Abstract
Mosaic trees contain more than one phenotype. The two Eucalyptus mosaic trees studied here (E. melliodora and E. sideroxylon) are predominantly susceptible to insect herbivory, with the leaves on a single large branch on each tree resisting herbivory. We used gas chromatography–mass spectrometry and high-pressure liquid chromatography to analyze the chemical profile of leaves of the mosaic trees, as well as leaves of adjacent non-mosaic con-specifics. We show that the leaves of the two phenotypes are distinctly different. Compared to the susceptible (S) leaves on the same tree, the resistant (R) leaves on the mosaic trees had low concentrations of sesquiterpenes (E. melliodora: 2 vs. 24 mg·g−1 dry matter; E. sideroxylon: 8 vs. 22 mg·g−1 dry matter), high concentrations of FPCs (E. melliodora: 5.4 vs. 0.3 mg·g−1 dry matter; E. sideroxylon: 9.8 vs. 0.2 mg·g−1 dry matter) but similar concentrations of nitrogen (E. melliodora: 15.4 vs. 16.8 mg·g−1 dry matter; E. sideroxylon: 13.1 vs. 14.0 mg·g−1 dry matter). The only difference between the two mosaic trees was in the levels of monoterpenes. The R leaves from the mosaic E. melliodora contained higher concentrations of monoterpenes compared to the S leaves (12 vs. 6 mg·g−1 dry matter). In contrast, the leaves from the E. sideroxylon mosaic contained much higher concentrations of monoterpenes with a reversed pattern (R: 26 vs. S: 45 mg·g−1 dry matter). There were qualitative differences too on the mosaic trees. The R leaves of both species contained much higher concentrations of the monoterpene, 1,8-cineole, whereas the S chemotype of E. sideroxylon only had high concentrations of phellandrenes. Furthermore, the chemical differences between leaves on the R and S branches of the mosaic trees resembled those between the leaves of R and S con-specific trees in the same population. We use these data and knowledge of secondary metabolite biosynthesis to propose that high-level transcriptional differences may control the profile of specialized metabolites in eucalypts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Long-lived organisms, such as forest trees, may accumulate somatic mutations throughout their lives that result in an adult supporting several genotypes. This is known as genetic mosaicism (Whitham and Slobodchikoff, 1981; Gill, 1986) – a hypothesis proposed to explain variable resistance to pests within a single plant. However, genetic variation probably will go unnoticed unless accompanied by phenotypic variation. There are excellent examples of such variation among horticultural species. For example, nectarines are a genetic variant of the peach (Prunus persica), and peach fruits have occurred on nectarine trees and vice versa since 1937 (McGregor, 1976). There are many other examples of intra-individual variation of plants that have profound influences on plant herbivore interactions (e.g., Herrera, 2009).
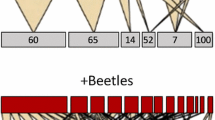
Two phenotypically variable mosaic Eucalyptus trees were identified in open woodland and pastoral environments in central New Soth Wales, Australia (NSW): an E. melliodora (Edwards et al., 1990) and an E. sideroxylon (Edwards et al., 1993) (Fig. 1). The two species are members of the “box-ironbark group” of Eucalyptus in the sub-genus Symphyomyrtus, section Adnataria, sub-section Terminales, series Melliodorae (Brooker, 2000; Slee et al., 2006). Despite their close affinities, they tend to occupy different ecological niches with E. melliodora preferring acidic clay, whereas E. sideroxylon occurs on more alkaline sandy soils (Brooker, 2000). Other unusual phenotypes grow nearby. Approximately 100 m from the mosaic E. melliodora is another of that species with unusual bark on its trunk (Fig. 1c). Chemical mosaics also occur in E. camaldulensis (Edwards et al., 1993) and in E. radiata (Penfold and Morrison, 1937), which suggests that genetic mosaicism in eucalypts may be more common than appreciated.
