Abstract
The potential for pheromone-based mating disruption of the Brassica pest Contarinia nasturtii was tested, both in small-scale plots with Brussels sprouts and in commercial-scale fields with either broccoli or cauliflower. Experiments in the small-scale plots used laboratory-reared insects released into a previously uninfested area, whereas large-scale experiments used a high natural population of C. nasturtii. Effectiveness of mating disruption was evaluated by the reduction of male captures in pheromone traps, and by reduction of crop damage caused by C. nasturtii. Dental cotton rolls (small-scale experiment) and polyethylene caps (large-scale experiment), containing 50 μg (2S, 9S)-diacetoxyundecane, 100 μg (2S,10S)-diacetoxyundecane, and 1 μg (2S)-acetoxyundecane, spaced 2 m apart, served as dispensers in the test plots. In both experiments, mean catches of C. nasturtii males in pheromone traps were reduced to near zero in treated plots, with control plots averaging 71 males/trap. In the large-scale experiments, no males were caught in pheromone traps over a period of 41 days after mating disruption was applied; one male was caught from days 42–60. In the small-scale trials, crop damage was reduced by 59 %, compared to the untreated control plot. In the large-scale experiments, damage was reduced on average by 91 %. This study shows successful field application of the mating disruption technique for control of a member of the dipteran family Cecidomyiidae, and demonstrates that pheromone-based mating disruption has potential for management of C. nasturtii populations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Cecidomyiidae, gall midges, are a dipteran family containing about 5000 species (Gagné, 1989), of which a number are important agricultural pests. The swede midge, Contarinia nasturtii (Kieffer), is a serious pest infesting Brassicaceae, e.g., broccoli, cauliflower, cabbage, and Brussels sprouts. Even relatively low population levels of this midge can cause high levels of damage (Harris, 1966; Darvas et al., 2000). The midge is widespread and economically important in Europe, and was introduced into Canada in the mid-1990s (Hallett and Heal, 2001), and later into the USA (Kikkert et al., 2006). The initial infestations in Ontario, Canada caused up to 85 % crop losses on some farms (Hallett and Heal, 2001). Contarinia nasturtii has spread to several important vegetable and canola growing regions in North America (Chen et al., 2011).
A pheromone-based monitoring system has been developed for adult males of C. nasturtii, using a blend of (2S,9S)-diacetoxyundecane, (2S,10S)-diacetoxyundecane, and (S)-2-acetoxyundecane (Hillbur et al., 2005; Boddum et al., 2009). Effective control of this pest requires a number of insecticide applications at weekly or even shorter intervals (Baur et al., 2005; Chen et al., 2007, 2011). Sustainable, cost-effective methods need to be developed to control the pest at low and high infestations.
Female C. nasturtii lay eggs in clusters on young plant tissue (Barnes, 1946; Readshaw 1961). The larvae feed at the growing tips of a plant, leading to gall-like distortions, turning growing tips, deformed plant tissue, and corky brown scars. In Brussels sprouts, cauliflower, broccoli, and other Brassica vegetable crops, even the slightest damage leaves the yield unmarketable. When larvae are fully developed, they move into the soil, form cocoons, and either pupate, before emerging to start the next generation cycle, or begin a diapause that can be one or two winters long. Depending on the regional climate, C. nasturtii has three to four generations per year (Readshaw 1961; Hallett et al., 2009b). In spring, diapausing larvae leave the winter cocoon and create a summer cocoon in which they pupate and then eclose. Emergence is determined by temperature and moisture (Readshaw, 1966).
Common characteristics for cecidomyiid midges are their small size (a few mm) and the short life span of adults (1–2 days) (reviewed by Hall et al., 2012). In many species, males mate several times, whereas females mate only once and produce monogenous progeny (ibid.). Given that C. nasturtii mate shortly after emergence (Barnes, 1946) and that the flight range of adults is relatively short (Samietz et al., unpublished data), there may be potential to control damage by this midge through use of pheromone mating disruption. Accordingly, the aim of this study was to trial an effective and environmentally acceptable control of C. nasturtii by applying synthetic sex pheromone of C. nasturtii to prevent mating, and consequently reproduction, of this pest in crops.
