mycorrhiza 18:251–256
Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH (2004) Airborne signals prime plants against insect herbivore attack. Proc Natl Acad Sci USA 101:1781–1785
Evelin H, Kapoor R, Giri B (2009) Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Ann Bot 104:1263–1280
Frost CJ, Appel HM, Carlson JE, de Moraes CM, Mescher MC, Schultz JC (2007) Within-plant signalling via volatiles overcomes vascular constraints on systemic signalling and primes responses against herbivores. Ecol Lett 10:490–498
Gange AC (1996) Reduction in vine weevil larval growth by mycorrhizal fungi. Mitt Biol Bund Forst 316:56–60
Gange AC (2001) Species-specific responses of a root- and shoot-feeding insect to arbuscular mycorrhizal colonization of its host plant. New Phytol 150:611–618
Gange AC, West HM (1994) Interactions between arbuscular mycorrhizal fungi and foliar-feeding insects in Plantago lanceolata L. New Phytol 128:79–87
Gange AC, Brown VK, Sinclair GS (1994) Reduction of black vine weevil growth by vesicular-arbuscular mycorrhizal fungi. Entomol Exp Appl 70:115–119
Gange AC, Bower E, Brown VK (1999) Positive effects of an arbuscular mycorrhizal fungus on aphid life history traits. Oecologia 120:123–131
Hartley SE, Gange AC (2009) Impacts of plant symbiotic fungi on insect herbivores: mutualism in a multitrophic context. Annu Rev Entomol 54:323–342
Hause B, Maier W, Miersch O, Kramell R, Strack D (2002) Induction of jasmonate biosynthesis in arbuscular mycorrhizal barley roots. Plant Physiol 130:1213–1220
Hause B, Mrosk C, Isayenkov S, Strack D (2007) Jasmonates in arbuscular mycorrhizal interactions. Phytochemistry 8:101–110
Heil M, Silvabueno JC (2007) Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc Natl Acad Sci USA 140:5467–5472
Howe GA, Jander G (2008) Plant immunity to insect herbivores. Annu Rev Plant Biol 59:41–66
Howe GA, Ryan CA (1999) Suppressors of systemin signaling identify genes in the tomato wound response pathway. Genetics 153:1411–1421
Jung HW, Tschaplinski TJ, Wang L, Glazebrook J, Greenberg JT (2009) Priming in systemic plant immunity. Science 324:89–91
Jung SC, Martinez-Medina A, Lopez-Raez JA, Pozo MJ (2012) Mycorrhiza-induced resistance and priming of plant defenses. J Chem Ecol 38:651–664
Karban R, Baldwin IT (1997) Induced responses to herbivory. University of Chicago Press, Chicago
Kessler A, Halitschke R, Diezel C, Baldwin IT (2006) Priming of plant defense responses in nature by airborne signaling between Artemisia tridentata and Nicotiana attenuata. Oecologia 148:280–292
Kiefer E, Heller W, Ernst D (2000) A simple and efficient protocol for isolation of functional RNA from plant tissues rich in secondary metabolites. Plant Mol Biol Rep 18:33–39
Kim J, Felton GW (2013) Priming of antiherbivore defensive responses in plants. Insect Sci 20:273–285
Koricheva J, Gange AC, Jones T (2009) Effects of mycorrhizal fungi on insect herbivores: a meta-analysis. Ecology 90:2088–2097
León-Morcillo RJ, Angel J, Martín-Rodríguez, Vierheilig H, Ocampo JA, García-Garrido JM (2012) Late activation of the 9-oxylipin pathway during arbuscular mycorrhiza formation in tomato and its regulation by jasmonate signalling. J Exp Bot 63:545–3558
Li CY, Liu GH, Xu CC, Lee GI, Bauer P, Ling HQ, Ganal MW, Howe GA (2003) The tomato suppressor of prosystemin-mediated responses 2 gene encodes a fatty acid desaturase required for the biosynthesis of jasmonic acid and the production of a systemic wound signal for defense gene expression. Plant Cell 15:1646–1661
Pozo MJ, Azcon-Aguilar C (2007) Unraveling mycorrhiza-induced resistance. Curr Opin Plant Biol 10:393–398
Pozo MJ, Verhage A, García-Andrade J, García JM, Azcon-Aguilar C (2009) Priming plant defence against pathogens by arbuscular mycorrhizal fungi. In: Azcón-Aguilar C, Gianinazzi S, Barea JM, Gianinazzi-Pearson V (eds) Mycorrhizas - functional processes and ecological impact. Springer- Verlag, Berlin Heidelberg, pp 123–135