Photographs of mosaic trees. a Eucalyptus melliodora and b E. sideroxylon supporting leaves of two ecotypes (Edwards et al., 1993; Edwards et al., 1990). c a bark-mosaic E. melliodora with typical box-bark on the lower trunk, which changes to an iron-bark before returning to a box-bark on the newest branches (left). d the typical pattern of leaf damage by Christmas beetles (Anoplognathus spp.)
Central NSW periodically experiences outbreaks of several insect species, which result in widespread defoliation of dominant trees (Edwards et al., 1990). Damage by several species of Christmas beetles (Anoplognathus spp.) revealed the two phenotypic mosaics that we studied because the beetles ate most leaves but left leaves on the resistant (R) branches virtually untouched (Edwards et al., 1990, 1993). Those workers showed that both the composition and concentration of several monoterpenes differ between the leaves of the R and susceptible (S) ecotypes (Edwards et al., 1990, 1993). They proposed that heavy insect feeding released a favorable mutation in the meristematic tissue (the resistant ecotype) that persisted to form an entire branch. Herbivorous insects frequently feed on meristematic tissue. They will either destroy the meristem and alter the growth form of the plant or consume part of the meristem whereby different cells form the developing branch (Marcotrigiano, 2000). One possible scenario is that exposure of damaged cells to reactive oxygen species causes mutations to the nuclear DNA (Halliwell, 1999). If the genotype of these cells differs from the rest of the plant, then a genetic mosaic will arise. A phenotypic mosaic, like the Eucalyptus spp described, may result if this genotypic difference also encodes a phenotypic change. Understanding the chemical changes in the mosaics will enable us to develop hypotheses regarding the specific type of mutation. For example, changes in monoterpenes, such as those found by Edwards et al. (1990, 1993), would suggest a mutation in the monoterpene biosynthetic pathway. In contrast, widespread changes in several chemical traits would suggest that the mutation is at a higher control point, such as a transcription factor.
In this study, we report the profiles of three biosynthetically distinct groups of foliar metabolites − the monoterpenes, sesquiterpenes, and the formylated phloroglucinol compounds (FPCs), from both phenotypes of the mosaic trees and from non-mosaic individuals in the same population.
In particular, we ask: (i) What similarities and differences are there in the chemical profiles of the two eucalypt species? (ii) Do the mosaic trees retain their chemical profiles over a period of several years? (iii) Do the chemical profiles of the leaves of R and S trees differ in all three groups of metabolites? (iv) Do the chemical profiles of leaves from non-mosaic individuals mirror the profiles of the leaves from the R and S branches of the mosaic trees?
By answering these questions, we propose a mechanism for the evolution and maintenance of multiple chemotypes in a single individual using the genetic mosaic hypothesis (GMH) framework.
Methods and Materials
Plant Material
Foliage samples were collected at two sites in south-eastern NSW – Yeoval (32°45′00″S; 148°39′00″E), where there is a chemically mosaic E. melliodora, and at Cumnock (32°55′00″S; 148°46′01″E), where a mosaic E. sideroxylon grows (Edwards et al., 1990, 1993). In 2005 and 2009, we collected leaf samples (ca 60 g) using an elevated platform mounted on a truck. Each of the mosaic trees has one large R branch from which smaller R branches originate. Therefore, we collected a sample from 5 of these smaller R branches and 5 samples from random points in the S part of the canopy. In 2005, we also collected a sample of foliage from two neighboring trees of E. melliodora (M11, M12) and of E. sideroxylon (S14, S15). Edwards et al. (1990, 1993) had already identified these as R (M12, S15) and S (M11 and S14). The collection in 2009 was larger. Apart from sampling the mosaic trees again, we also collected 3 – 5 samples from each of 10 non-mosaic E. melliodora and from 11 non-mosaic E. sideroxylon growing within a 50 m radius of the mosaic tree. These included M11, M12, S14, S15, and several E. melliodora (M2-M8, M10) and E. sideroxylon (S2, S8, S16-S22) of unknown ecotype. Each leaf sample was sub-sampled. We immediately placed a weighed amount (ca 0.5 g) into a sealed vial containing 5 ml of 100 % ethanol (Southwell and Russell, 2002) with 0.254 g·l−1 tetradecane (Sigma-Aldrich, Sydney Australia) as an internal standard for terpene analysis. A second sub-sample of leaves was stored in a sealed polythene bag and kept frozen on a bed of CO2 pellets. Approximately 1 g of these leaves was dried at 50 °C for 72 h to determine water content. Another sample (ca 20 g) was freeze-dried and ground to pass a 1 mm screen in a Tecator Cyclotec mill for analysis of formylated phloroglucinol compounds (FPCs) and nitrogen. We retained the remaining portion of the sample at −20 °C pending further work.
