Abstract
Oplostomus haroldi Witte belongs to a unique genus of afro-tropical scarabs that have associations with honey bee colonies, from which they derive vital nutrients. Although the attributes of the honey bee nest impose barriers to communication among nest invaders, this beetle still is able to detect conspecific mates for reproduction. Here, we show, through behavioral studies, that cuticular lipids serve as mate discrimination cues in this beetle. We observed five steps during mating: arrestment, alignment, mounting, and copulation, and a post-copulatory stage, lasting ~40–70 % of the total mating duration, that suggested mate guarding. Chemical analysis identified the same nine straight-chain alkanes (C23–C31), six methyl-branched alkanes (6), and five mono-unsaturated alkenes in the cuticular lipids of both sexes. Methyl alkanes constituted the major component (46 %) of male cuticular lipids, while mono-unsaturated alkenes were most abundant (53 %) in females. (Z)-9-Pentacosene was twice as abundant in females than in males, and ~20 fold more concentrated in beetles than in worker bees. In mating assays, (Z)-9-pentacosene elicited arrestment, alignment, and mounting, but not copulation, by male beetles. These results represent the first evidence of a contact sex pheromone in a scarab beetle. Such contact pheromones may be an essential, cryptic mechanism for arthropods associated with eusocial insects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The honey bee, Apis mellifera L., ranks among the top 5 % of most-studied insects, due to its importance as a pollinator of crops and provider of ecosystem services (Graham 2005). The honey bee colony is a rich source of nutrients (pollen, brood and honey), each with an unique odor (Suazo et al., 2003; Torto et al. 2005, 2007). These odors, combined with those released by bees, provide a critical chemical signature that permits many arthropods, including mites, flies, beetles, wasps, and moths, to associate with honey bee colonies, thus exploiting resources (Torto et al. 2005, 2010). Most cetonine scarabs are pollen feeders. Therefore, it is not unusual to find scarabs, such as Oplostomus spp., associated with honey bee colonies (Fombong et al. 2012a). A few examples of scarab-honey bee associations are known for African honey bees, e.g., Oplostomus fuligenus Olivier and Oplostomus haroldi Witte (Donaldson 1989; Njau et al. 2009; Torto et al. 2010; Fombong et al. 2012b).
Recently, it was reported that O. haroldi was found mainly on brood frames which, in the honey bee hive, is the situation most populated and protected by worker bees (Torto et al. 2010; Fombong et al. 2012b). Although the benefits of being on the brood frame confer easy access to food, finding a suitable mate for reproduction in this crowded environment is likely to pose a challenge for the beetle; e.g., distingishing conspecific odors among the bouquet of hive odors. Despite this, we found mating couples of the beetle on brood frames during a survey of honey bee colonies in Kenya (Supplemental Material S1), suggesting that intraspecific communication in the beetle may involve an efficient cryptic signaling mechanism that minimizes detection by worker bees. Therefore, we hypothesized that mate signaling in this insect is determined by contact semiochemicals. To test this, we carried out experiments to determine: a) specific behaviors involved in mating, b) the mode of mate recognition, and c) the nature of the signal. Knowledge of the behavior and underlying mechanisms of mate identification in this honey bee pest could help in developing effective tools for its management.
Methods and Materials
Source of Experimental Insects
We used O. haroldi beetles collected from A. mellifera colonies at various sites (Matuu, 01° 5′ 6.3″ S, 037° 28′ 13.1″ E; Taita, 03° 28′ 30.7″ S, 038° 20′ 17.9″ E; and Watamu, 03° 18′ 24.3″ S, 040° 1′ 4.4″ E) in Kenya between Jan. 2010–Feb. 2011, and maintained these in the laboratory as described in Torto et al. (2010). The age and mating status of beetles were not known at the time of collection. Prior to bioassays, we transfered beetles into rearing containers with moist cotton wool, to allow them to rub off any food particles that may have been stuck to their bodies, replacing the moist cotton wool periodically with fresh cotton.
Worker honey bees used were collected from colonies maintained in Langstroth hives at the International Centre of Insect Physiology and Ecology apiary, located within the Nairobi campus (01° 13′ 25.3″ S, 036° 53′ 49.2″ E).
Mating Behavior of O. haroldi: Role of Air-borne Volatiles
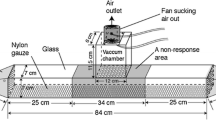
We investigated the role of air-borne volatiles in mate recognition, using a dual-choice olfactometer, similar to that described by Torto et al. (2010). Briefly, air from a pressurized air tank was cleaned by passing it through a column of activated charcoal, passed through test odor sources held in 2 l glass jars fitted with airtight lids, and delivered into the olfactometer (100 × 30 × 30 cm). A vacuum fan at the centre of the olfactometer pulled air out, at a flow rate of 0.71 ml.min−1. The bioassay arena was illuminated by two 40 W fluorescent bulbs, placed 1 m above the setup.