Ramadan A, Muroi A, Arimura G (2011) Herbivore-induced maize volatiles serve as priming cues for resistance against post-attack by the specialist armyworm Mythimna separata. J Plant Interact 6:155–158
Rasmann S, De Vos M, Casteel CL, Tian D, Halitschke R, Sun JY, Agrawal AA, Felton GW, Jander G (2012) Herbivory in the previous generation primes plants for enhanced insect resistance. Plant Physiol 158:854–863
Rillig MC, Mummey DL (2006) Mycorrhizas and soil structure. New Phytol 171:41–53
Schaller F, Schaller A, Stintz A (2005) Biosynthesis and metabolism of jasmonates. J Plant Growth Regul 23:179–199
Slaughter A, Daniela X, Flors V, Luna E, Hohn B, Mauch-Mani B (2012) Descendants of primed Arabidopsis plants exhibit resistance to biotic stress. Plant Physiol 158:835–843
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Academic, London
Smith SE, Facelli E, Pope S, Smith FA (2010) Plant performance in stressful environments: interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant Soil 326:3–20
Song YY, Zeng RS, Xu JF, Li J, Shen X, Yihdego WG (2010) Interplant communication of tomato plants through underground common mycorrhizal networks. PLoS One 5:e13324
Song YY, Wang RL, Wei XC, Lu YJ, Wu GZ, Su YJ, Zeng RS (2011) Mechanism of tomato plants enhanced disease resistance against early blight primed by arbuscular mycorrhizal fungus Glomus versiforme. Chin J Appl Ecol 22:2316–2324 (in Chinese)
Ton J, D’alessandro M, Jourdie V, Jakab G, Karlen D, Held M, Mauch-Mani B, Turlings TCJ (2006) Priming by airborne signals boosts direct and indirect resistance in maize. Plant J 49:16–26
van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T, Wiemken A, Sanders IR (1998) Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 365:69–72
van Hulten M, Pelser M, van Loon LC, Pieterse CMJ, Ton J (2006) Costs and benefits of priming for defense in Arabidopsis. Proc Natl Acad Sci USA 103:5602–5607
van Wees SCM, van der Ent S, Pieterse CMJ (2008) Plant immune responses triggered by beneficial microbes. Curr Opin Plant Biol 11:443–448
Vogelsang KM, Reynolds HL, Bever JD (2006) Mycorrhizal fungal identity and richness determine the diversity and productivity of a tallgrass prairie system. New Phytol 172:554–562
Waldbauer GP, Cohen RW, Friedman S (1984) An improved procedure for laboratory rearing of the corn earworm, Heliothis zea (Lepidoptera: Noctuidae). Great Lakes Entomol 17:113–118
Wooley SC, Paine TD (2011) Infection by mycorrhizal fungi increases natural enemy abundance on tobacco (Nicotiana rustica). Environ Entomol 40:36–41
Worrall D, Holroyd GH, Moore JP, Glowacz M, Croft P, Taylor JE, Paul ND, Roberts MR (2012) Treating seeds with activators of plant defence generates long-lasting priming of resistance to pests and pathogens. New Phytol 193:770–778
Zeng RS (2006) Disease resistance in plants through mycorrhizal fungi induced allelochemicals. In: Inderjit, Mukerji KG (eds) Allelochemicals: biological control of plant pathogens and diseases. Springer, Dordrecht, pp 181–192
Zvereva EL, Kozlov MV, Niemela P, Haukioja E (1997) Delayed induced resistance and increase in leaf fluctuating asymmetry as responses of Salix borealis to insect herbivory. Oecologia 109:368–373
Acknowledgments
This research was supported by the National 973 Program of China (2011CB100400), National Natural Science Foundation of China (31070388, 31028018, 31100286), Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2010), Guangdong Natural Science Foundation of China (S2011040004336), China Postdoctoral Science Foundation (201104341, 20100480762), and Ph.D. Foundation of the Ministry of Education of China (20104404110004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, Y.Y., Ye, M., Li, C.Y. et al. Priming of Anti-Herbivore Defense in Tomato by Arbuscular Mycorrhizal Fungus and Involvement of the Jasmonate Pathway. J Chem Ecol 39, 1036–1044 (2013). https://doi.org/10.1007/s10886-013-0312-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-013-0312-1