Analyses
Terpenes
Terpenes were separated using gas chromatography on an Agilent 6890 GC and detected with an Agilent 5973 Mass Spectrometer. The Alltech AT-35 column (35 % phenyl, 65 % dimethylpolyoxylane) (Alltech, Wilmington, DE, USA) was 60 m long with an internal diameter of 0.25 mm and a stationary phase film thickness of 0.25 μm. The carrier gas was helium, and we injected 1 μl of the ethanol extract at 250 °C at a 1:25 split ratio. The temperature program for the 25 min elution was 100 °C for 5 min, ramping to 200 °C at 20 °C·min-1, followed by a ramp to 250 °C at 5 °C·min−1, where it remained for 4 min. We identified peaks by comparing mass spectra to reference spectra in the National Institute of Standards and Technology library (Agilent Technologies, Deerfield, IL, USA) and verified major peaks with authentic standards. One sample from each individual was reanalyzed on the final day of chromatography to confirm that retention times remained stable. The area under each peak was measured manually with the help of MSD Chemstation Data Analysis (Agilent Technologies, Deerfield, IL, USA) and converted to a relative concentration by comparison with the internal standard.
Formylated phloroglucinol compounds
Formylated phloroglucinol compounds (FPC) were extracted by sonicating 50 mg of freeze-dried, ground leaf with a known mass (ca 4.0 g) of solvent (7 % water in acetonitrile containing 0.1 % trifluoroacetic acid and 0.300 g·l−1 of the internal standard, 2-ethylphenol) for 5 min (Wallis and Foley, 2005). The resulting mixture was filtered (0.2 μm) directly into an auto sampler vial, and then we injected 20 μl onto a Wakosil 250 × 4 mm GL 3C18RS (SGE, Ringwood, Victoria) column maintained at 37 °C with a flow rate of 0.75 ml·min−1on a Waters Alliance Model HPLC. We eluted the FPCs under gradient conditions with 0.1 % trifluoroacetic acid in water (A) and 0.1 % trifluoroacetic acid in acetonitrile (B) as follows: 40 % A/60 % B for 5 min, linear gradient to 90 % B/10 % A at 60 min, held for 10 min and returned to starting conditions over 10 min. We measured the peak response at 275 nm and quantified the FPCs present using authentic standards purified in the laboratory (Eschler and Foley, 1999). The coefficient of variation between duplicate measurements was typically less than 4 %.
Total nitrogen
We determined the total nitrogen concentration on duplicate samples (200 ± 10 mg) of freeze-dried ground leaf using a Leco CNS-2000 combustion analyzer. Duplicates that differed by more than 2 % were repeated. We re-analyzed 8 random samples to ensure repeatability (Wallis et al., 2011).
Terminology – Ecotypes and Chemotypes
In 1990 and 1993, Edwards et al. made comprehensive observations of herbivory, focusing on Christmas beetle (Anoplognathus spp) damage. From this we can define the S ecotype as leaves that suffer heavy herbivory, while leaves of the R ecotype suffer little or no herbivory. This can occur for two reasons. First, all ecotypes have the same suite of chemicals, but differences in concentrations convey resistance or susceptibility. Alternatively, the R and S ecotypes may contain different suites of chemicals. Either way, it is likely the ecotypes will have a different chemical profile or chemotype, defined as consistent and discontinuous differences in chemical composition that occur in the same species (Keszei et al., 2008; Kleine and Müller, 2011).