The treatment odor sources included 10 females and 10 males, with clean air as a control. In preliminary tests, run for 30 min each, we observed that all respondents chose within the first 3–5 min.; thereafter, the position of the beetle in the olfactometer did not change. Consequently, we reduced the bioassay period to 10 min. and counted the number of beetles that responded to the odor sources after this period. Test beetles were released singly into the olfactometer and observed for 10 min, after which they were removed. Each beetle was used only once. The odor sources were reversed after every five replicates to minimize positional bias. Between tests, the olfactometer was cleaned with acetone to eliminate any residual odor. All bioassays were conducted between 09:00 and 16:00 h (the time of day when adults are most active and display activities such as feeding and host searching).
Mating Behavior of O. haroldi: Role of Contact Chemicals
To establish whether contact chemicals were involved in mating, we placed a male and female in glass Petri dishes (9 cm diam., 2 cm deep), lined with filter paper, and videotaped the beetles with a SONY™ digital camera. This procedure was repeated a further nine times to obtain 10 replicates. All recordings were carried out between 0900 and 1600 h. The recordings were analyzed to categorize the specific behaviors involved, and to determine whether contact chemicals were involved in mate finding. All observations were conducted under laboratory lighting (white light), at 25 ± 2 °C and 47 ± 5 % RH. Preliminary observations showed that mating took place under both red and white light, suggesting that light played a minimal role in mating.
Role of Cuticular Lipids
We carried out two experiments to establish the involvement of cuticular lipids in mating. Prior to each experiment, we pre-cleaned two groups of males and females (N = 20), as described above, and transferred them into empty plastic rearing bowls to allow them to copulate. Beetles that coupled (~80–90 %) were decoupled immediately and transferred into separate bowls for use in subsequent bioassays. This ensured that only sexually active beetles were used in bioassays.
In the first experiment, pairs of freeze-killed females of identical size, shape, and color (to exclude visual cues, since O. haroldi possesses different body colorations, see Supplemental Material S1), were placed at opposite ends of a filter paper in a Petri dish. One of the females was washed thrice in 3 ml of analytical grade pentane for 10 min to remove its cuticular lipids, while the other remained unwashed. The pair was presented to males (N = 25) and the behavior recorded.
In the second experiment, dead females were washed in pentane, and female extract applied to the body of one of a pair of females at various female equivalent (FE) doses (0.1, 0.2, 0.4, 0.8, 1, and 2 FE). Female pairs (treated and untreated) were presented to individual males (N = 20), and the responses in the test arena recorded on a SONY™ cybershot digital camera. The percentages of males making mouth contact, mounting, and attempting to copulate with each of the dead females were recorded. Test females were changed aftter five replicates. If a mounted male attempted to copulate with a dead female by extruding its aedeagus, it was prevented from doing so, and the bioassay considered complete if the male had previously made mouth contact with the other female. All bioassays were conducted between 09:00–16:00 h under laboratory conditions identical to those described in the previous experiment.
Role of Mouth Palps in Mating
Preliminary observations revealed that mouth, but not antennal, contact was crucial to mate discrimination, suggesting the involvement of mouth palps in mating. To establish the role of the palps in mating, we removed the labial palps of one group of 5 males and the maxillary palps of another group of 5 males, using a pair of sterilized fine scissors, under a stereomicroscope. Prior to each assay, 10 males were placed individually in separate glass Petri dish arenas (as previously described), containing 3 female beetles, and the males allowed to interact with the females. Once a male was observed attempting to copulate with a mounted female, the pair was separated, and the female was placed in another Petri dish, while the male was returned to the arena. A fresh female from the laboratory colony then was placed in the Petri dish to replace the one that had been mounted. We repeated this procedure until we obtained 3 females that a male had attempted copulating with, for each of the 10 males. This procedure was carried out to minimize variation in male behavioral responses toward females. This yielded a total of 30 females (3 mounted by each male), set aside in groups of 3, according to the males that mounted them, for subsequent bioassays.
Each male from the two groups of labial and maxillary palp-excised beetles was then placed in the Petri dish arena, together with the 3 females it had previously attempted to copulate with, and allowed to mate. The number of males in each group mounting and copulating with females was recorded. All bioassays were recorded with a digital camera and conducted under temperature and relative humidity conditions similar to that used in the mating bioassays.