Methods and Materials
Pheromone Dose and Dispensers
Dispenser spacing was set to 2 × 2 m, providing a radius of about 1 m around each dispenser, in order to match the low flight ability of the midges (Samietz et al., unpublished data). Each dispenser was loaded with a blend of 50 μg (2S, 9S)-diacetoxyundecane, 100 μg (2S,10S)-diacetoxyundecane, and 1 μg (2S)-acetoxyundecane, diluted in 100 μl of hexane, resulting in a dose 100 times higher than the dose used for monitoring (Hillbur et al., 2005). Freshly loaded dispensers were placed in the crop 3 d prior to experiments.
The pheromone used in the small-scale experiments was loaded in dispensers made of whole dental cotton rolls (14 mm diam., length 40 mm; IVF Hartmann, Neuhausen, Switzerland). Each dispenser was covered by a delta-shaped, 10 cm-wide sheet (10 cm high; open to the sides), made of white plastic foil, for rain protection, and mounted at a height of 45 cm.
In the large-scale experiments, polyethylene vial caps (12 mm diam., height 8.5 mm, Semadeni, Ostermundigen, Switzerland) were used, as polyethylene is a longer-lasting dispenser than cotton in the field (Boddum et al., 2009). The vial caps were pressed through holes, matching their diameter, in white plastic cross-slit labels (Kreuzschnittetiketten 4 × 8 cm; Baumann Saatzuchtbedarf, Waldenburg, Germany). One dispenser was adjusted to one plastic cross-slit stick of 40 cm length (Kreuzschnittetikettenstäbe; Baumann Saatzuchtbedarf, Waldenburg, Germany). Another cross-slit label (c.f. above), without a hole, was slipped on the same stick exactly above the dispenser, in order to protect it from rain and sunlight (especially ultraviolet radiation). The ready-made sticks were fixed about 15 cm deep into the soil; i.e., dispensers were at a height of about 25 cm above ground. Again, dispensers were spaced in a rectangular 2 × 2 m grid over the entire plot.
The field doses of pheromones were estimated based on approximate release rates from trapping dispensers in the field (S. Rauscher, unpublished data). Additional field experiments showed that the release-rate ratio of the three components was relatively constant over a period of 32 d in the field (Boddum et al., 2009). Polyethylene dispensers were highly attractive in the field for a period of 46 d, despite comparatively low release rates toward the end of the period (Boddum et al., 2009). In dental cotton dispensers, the amounts of pheromone components released during the field trials over 70 d were estimated to average 1.78 mg·ha−1·day−1 (2S, 9S)-diacetoxyundecane, 3.57 mg·ha−1·day−1 (2S,10S)-diacetoxyundecane, and 35.7 μg·ha−1·day−1 (2S)-acetoxyundecane. In the polyethylene dispensers, the amounts released over 100 d were estimated to average 1.25 mg·ha−1·day−1 (2S, 9S)-diacetoxyundecane, 2.5 mg·ha−1·day−1 (2S, 10S)-diacetoxyundecane, and 25 μg·ha−1·day−1 (2S)-acetoxyundecane.
Small-Scale Experiments
Two equally designed and planted plots of Brussels sprouts (Brassica oleracea var. gemmifera cv. Helemus) were used with released midges. The plots were located on the ACW experimental farm at Wädenswil (Switzerland). Dimensions of the plots were 16 × 16 m, with a distance of 1 m between rows, and about 40 plants per row (640 plants per plot). Neither of the plots had Brassica planted for several years prior and, as a consequence, had no natural population of C. nasturtii. The two plots were located about 500 m apart and were separated by a row of greenhouses, which probably precluded midge movements from one plot to the other and pheromone contamination of the untreated plot. One plot was treated with pheromone for mating disruption; the other was untreated and used as a control.