Statistical Analyses
We used GenStat 12th Edition Lawes Agricultural Trust (Rothamsted Experimental Station) for all analyses. We first used principal component analysis (PCA) to identify and describe the chemical variation within and between trees, using the monoterpene and sesquiterpene data expressed as a proportion of each pool, respectively (Butcher et al., 1994; Ramanoelina et al., 1994; Trilles et al., 2006; Padovan et al., 2010). Due to the limited data from 2005, which precluded other analyses, we also used the PCA to examine whether samples from the two collections grouped together, suggesting chemical stability over time. We then used the linear mixed model with restricted maximum likelihood (REML) to measure variation in monoterpenes, cineole, sesquiterpenes, FPCs, and nitrogen between the two species, trees within species, branches within trees, and ecotypes. The fixed model was “ecotype” plus “branch within tree within species”, and the random model “branch within tree”. Finally, we compared the chemistry of the leaves from R and S branches on the mosaic trees with that of leaves from R and S neighboring trees after first restricting the data to either “R” or “S” ecotypes. In this case, the fixed model was “mosaic” and the random model again “branch within tree”.
Results
Chemical Profile of Leaves of Two Ecotypes
We categorized four groups of monoterpenes (Groups A-D) and three groups of sesquiterpenes (Groups E-G) (Table 1) based on the carbocation precursor described in the terpene synthase reaction mechanisms proposed by Keszei et al. (2008). The chemical profile of the leaves of the R ecotype contains a high proportion of group C monoterpenes, a high concentration of FPCs, and a low concentration of sesquiterpenes, while that of the S ecotype, contains mainly group A compounds, a high concentration of sesquiterpenes and a low concentration of FPCs (Fig. 2).
A graphical representation of the chemical variation in monoterpenes, sesquiterpenes and formulated phloroglucinol compounds (FPCs) in the two mosaic trees (Eucalyptus melliodora on the left and E. sideroxylon on the right). The pie charts represent the relative proportion of specific groups of compounds and the size of the pie chart indicates total concentration in that sample. The pie charts for E. melliodora correspond to the larger scale on the left, whereas those for E. sideroxylon use the smaller scale on the right to clarify the pattern. Arrows indicate very low concentrations
Principal component analysis, using proportional data, indicated that monoterpenes were the best predictor of ecotype because, for both E. melliodora and E. sideroxylon, there was a clear distinction between the R and S ecotypes on the x-axis (Figs. 3a, b). This axis explained 94 and 80 % of the variation for E. melliodora and E. sideroxylon, respectively. It depended on 1,8-cineole in the negative direction and both β-phellandrene and p-cymene in the positive direction. The species differed a little on the y-axis, both by the amount of variation explained (3 % for E. melliodora and 18 % for E. sideroxylon) and by the compounds explaining the variation. For E. melliodora, β-pinene in the negative direction and α-pinene in the positive direction explained the variation. In contrast, α-pinene in the negative direction and 1,8-cineole and β-phellandrene in the positive direction explained the variation in E. sideroxylon.
Graphical representation of the principal component analysis of foliar terpenes. a Eucalyptus melliodora monoterpenes. b E. sideroxylon monoterpenes. Filled symbols represent data obtained from the 2009 collection (labelled -09), and open symbols represent data obtained from the 2005 collection (labelled -05). The resistant branches of the mosaic trees are labelled M1R and S1R (triangle) and the susceptible branches are labelled M1S and S1S (diamond). There are two non-mosaics from each species that we collected from in both years – the resistant M12 and S15 (square) and the susceptible M11 and S14 (circle). The trees of unknown chemotype from the larger 2009 collection are labelled EUmel09 and EUsid09 (cross)
The PCA using sesquiterpenes did not separate the ecotypes even though the first principal component in E. sideroxylon explained 50 % of the variation. Instead, despite the same compounds influencing the variation in both species, these compounds (bicyclogermacrene, aromadendrene, spathulenol, and β-caryophyllene) tend to occur in the leaves of all trees and often are the most abundant sesquiterpenes. Thus, the resultant PCA (not shown) indicated a continuous rather than discrete distribution of sesquiterpenes.