External Morphology of Mouth Palps
We studied the external morphology of the mouth palps and their sensilla by scanning electron microscopy (SEM). Adult male and female beetles were killed by freezing and preserved in ethanol (70 %). The anterior portion of the head, including the mouthparts, was excised using micro-dissection scissors and cleaned by sonication in distilled water with a small quantity of detergent. The cleaned parts were air dried and mounted on SEM stubs, using double-sided adhesive discs and graphite paste (Electron Microscope Sciences, Hatfield, PA, USA). The mounted specimens were held for 2–3 d in a desiccator over silica gel for final drying, then sputter coated with gold-palladium in a Denton Vacuum Desk V (Denton Vacuum, Inc., Moorestoen, PA, USA). Specimens were viewed and micrographs taken at 10.0 kV using a Hitachi H4000 scanning electron microscope (Hitachi High Technologies America, Schaumberg, IL, USA).
Collection and Analysis of Cuticular Lipids (CLs)
To identify the nature of the mating signal, we freeze killed individual adult males (N = 11), females (N = 9) and worker honey bees (N = 5), and extracted cuticular lipids in 3 ml of pentane for 10 min. The extracts were concentrated under N2 gas to ~500 μl and stored at −20 °C until analyzed. Extracts were analyzed by coupled gas chromatography/mass spectrometry (GC/MS) on an Agilent Technologies 7890A GC, equipped with an HP-5 capillary column (30 m × 0.25 mm × 0.25 μm film thickness) and a 5795C MS. We injected 1 μl of each sample, containing 50 ng of an internal standard (1-tridecene) splitlessly, with helium as carrier gas at a flow rate of 1.0 ml.min−1. The oven temperature was initially held at 35 °C for 5 min, increased to 280 °C at 10 °C min−1, and then held for 30.5 min. Samples also were analyzed on the same equipment using a Carbowax-20 M capillary column (15 m × 0.3 mm × 0.3 μm film thickness). For this column, the oven temperature was initially held at 35 °C for 5 min, increased to 220 °C at 10 °C min−1, and then held for 30.5 min. Spectra were recorded at 70 eV in the electron impact (EI) ionization mode. The mass range scanned was 35–550, at 0.7 scan.sec−1, following a solvent delay of 3 min.
Identification of Straight Chain and Methyl-Branched Alkanes
Tentative identification of peaks was made by comparing mass spectral data with spectra in the NIST 05a and Chemoecol mass spectral libraries. A C23-C31 hydrocarbon mixture in heptane (~200 ng/μl) was coinjected with the sample to calculate retention indices (RIs) of cuticular components, as described by Millar and Haynes (1998). The identities of saturated hydrocarbons were confirmed by comparing mass spectra and RIs with those of authentic standards, and by co-injection. Methyl-branched alkanes were tentatively identified by their mass spectral fragmentation patterns, on both the HP-5 and Carbowax-20 M columns.
Determination of Double Bond Position in Mono-Unsaturated Hydrocarbons
The position of double bonds in cuticular components tentatively identified as mono-unsaturated alkenes was determined by a single step derivatization reaction of the crude extract. This reaction involved addition of 100 μl of dimethyl disulfide (DMDS) to an aliquot (100 μl) of cuticular extract, obtained from 100 beetles of mixed sex, as described in the previous section. Iodine solution (10 μl of 30 mg.ml−1 I2 in diethyl ether) was added to catalyze the reaction. The reaction mixture then was heated at 50 °C for 12 h. Subsequently, 10 μl of 0.5 M aqueous sodium thiosulphate were added to the reaction mixture to neutralize the I2. The supernatant, containing the DMDS adducts, was decanted and analyzed by GC/MS. Aliquots (50 μl) of 200 ng.μl−1 of authentic (Z)-9-pentacosene, (Z)-9-hexacosene, (Z)-9-heptacosene, and (Z)-9-nonacosene (see section on chemicals below) were subjected to a similar procedure, and the resulting DMDS adducts were analyzed by GC/MS on the HP-5 and Carbowax-20 M columns. Mass spectra of adducts from both the crude extract and standards were compared by matching retention times, fragmentation patterns, and position of double bonds, determined from characteristic diagnostic ions. The identities of unsaturated components in the crude extracts were confirmed by co-injection with authentic standards, and by comparison of mass spectral data and RIs with these standards.
Chemicals
Hydrocarbon standards were obtained from various sources: n-tricosane, n-tetracosane (Alltech Associates Inc. IL, USA), n-pentacosane (Kok lab. Inc., NY, USA), n-hexacosane, n-heptacosane, n-octacosane, n-nonacosane, n-triacontane, n-Hentriacontane, n-dotriacontane (Analabs Inc., CT, USA). n-Pentane (99 % purity) and n-hexane (>99.9 % purity) were purchased from Allied Signal (Seelze, Germany) and Sigma-Adrich (Steinheim, Germany), respectively. Diethyl ether (99.7 % purity) and DMDS were purchased from Sigma-Adrich (Steinheim, Germany). The mono-unsaturated hydrocarbons, (Z)-9-pentacosene, (Z)-9-hexacosene, (Z)-9-heptacosene, and (Z)-9-nonacosene, were donated by Prof. J. Millar (University of California Riverside, CA, USA). The purities of these chemicals were >95 % by GC/MS.