Contarinia nasturtii were released into plots by placing 12 pots in each plot. Each pot contained substrate with 150–250 pupae of laboratory-reared midges. The total expected emergence of adult midges was about 1000 males and 1000 females per plot per release, hatching over a period of approximately 1 week.
In the initial experiment, starting 20 July 2004, pots were placed in a row 30 m west of the treated and control plots. Plants in the plots, planted 10 weeks before, were about 50 cm high. This experiment tested whether mating disruption in a target field was effective when the emergence area was not treated with pheromone.
In a second experiment, starting 9 August 2004, pots were randomly distributed within the treated and control plots. Plants in plots were planted 13 weeks earlier, and were about 65 cm high. The experiment tested whether mating disruption in a target field is effective when midges emerge within the treated plot. The general effectiveness of mating disruption was tested by pheromone-trap shutdown. For this, four pheromone traps were randomly distributed over each of the treated and control plots. The sticky inserts of the traps were checked, changed, and catches determined at 3- or 4-d intervals over 6 weeks.
Damage for both experiments was scored at harvest time (end of September 2004). Randomly starting within each row, every 5th plant was inspected for damage; i.e., about 130 plants were examined from each of the control and treated plots. Each plant was scored for early damage by 1st-release midges (leaf axillary sprouts on lower plant half) and later damage by 2nd-release midges (leaf axillary sprouts on higher plant half, not yet formed at time of 1st release of midges). Damages scored for each plant were the number of distorted sprouts and the number of sprouts with cork damage.
Large-Scale Experiments
Large-scale field experiments were carried out in commercial Brassica fields in the Seeland area between Müntschemier and Kerzers in Switzerland. This area is one of the most important Swiss vegetable growing areas, with many medium-size vegetable fields. The breeding conditions for C. nasturtii are good in this area, due to the bog soil and moist conditions supported by irrigation, with the population densities being high.
The first experiment was conducted in a 3000-m2 broccoli field (Brassica oleracea var. italica cv. Fiesta) in Müntschemier, in two plots planted 29 July 2005 (plot 1.1, 100 × 15 m) and 12 July 2005 (plot 1.2, 100 × 15 m), respectively. The treated area within plots was 34 × 34 m (1156 m2), using 16 × 16 (256) pheromone dispensers. The treated plot produced 504 plants at harvest, while the control plot, without any treatment, was 16 × 30 m (480 m2) and produced 980 plants at harvest.
The second experiment was conducted in a 8000-m2 broccoli field (Brassica oleracea var. italica cv. Fiesta) in Kerzers, planted 1 August 2005 (plot 2). The treated area was 34 × 42 m (1428 m2), and used 16 × 20 (320) pheromone dispensers. It produced 635 plants at harvest. The control area, without any treatment, was 16 × 40 m (640 m2) and produced 677 plants at harvest.
The third experiment was conducted in a 5250-m2 cauliflower field (Brassica oleracea var. botrytis cv. Fremont) in Ried, planted 2 August 2005 (plot 3). The treated area was 34 × 42 m (1428 m2), used 16 × 20 (320) pheromone dispensers, and produced 338 plants at harvest. The control area, without any treatment, was 10 × 35 m (350 m2), and produced 346 plants at harvest.
All pheromone dispensers were placed in the fields on 2 August 2005. Damage scoring was carried out on 30 September 2005 (the two broccoli fields, plots 1.1, 1.2, and 2) and on 18 October 2005 (the cauliflower field, plot 3), scoring each plant (sample sizes given above) in the treated and control plots into the following categories: A—no damage, B—cork damage, C—deformed inflorescence, D—without inflorescence. Only category A would be marketable in Switzerland.
Statistics
Pheromone-trapping results were compared using repeated measures analyses of variance (RM-ANOVA), with sampling times being the repetitions in the analysis, followed by a pairwise comparison of treated and control plots by Dunn-Sidak tests. Damage in the small-scale experiments was analyzed by analyses of covariance (ANCOVA), with treatment as factor and spatial arrangement (crop row from immigration side) as covariate. Proportions of plants in the different damage categories of the field-scale trials were compared by χ 2 tests for analyzing the tabulated counts. Efficacy of pest control in each trial was calculated as relative reduction of damage in the treated vs. the control plot (Abbot’s formula). All statistics were performed with XLStat Pro V2011.204 (Addinsoft, Andernach, Germany).