Pheromonal Role of (Z)-9-Pentacosene
To test the role of (Z)-9-pentacosene, we applied different doses of the compound on solvent-washed males in experimental setups similar to that previously described for the mating experiments. A stock solution of (Z)-9-pentacosene (1 μg/μl) in pentane was prepared. One, 2, and 4 female equivalents of this compound, corresponding to 46, 93, and 186 μg, respectively, were tested for stimulating mating activity in males. Two freeze-killed males, similar in size and body color, were stripped of their cuticular lipids, as described for females in the mating bioassays, and fastened opposite one another on filter paper discs, using masking tape, in a glass Petri dish with a lid. Males, rather than females, were used in this bioassay, as female dummies (e.g., solvent-cleaned plastic pieces or glass rods simulating the shape and size of females) treated with female extract failed to elicit consistent responses from males. For controls, an identical volume of solvent was applied on the other male. Each treatment and control was presented to 15 males and the responses of the males, including arrestment, alignment, mounting, and copulation, were recorded on a digital video camera.
Statistical Analyses
We computed the duration of each stage of mating from the recordings and expressed these as a percentage of the total duration for each mating couple. The durations were subjected to a one way Kruskal-Wallis analysis of variance (ANOVA) and Tukey HSD test for mean separation, as the data did not pass equal variance and homogeneity tests. Because the first two stages of the mating sequence were very short, they were pooled together for statistical analysis. We used a one-sample chi square test to compare the responses of males to test and control treatments in olfactometer and Petri-dish bioassays. We pooled together, by sex, the relative proportions of each cuticular lipid component for each insect and compared sex differences by a Mann-U Whitney test. We also compared the amount of (Z)-9-pentacosene present in the CLs of the beetle and honey bee in relation to mass. All statistical analyses were carried out at an α level of 0.05 using Genstat VSN version 4 (Rothamstead, UK) software.
Results
Mating Behavior of O. haroldi
In dual-choice olfactometer assays, the numbers of male and female beetles responding to male odors or the control were not different (males: χ 2 = 2.65, P = 0.10, male odor N = 7, control odor N = 11; and females: χ 2 = 0, P = 1, male odor N = 8, control odor N = 8). Similarly, responses of both sexes to female odors were not different from the control (males: χ 2 = 0.52, P = 0.47, female odor N = 6, control odor N = 7; females: χ 2 = 0.44, P = 0.51, female odor N = 8, control odor N = 7).
Analysis of the mating behavior, depicted in Fig. 1 and listed in Table 1 (based on recordings of 10 mating couples), showed four stages displayed by all couples: a male repeatedly palpated a female’s body with the mouth palps upon contact (arrestment), aligned with a female’s body (alignment), then mounted and gripped the female between the prothorax and mesothorax with the forelegs (mounting), before attempting to copulate (copulation). Following copulation, a male occasionally palpated a female’s pronotum, while simultaneously cleaning the aedeagus (by multiple retractions and protrusions of the organ; mean no. of times ± S.E = 6.3 ± 0.7, mean duration ± S.E. = 150.8 ± 49.8 sec) in a post-copulatory stage before dismounting. The entire mating took between 11 and 59.3 min),with the post-copulatory stage lasting 40–70 % of the total duration. The durations of arrestment + alignment, mounting, copulation, and post-copulation were different from each other (H = 3,535, P < 0.001, N = 10), with the first two and post-copulatory stages being the shortest and longest, respectively.
The sequence of behaviors that leads to copulation in Oplostomus haroldi: (a) arrestment of male following mouth contact with female elytra, (b) alignment of male to female’s body, (c) mounting of male on female’s back, characterized by male’s tight foreleg grip of female between the prothorax and mesothorax, and (d) copulation in progress with male aedeagus inserted in female abdominal tip
Role of CLs
Males showed a higher preference for dead, unwashed females than solvent-washed, dead females (one sample χ 2 = 2.7, P = 0.1 for mouth contact; χ 2 = 12.8, P < 0.001 for mounting; χ 2 = 4, P = 0.046 for copulation; Fig. 2a). Similarly, males preferred solvent-washed females coated with female extract over solvent-washed females in a dose-dependent manner, especially for mounting at 0.2 FE (χ 2 = 11, P < 0.001) and 2.0 FE (χ 2 = 5, P = 0.025), and copulation at 0.2 FE (χ 2 = 11, P < 0.001) (Fig. 2b and c). At a dose of 0.1 FE, no mounting or copulation was recorded; while at 2.0 FE only mounting was observed in respondents (Fig. 2). Responses to control treatments were random, following no specific pattern, with mounting and mating also observed.