Results
Small-Scale Experiments
Application of pheromone resulted in a complete reduction of trap catch in pheromone traps in the plot where midges were released inside the plot (Table 1); no midges were caught over four continuous weeks (RM-ANOVA, treatment by repetition: F 7,42 = 100.3, P < 0.001). During the same period, traps in control plots caught more (282) midges (Table 1; Dunn-Sidak tests, P < 0.05). Five weeks after release, a single male was caught in the treated plot (Table 1).
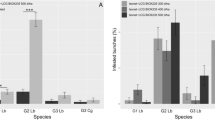
In total, 79.4 % of all plants in controls of the small-scale trials were damaged by C. nasturtii, indicating that the release of midges simulated high population pressure. The spatial distribution of damage in the control and pheromone-treated plots is shown in Fig. 1. The total damage across the sampled plants (Fig. 1a) was influenced by treatment (ANCOVA factor: F 1,260 = 37.5, P < 0.001), but not by spatial arrangements of rows from the initial immigration side, i.e., in the first experiment with release from distance (ANCOVA covariate: F 1,260 = 0.227, P = 0.634). The same result was obtained when analyzing separately for early damage caused by midges released from distance (Fig. 1b; ANCOVA factor: F 1,260 = 6.62, P = 0.011, covariate: F 1,260 = 0.001, P = 0.973), and also for late damage caused by midges released from inside the plots (Fig. 1 c; ANCOVA factor: F 1,260 = 51.3, P < 0.001, covariate: F 1,260 = 0.459, P = 0.499).
The pheromone treatment resulted in a reduction of damage by C. nasturtii, both when midges were released at a distance from the plots, and also when they were released inside plots (Table 2). When midges were released from a distance, crop damage was reduced by 68 % (ANCOVA factor: F 1,260 = 4.00, P < 0.05, covariate: F 1,260 = 0.237, P = 0.627), when measured by the number of distorted sprouts, and by 80 % (ANCOVA factor: F 1,260 = 5.22, P < 0.05, covariate: F 1,260 = 0.002, P = 0.962), when measured by cork-damaged sprouts. Damage was also reduced when midges were released within the field; by 75 %, when measured by the number of distorted sprouts (ANCOVA factor: F 1,260 = 49.2, P < 0.001, covariate: F 1,260 = 1.39, P = 0.238), by and 34 %, when measured by the cork-damaged sprouts (ANCOVA factor: F 1,260 = 11.7, P < 0.001, covariate: F 1,260 = 2.99, P = 0.082). The overall reduction of damage in the two release experiments was 59 % (Table 2; ANCOVA factor: F 1,260 = 37.5, P < 0.001).
Large-Scale Experiments
In the commercial fields, no C. nasturtii males were caught in pheromone traps over the first 41 days after the mating disruption treatment had been applied; from days 42–60, one male was caught (Fig. 2; Dunn-Sidak tests, P < 0.05). In the untreated control, 473 males were caught during the first 41 days, and 239 males caught from days 42–60. Over the entire period, the difference in trap catches between treated and control plots was highly significant (RM-ANOVA, treatment by repetition: F 8,48 = 5.27, P < 0.001).