Male Oplostomus haroldi responses to female treatments in mating bioassays: (a) to solvent-washed and unwashed, freeze-killed (N = 25) females, (b) mounting responses to solvent-washed, freeze-killed females, untreated or treated with different doses (each N = 20) of female cuticular extract, and (c) copulation responses to solvent-washed, freeze-killed females, untreated or treated with different doses (each N = 20) of female cuticular extract. * indicates a difference in male responses to a treatment pair (P < 0.05)
Role of Mouth Palps in Mating and their External Morphology
All the labial-palpectomised males (N = 5, 100 %) mounted successfully, with three (60 %) copulating with females. No maxillary-palpectomised males (N = 5, 100 %) mounted or copulated with females.
SEM revealed fields of identical sensilla on both palps. These were long and short trichoid sensilla, distributed along the sides of the palps, uniporous basiconic sensilla, and unreported sensilla surrounded by collars (enclosed sensilla) (Fig. 3). The basiconic and enclosed sensilla occurred only at the tip of the palps, with more of the former than the latter on both palps. The maxillary palp tip had more sensilla than its labial counterpart.
Scanning electron micrographs of Oplostomus haroldi, illustrating the sensilla found on the labial and maxillary palpi. (a) Frontal view of the mouthparts showing the labial palp (circled in grey) and the maxillary palp (circled in white), (b) tip of a maxillary palp showing the concavity containing numerous sensilla, (c) tip of a labial palp showing the smaller number of sensilla, (d) types of sensilla found on both labial and maxillary palps (white arrow showing enclosed sensillum and grey arrow indicating a basiconic sensillum), (e) basiconic sensillum, and (f) enclosed sensillum
Identification of CLs
We identified the same 20 high molecular weight hydrocarbons, chain length C23–C31, in cuticular extracts of both sexes of the beetle (Table 2; Fig. 4). These CLs were completely stripped from beetles after the third solvent wash, as shown in Supplemental Material S2. A total of nine straight-chain alkanes, six methyl-branched alkanes, and five mono-unsaturated alkenes (Table 2; Fig. 4) constituted the total CL composition, varying quantitatively between males and females (Table 2). In males, straight-chain alkanes, their methyl-branched counterparts, and mono-unsaturated alkenes constituted 35.1, 46.1, and 18.8 %, respectively, of the total cuticular lipids. However, in females, alkenes were most abundant (53 %), with straight-chain alkanes and methyl-branched alkanes comprising 34.2 % and 12.8 %, respectively.
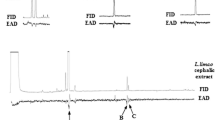
Representative total ion chromatogram of, (a) adult Oplostomus haroldi cuticular lipids before and after reaction with dimethyl disulfide (DMDS). See Table 1 for list of identified compounds represented by peaks 1–24; (b) expanded view of peaks 4–6 showing Z- and E-isomers of Δ9-pentacosene (the latter forming the front shoulder), and (c) DMDS adducts of Z- and E-isomers of Δ9-pentacosene (peaks 21a and b)
DMDS reaction with the cuticular extracts resulted in the disappearance of the components represented by peaks 4a, 4b, 7, 10, 15, and 19 (Fig. 4), indicating the presence of unsaturated components in the extract. Five DMDS adducts were detectable (represented by peaks 21a, 21b, 22, 23, and 24), with parent ions at m/z 444, 444, 458, 472, and 500, respectively. Comparison of the mass spectra of the DMDS adduct of authentic (Z)-9-pentacosene to that of the component in the crude extract represented by peak 21a gave identical fragmentation patterns, with intense m/z 173 and 271 resulting from a cleavage between C9 and C10 following the addition of a methyl sulfide group on each side of the double bond. Peaks 4a and b were identified as two isomers (Z- and E-) of the same compound, based on their identical parent ions (Table 2) and partial separation on the HP-5 capillary column (Fig. 4). On the Carbowax column, both peaks eluted nearly together; comparing RIs and co-injection with authentic (Z)- isomer, showed the (E)-isomer to elute before the (Z)-isomer. Similar analysis for peaks 22–24 showed intense peaks at m/z 173 and 285 (Peak 22), 173 and 299 (Peak 23), and 173 and 327 (Peak 24) (Table 2). The stereochemistries of the mono-alkenes, peaks 4, 7, 10, and 15, were determined by comparison of RIs with those of authentic standards and coinjections with the crude sample on the HP-5 column.
With the exception of n-tricosane, (Z)-9-pentacosene, (Z)-9-hexacosene, and (Z)-9-heptacosene, which were more abundant in females than males, all other cuticular components were more abundant in males (Table 3). Among the four components more prevalent in females than males, (Z)-9-pentacosene was most obviously (twice) more abundant (P = 0.048) in females than in males (Table 3). A comparison of the levels of this compound in both female beetle and worker honey bees, after correcting for difference in weight, showed that it was 19-fold more concentrated in the beetle than in the bee (Supplemental Material S3).
Pheromonal Role of (Z)-9-Pentacosene
In dose–response assays with (Z)-9-pentacosene, the proportion of males arrested by the test and control females did not differ (one sample χ 2 < 3.841, P > 0.05; Table 4). However, at 1 and 2 FE, the proportion of males that displayed alignment and mounting to both treatments differed (Table 4). Overall, (Z)-9-pentacosene elicited arrestment, alignment, and mounting, but not copulation, by males.
Discussion
Our study on the scarab honey bee pest O. haroldi provides the first demonstration of a contact pheromone used in mate finding in the family Scarabaeidae; the pheromone is present on the cuticle of females. This finding is consistent with contact semiochemicals in other families of Coleoptera (Geiselhardt et al. 2009; Lelito et al., 2009). Although Mini (2000) showed a possible involvement of body chemicals as mating cues for the scarab Oryctes rhinoceros L. (Coleoptera: Scarabaeidae), the mode of detection and identity of any potential semiochemicals were not explored. Previous pheromone studies on scarabs have reported only the existence of volatile, airborne aggregation or sex pheromones (Leal 1998; Larsson et al. 2003; Bengtsson et al. 2010). Our olfactometer bioassays on O. haroldi did not implicate any volatile pheromones in sex attraction in this beetle. In O. haroldi, males approached females in a sequence of behaviors consisting of arrestment, alignment, mounting, and copulation, similar to those described in other beetle species utilizing contact semiochemicals for mate discrimination (Ginzel et al. 2003; Geiselhardt et al. 2009, Lelito et al., 2009; Ginzel 2010), but differing in that male O. haroldi perceive the pheromone via the mouth, as opposed to through antennal contact.
We identified straight-chain alkanes, methyl-branched alkanes, and mono-saturated alkenes in the cuticular extracts of both sexes, with (Z)-9-pentacosene especially abundant in female extract. We hypothezise that quantitative differences in cuticular lipid components may constitute the baseline for sex discrimination in O. haroldi, as demonstrated by the partial mating sequence elicited by (Z)-9-pentacosene. That (Z)-9-pentacosene elicited arrestment, alignment, and mounting suggests that it is a key component of the contact sex pheromone of the beetle. However, its failure to elicit copulatory responses from males suggests that other components are necessary in order to elicit the full behavioral mating repertoire. The difference in the relative concentrations of (Z)-9-pentacosene between the beetle and honey bee, and the variation of male beetle responses to different doses of this compound, suggest that O. haroldi may use concentrations of specific cuticular hydrocarbon components to find a mate inside the crowded hive.
A similar scenario, in which both sexes share qualitatively similar cuticular lipid profiles, has been reported for other insects, including the cerambycid Megacyllene robiniae (Ginzel et al. 2003), the mustard leaf beetle, Phaedon cochleariae (Geiselhardt et al. 2009), the emerald ash borer, Agrilus planipennis (Lelito et al., 2009), various Drosophila spp. (Howard and Blomquist 2005), the German cockroach, Blatella germanica (Eliyahu et al. 2008), the silverfishes Lepisma saccharina and Ctenolepisma longicaudata (Woodbury and Gries, 2007), and the termite Coptotermes formosanus (Raina et al. 2003). Interestingly, (Z)-9-pentacosene has been reported to play a similar role, to that which it plays in O. haroldi, in the bug Lygocoris pabulinus (Drijfhout and Groot 2001).
The prolonged duration of the post-copulatory stage of mating in O. haroldi suggests mate guarding. Similar behavior, described as either intrinsic or adaptive, has been reported in other beetle species (Alcock 1991; Shivashankar and Pearson 1994; Facundo et al. 1999; Harari et al. 2003; Flay et al. 2009; Luo et al. 2011) and confers direct (through elimination of other male competitors) or indirect (through propagation of a male’s genetic make-up) benefits to a guarding male. As O. haroldi populations that infest honey bee colonies are male biased (Torto et al. 2010), mate guarding in this beetle likely confers both direct and indirect benefits.
The excision experiment identified the maxillary palps as the structure that detected the contact pheromone, even though identical types of sensilla were found on the maxillary and labial palps. This may imply that these sensilla serve multiple gustatory roles in O. haroldi. Particularly intriguing was the record of an enclosed sensillum, not previously reported in insects (Ryan 2002). Further investigation is needed to describe the internal structure and function of this sensillum. Perception of the contact pheromone is likely to be via the basiconic sensillum, as uniporous sensilla types have been implicated in contact chemoreception in several insects (Ryan 2002). Utilization of the maxillary palps for mate discrimination and reproduction may represent an ecologically significant adaptation by O. haroldi to its unwelcoming host environment as: (a) it allows for protection of the antennae, beneath the eyes, during thanatosis (a defensive tactic employed against bee aggression), (b) it offers greater sensitivity for mate detection, and (c) it combines greater flexibility and protection, as it originates in the buccal cavity; in contrast, the labial palps are fixed to the side of the labium, with fewer sensilla, and are less protected. Protection of the antennae is particularly advantageous as these are utilized to perceive honey bee odors during host searching (Fombong et al., unpublished data.), for dispersal between host colonies, and possibly for location of oviposition sites by females.