Mean number (± SE) of Contarinia nasturtii males caught in pheromone traps in two large-scale fields with pheromone dispensers for mating disruption, and in two control fields (a plot 1, b plot 2). In each field plot, four pheromone traps were placed randomly. Asterisks indicate differences (RM-ANOVA, Dunn-Sidak tests, P < 0.05) in trap catches between pheromone treated plots and untreated control plots
Untreated control plots of the field trials had rates of damaged plants between 7.4–19.1 % (average 12.4 ± 2.5 %, SE). All three fields included in the large-scale experiment showed a reduction in damage by C. nasturtii (Fig. 3, χ 2-tests, P < 0.01). Total damage rates in the untreated control ranged from 10–19 % (Fig. 2a). The reduction in crop damage due to pheromone treatment was 90.9 %, on average (Fig. 3a). When discriminating for damage type, cork damage (corky scars on plant tissue), deformed inflorescence, and missing inflorescence, the different plots had similar patterns, except for the 1st planting in Müntschemier, in which plants in the pheromone-treated areas showed no deformed or missing inflorescences, implying a 100 % mating disruption efficacy for these damage types (Fig. 3b–d).
Damage rates and treatment efficacies in large-scale fields with pheromone dispensers for mating disruption of Contarinia nasturtii and in untreated control fields. Proportion of damaged Brassica plants in total (a) and in the different damage categories (b–d). All damage categories as well as total damage show differences (X 2-tests, P < 0.01) between pheromone-treated plots and untreated control plots
Discussion
Our study showed that the tested release rates of (2S,9S)-diacetoxyundecane, (2S,10S)-diacetoxyundecane and (2S)-acetoxyundecane prevented male capture of C. nasturtii in pheromone traps, and also reduced plant damage caused by this pest. This demonstrates the potential of pheromone-based mating disruption for control of these cecidomyiid midges, supporting previous pheromone-based control studies, using attract and kill, mass trapping, and mating disruption techniques, on apple leaf curling midge, Dasineura mali, and raspberry cane midge, Resseliella theobaldi, (reviewed by Hall et al., 2012),
Polyethylene dispensers generated complete trap shutdown over a period of 41 days, and close to 100 % reduction over 60 days in plots containing released C. nasturtii. In plots with natural infestations of C. nasturtii, treatment reduced damage by 91 % (cauliflower), 94 %, and 88 % (broccoli) relative to untreated controls. A reduction of damage in treated plots was also achieved when midges were released at a distance from the plots, suggesting that a substantial proportion of matings must have occurred away from the site of emergence/release (i.e., probably within control plots). This is promising with respect to the potential of using mating disruption, as the technique may be effective against midges moving into a crop for mating and subsequent oviposition. This also suggests that males, as well as females, may be attracted to the Brassica host plant, in contrast to what has been suggested for the related pea midge, C. pisi (Wall et al., 1991).
Pyrethroid, spinosyd, and neonicotinoid insecticides are efficient against C. nasturtii in the laboratory and in the field, as long as population densities are moderate (Wu et al., 2006; Chen et al., 2007; Hallett et al., 2009a). Damage levels in Brassica vegetable cultures are kept within the range of marketable quality at the beginning of the season by regular application of these insecticides (Wu et al., 2006; Hallett et al., 2009a). Under high population densities, later in season, however, the efficacy of insecticides is much lower than what we achieved by pheromone mating disruption in the present study (Hallett et al., 2009a). Our large-scale trials, comparable to high population pressures under natural field conditions, showed a 90 % reduction in damaged plants compared to control plots in which nearly 20 % of the plants were damaged (plot 3).
However, mating disruption is likely to be relatively expensive, due to the relatively high costs of synthetic pheromone. The three components tested were all pure S-enantiomers, for which the syntheses are laborious and time-consuming (Hillbur et al., 2005). A possible cheaper option would be to use mixtures of stereoisomers of the three components, with total amounts increased to account for the lower amounts of the correct enantiomers in the racemates. The presence of R-enantiomers of two (2,9-diacetoxyundecane and 2-acetoxyundecane) of the three components in the attractive three component blend of S-enantiomers has little or no effect on male orientation and pheromone trap catch in the field (Boddum et al., 2009). We would expect, at least for these two components, that mating disruption with racemates should also work by interfering with mate location. In contrast, the presence of the non-natural stereoisomers of 2,10-diacetoxyundecane, i.e., (2R,10R)-diacetoxyundecane or meso-2,10-diacetoxyundecane, has a strong inhibitory effect on male orientation and trap catch (Boddum et al., 2009). Nevertheless mating disruption could be effective with a cheap mixture of stereoisomers, based on a combination of disruptive and repellent effects (Bengtsson et al., 1994; Suckling and Burnip, 1996; Eizaguirre et al., 2007).