In summary, our data provide evidence that the honey bee pest O. haroldi utilizes a contact, rather than a volatile, pheromone for mating. The contact pheromone is perceived by males via sensilla present on the maxillary palp. The evolution and utilization of contact pheromones in certain arthropods may represent an ecological adaptation that enables these arthropods to overcome chemical complexity in challenging environments.
References
Alcock, J. 1991. Adaptive mate-guarding by males of Ontholestes cingulatus (Coleoptera: Staphylinidae). J. Insect Behav. 4:763–771.
Bengtsson, J. M., Chinta, S. P., Wolde-Hawariat, Y., Negash, M., Seyoum, E., Hansson, B. S., Schlyter, F., Stefan, S. and Hillbur, Y. 2010. Pheromone-based mating and aggregation in the sorghum chafer, Pachnoda interrupta. J. Chem. Ecol. 36:768–777.
Donaldson, J. M. I. 1989. Oplostomus fuligineus (Coleoptera Scarabaeidae): Life cycle and biology under laboratory conditions, and its occurrence in bee hives. Coleopt. Bull. 43:177–182.
Drijfhout, F. P. and Groot, A. T. 2001. Close range attraction in Lygocoris pabulinus (L). J. Chem. Ecol. 27:1133–1149.
Eliyahu, D., Nojima, S., Mori, K., and Schal, C. 2008. New contact sex pheromone components of the German cockroach, Blattella germanica, predicted from the proposed biosynthetic pathway. J. Chem. Ecol. 34:229–237.
Facundo, H. T., Linn Jr., C. E., Villani, M. G., and Roelofs, W. L. 1999. Emergence, mating and postmating behaviours of the oriental beetle (Coleoptera: Scarabaeidae). J. Insect Behav. 12:175–192.
Flay, C. D., He, X. Z., and Wang, Q. 2009. Influence of male density on the courtship and mating duration of male rice weevils, Sitophilus oryzae. New Zeal. Plant Protect 62:76–79.
Fombong, A. T., Haas, F., Ndegwa, P. N., and Irungu, L. W. 2012a. Life history of Oplostomus haroldi Witte (Coleoptera: Scarabaeidae) under laboratory conditions and a description of its third instar larva. Int. J. Trop. Insect Sci. 32:56–63.
Fombong, A. T., Mumoki, F. N., Muli, E., Masiga, D. K., Arbogast, R. T., Teal, P. E. A., and Torto, B. 2012b. Occurrence, diversity and pattern of damage of Oplostomus species (Coleoptera: Scarabaeidae), honey bee pests in Kenya. Apidologie. doi:1007/S1359-012-0149-6.
Geiselhardt, S., Otte, T., and Hilker, M. 2009. The role of cuticular hydrocarbons in male mating behaviour of the mustard leaf beetle Phaedon cochleariae (F.). J. Chem. Ecol. 35:1162–1171.
Ginzel, M. D. 2010. Hydrocarbons as Contact Pheromones of Longhorned Beetles (Celeoptera: Cermbycidae), pp. 375–389, in G. J. Blomquist and A. G. Bagnères (eds.), Insect Hydrocarbons: Biology, Chemistry and Chemical Ecology. Cambridge Press, New York.
Ginzel, M. D., Millar, J. G., and Hanks, L. M. 2003. (Z)-9-Pentacosene-contact sex pheromone of the locust borer, Megacyllene robiniae. Chemoecology 13:135–141.
Graham, J. M. 2005. The Hive and the Honey Bee. Dadant, Hamilton.
Harari, A. R., Landolt, P. J., O’Brien, C. W., and Brockmann, H. J. 2003. Prolonged mate guarding and sperm competition in the weevil Diaprepes abbreviatus (L.). Behav. Ecol. 14:89–96.
Howard, R. W. and Blomquist, G. J. 2005. Ecological, behavioural and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 50:371–393.
Larsson, M. C., Hedin, J., Svensson, G. P., Tolasch, T., and Wittko, F. 2003. Characteristic odor of Osmoderma eremita identified as a male-released pheromone. J. Chem. Ecol. 29:575–587.
Leal, W. S. 1998. Chemical ecology of phytophagous scarab beetles. Annu. Rev. Entomol. 43:39–61.
Lelito, J. P., Boroczky, K., Jones, T. H., Fraser, I., Mastro, V. C., Tumlinson, J. H., and Baker, T. C. 2009. Behavioral evidence for a contact pheromone in the emerald ash borer, Agrilus Planipennis. J. Chem. Ecol. 35:104-110.
Luo, S.-L., Zhuge, P.-P., and Wang, M.-Q. 2011. Mating behaviour and contact pheromones of Batocera horsfieldi (Hope) (Coleoptera: Cerambycidae). Entomol. Sci. 14:359–363.
Millar, J. G. and Haynes, K. F. 1998. Methods in Chemical Ecology: Chemical Methods. Kluwer Academic Publishers, Massachusetts.
Mini, A. 2000. Role of palp-tip sensilla of male Oryctes rhinoceros (Coleoptera: Scarabaeidae) in their sexual activation. J. Exp. Zool. India 3:73–82.
Njau, M. A., Mpuya, P. M., and Mturi, F. A. 2009. Apiculture potential in protected areas: The case study of Udzungwa Mountains National Park, Tanzania. Int. J. Biodivers. Sci. Mgt. 5:95–101.
Raina, A. K., Bland, J. M., Dickens, J. C., Park, Y. I., and Hollister, B. 2003. Premating behaviour of dealates of the formosan subterranean termite and evidence for the presence of a contact sex pheromone. J. Insect Behav. 16:233–245.
Ryan, M. F. 2002. Insect Chemoreception: Fundamental and Applied. Kluwer Academic Publishers, London.
SUAZO, A., TORTO, B., TEAL, P. E. A., and TUMLINSON, J. H. 2003. Response of the small hive beetle (Aethina tumida) to honey bee (Apis mellifera) and beehive-produced volatiles. Apidologie. 34: 525-533.
Shivashankar, T. and Pearson, D. L. 1994. A comparison of mate guarding among five syntopic tiger beetle species from peninsular India. Biotropica 26:436–442.
WOODBURY, N., and GRIES, G. 2007. Pheromone-based arrestment behavior in the common Silverfish, Lepisma saccharina, and giant Silverfish, Ctenolepisma longicaudata. J. Chem. Ecol. 33: 1351-1358.
Torto, B., Suazo, A., Teal, P. E. A., and Tumlinson, J. H. 2005. Response of the small hive beetle (Aethina tumida) to a blend of chemicals identified from honeybee (Apis mellifera) volatiles. Apidologie 36:523–532.
Torto, B., Boucias, D. G., Arbogast, R. T., Tumlinson, J. H., and Teal, P. E. A. 2007. Multitrophic interaction facilitates parasite-host interaction between an invasive beetle and the honey bee. Proc. Natl. Acad. Sci. USA 104:8374–8378.
Torto, B., Fombong, A. T., Mutyambi, D. M., Muli, E., Arbogast, R. T., and Teal, P. E. A. 2010. Aethina tumida (Coleoptera: Nitidulidae) and Oplostomus haroldi (Coleoptera: Scarabaeidae): Occurrence in Kenya, distribution within honey bee colonies and responses to host odors. Ann. Entomol. Soc. Am. 103:389–396.
Acknowledgments
The authors thank Dr. Salifu D. for statistical advice, Mokua E. and Obege E. for research assistance, Prof. J. G. Millar, University of California-Davis for providing us with authentic (Z)-9 alkene standards, and Kelley K. and Backer-Kelley K., University of Florida ICBR-EMBL, for the scanning electron microscopy. The German Academic Exchange Service (DAAD) funded ATF while project funding came from the United States Department of Agriculture – Agricultural Research Service (USDA-ARS project no. SCA-586615-7-119F).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Section of a honey bee frame in a colony infested by Oplostomus haroldi showing 3 beetle couples (circled in red). An average of 10.3 mating couples (N = 3, where N is number of honey bee colonies inspected) were observed during surveys in February 2010. (Most worker bees on frame had either flown-off in defense of the colony or as a result of the smoke used to calm them during inpsection) (DOCX 5,758 kb)
Fig. S2
A comparative GC-MS trace of the CLs profile of a female Oplostomus haroldi adult washed in solvent (n-Pentane) showing the removal of CLs after the third solvent wash. (DOCX 40 kb)
Fig. S3
Peak 1 represents (Z)-9-pentacosene which is 19.2 times higher in the beetle based on comparisons corrected for the weight difference between the beetle (N = 3) and worker honey bees (N = 3). (DOCX 52 kb)
Rights and permissions
About this article
Cite this article
Fombong, A.T., Teal, P.E.A., Arbogast, R.T. et al. Chemical Communication in the Honey Bee Scarab Pest Oplostomus haroldi: Role of (Z)-9-Pentacosene. J Chem Ecol 38, 1463–1473 (2012). https://doi.org/10.1007/s10886-012-0211-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-012-0211-x