Although the influence of dispenser spacing and active dose range remains to be investigated, the synthetic pheromone blend tested in this study shows potential for managing C. nasturtii populations. Its use may substitute for, or at least reduce the input of, chemical insecticides (Wu et al., 2006; Hallett et al., 2009a), or enhance other control approaches for this pest, such as cultural techniques or host plant resistance (Wyss and Daniel, 2004; Chen and Shelton, 2007; Hallett, 2007; Chen et al., 2009).
References
Barnes, H. F. 1946. Gall Midges of Economic Importance, Vol. I: Gall Midges of Root and Vegetable Crops. Crosby, Lockwood & Son, London.
Baur, R., Rauscher, S., Eder, R., and Samietz, J. 2005. Kohldrehherzgallmücke: Überwachung des Fluges mit Pheromonfallen. Der Gemüsebau 2(05):18–19.
Bengtsson, M., Karg, G., Kirsch, P. A., Lofqvist, J., Sauer, A., and Witzgall, P. 1994. Mating disruption of pea moth Cydia nigricana F. (Lepidoptera, Tortricidae) by a repellent blend of sex-pheromone and attraction inhibitors. J. Chem. Ecol. 20:871–887.
Boddum, T., Skals, N., Wiren, M., Baur, R., Rauscher, S., and Hillbur, Y. 2009. Optimisation of the pheromone blend of the swede midge, Contarinia naturtii, for monitoring. Pest Manag. Sci. 65:851–856.
Chen, M. and Shelton, A. M. 2007. Impact of soil type, moisture and depth on swede midge pupation and emergence. Environ. Entomol. 36:1349–1355.
Chen, M., Zhao, J. Z., and Shelton, A. M. 2007. Control of Contarinia nasturtii (Diptera: Cecidomyiidea) by foliar sprays of acetamiprid on cauliflower transplants. Crop Prot. 26:1574–1578.
Chen, M., Li, W. W., and Shelton, A. M. 2009. Simulated crop rotation systems control swede midge, Contarinia nasturtii. Entomol. Exp. Appl. 133:84–91.
Chen, M., Shelton, A. M., Hallett, R. H., Hoepting, C. A., Kikkert, J. R., and Wang, P. 2011. Swede midge (Diptera: Cecidomyiidae), ten years of invasion of crucifer crops in North America. J. Econ. Entomol. 104:709–716.
Darvas, B., Skuhrava, M., and Andersen, A. 2000. Agricultural and dipteran pests of the Palaearctic region, vol. 1, in L. Papp and B. Darvas (eds.), Contributions to a Manual of Palaearctic Diptera. Science Herald, Budapest.
Eizaguirre, M., Albajes, R., Lopez, C., Sans, A., and Gemeno, C. 2007. Inhibition of pheromone response in Sesamia nonagrioides by the pheromone of the sympatric corn borer, Ostrinia nubilalis. Pest Manag. Sci. 63:608–614.
Gagné, R. J. 1989. The Plant Feeding Gall Midges of North America. Cornell University Press, Ithaca.
Hall, D. R., Amarawardana, L., Cross, J. V., Francke, W., Boddum, T., and Hillbur, Y. 2012. The chemical ecology of cecidomyiid midges (Diptera: Cecidomyiidae). J. Chem. Ecol. 38:2–22.
Hallett, R. H. 2007. Host plant susceptibility to the swede midge (Diptera: Cecidomyiidae). J. Econ. Entomol. 100:1335–1343.
Hallett, R. H. and Heal, J. D. 2001. First nearctic record of the swede midge (Diptera: Cecidomyiidae), a pest of cruciferous crops from Europe. Can. Entomol. 133:713–715.
Hallett, R. H., Chen, M., Sears, M. K., and Shelton, A. M. 2009a. Insecticide management strategies for control of Swede midge (Diptera: Cecidomyiidae) on cole crops. J. Econ. Entomol. 102:2241–2254.
Hallett, R. H., Goodfellow, S. A., Weiss, R. M., and Olfert, O. 2009b. MidgEmerge, a new predictive tool, indicates the presence of multiple emergence phenotypes of the overwintered generation of swede midge. Entomol. Exp. Appl. 130:81–97.
Harris, K. M. 1966. Gall midges genera of economic importance (Diptera: Cecidomyiidae). Part I: introduction and subfamily Cecidomyiinae; supertribe Cecidomyiidi. Trans. R. Entomol. Soc. Lond. 118:313–358.
Hillbur, Y., Celander, M., Baur, R., Rauscher, S., Haftmann, J., Franke, S., and Francke, W. 2005. Identification of the sex pheromone of the swede midge, Contarinia nasturtii. J. Chem. Ecol. 31:1807–1828.
Kikkert, J. R., Hoepting, C. A., Wu, Q. J., Wang, P., Baur, R., and Shelton, A. M. 2006. Detection of Contarinia nasturtii (Diptera: Cecidomyiidae) in New York, a new pest of cruciferous plants in the United States. J. Econ. Entomol. 99:1310–1315.
Readshaw, J. L. 1961. The biology and ecology of the swede midge, Contarinia nasturtii (Kieffer) (Diptera: Cecidomyiidae). Ph.D. dissertation, University of Durham, Durham, United Kingdom.
Readshaw, J. L. 1966. The ecology of the swede midge, Contarinia nasturtii (Kieff.), (Diptera; Cecidomyiidae). I.—Life-history and influence of temperature and moisture on development. Bull. Entomol. Res. 56:685–700.
Suckling, D. M. and Burnip, G. M. 1996. Orientation disruption of Planotortrix octo using pheromone or inhibitor blends. Entomol. Exp. Appl. 78:149–158.
Wall, C., Biddle, A. J., Blood Smyth, J., Foot, G. E., Garthwaite, D. G., Morris, N., Owen, C. R., Sturgeon, D. M., and Weyman, G. 1991. Local, spatial and temporal distribution of pea midge, Contarinia pisi. Asp. Appl. Biol. 27:355–360.
Wu, Q. J., Zhao, J. Z., Taylor, A., and Shelton, A. M. 2006. Evaluation of insecticides and application methods against swede midge (Diptera: Cecidomyiidae), a new invasive insect pest in the United States. J. Econ. Entomol. 99:117–122.
Wyss, E. and Daniel, C. 2004. The effect of exclusion fences on the colonization of broccoli and kohlrabi by the swede midge, Contarinia nasturtii (Diptera: Cecidomyiidae). Mitt. Dtsch. Ges. Allg. Ang. Entomol. 14:387–390.
Acknowledgments
We thank Stefan Rauscher, Reinhard Eder, Jacob Rüegg, Hansueli Höpli, Teresa Koller, Regula Eder-Bauermeister, Martin Keller, and Mira Contesse for assistance with laboratory and field work. Support by the commercial growers Urs Johner-Leibundgut (Kerzers), Jakob Wettstein (Müntschemier), Manfred Wolf (Ried b. Kerzers), and the ACW experimental farm, especially Jürgen Krauss, is appreciated. The supply of pure pheromone components by Wittko Francke, Institute of Organic Chemistry, University of Hamburg is acknowledged.
This paper was largely compiled during a sabbatical of J.S. that was generously hosted by The New Zealand Institute for Plant and Food Research, Lincoln. The support, especially by Max Suckling and his group, is thankfully acknowledged. Y.H. thanks the Linnaeus grant IC-E3 (Formas, Sweden) for funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Samietz, J., Baur, R. & Hillbur, Y. Potential of Synthetic Sex Pheromone Blend for Mating Disruption of the Swede Midge, Contarinia nasturtii . J Chem Ecol 38, 1171–1177 (2012). https://doi.org/10.1007/s10886-012-0180-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-012-0180-0