The PCA identified the non-mosaic con-specifics growing near the mosaic trees as predominantly R-ecotypes. We identified no further S ecotypes among the eight unknown E. melliodora but two S ecotypes (S8 and S16) among the nine E. sideroxylon. Therefore, in the following comparisons of R and S ecotypes we had a mosaic tree from each species (M1 and S1) – that is, with both R and S ecotypes, 9 resistant and 1 susceptible E. melliodora and 8 resistant and 3 susceptible E. sideroxylon. This gave 12 samples for E. melliodora and 13 for E. sideroxylon.
Quantitative Variation (Table 2)
Ecotype explained most of the variation in the concentrations of 1,8-cineole, sesquiterpenes and FPCs (all P < 0.001), monoterpenes (P = 0.003) and nitrogen (P = 0.009), while “tree within species” explained significant variation for monoterpenes (P = 0.042) and sesquiterpenes (P = 0.019). “Branch within tree” did not contribute significant variation.
Testing the Experimental Hypotheses
-
(i)
What similarities and differences are there in the chemical profiles of the two eucalypt species (Table 2, Fig. 3)? The chemical profiles of the leaves of E. melliodora and E. sideroxylon are similar. We separated the same terpenes − 29 monoterpenes and 39 sesquiterpenes, from the leaves of both species. A REML analysis indicated that the leaves of the two species had similar concentrations of total monoterpenes and of 1,8-cinole, the most abundant monoterpene in the resistant trees and of FPCs. The species did differ, however, in two ways both associated with susceptible trees. First, susceptible E. sideroxylon but not susceptible E. melliodora had very high concentrations of phellandrenes. Secondly, leaves of E. sideroxylon had higher concentrations of sesquiterpenes due to extremely high concentrations in the leaves of one tree – S8.
-
(ii)
Do the mosaic trees retain their chemical profiles over a period of several years? The PCA results shown in Fig. 3, indicate that the leaves collected in 2005 group with those collected in 2009 for the resistant and susceptible branches of both E. melliodora and E. sideroxylon, suggesting that the mosaic ecotypes are stable over time. Similarly, leaves from the non-mosaic trees collected in both years (E. melliodora M11 and M12; E. sideroxylon S14 and S15) also group together indicating little change over time.
-
(iii)
Do the chemical profiles of the leaves of R and S trees differ in all three groups of metabolites (Table 2)? Trees identified as resistant or susceptible differed in all three chemical profiles. Leaves of the resistant trees have higher concentrations of monoterpenes (P = 0.003) and FPCs (P < 0.001) but lower concentrations of sesquiterpenes (P < 0.001). Also, the leaves of susceptible trees contained more nitrogen, but this was caused by a species*ecotype interaction. There were higher concentrations of nitrogen in susceptible E. melliodora compared to resistant trees (S = 17.1 mg·g−1 DM; R = 14.2 mg·g−1 DM) but little difference in E. sideroxylon (S = 14.3 mg·g−1 DM; R = 14.0 mg·g−1 DM).
-
(iv)
Do the chemical profiles of leaves from non-mosaic individuals mirror the profiles of the leaves from the R and S branches of the mosaic trees (Table 2, Fig. 3)? We examined this in two ways – a REML analysis and visually, using a PCA with proportional monoterpene data. Because there was no difference between species for monoterpenes and FPCs, we removed the species identifier to give two mosaic trees in the analysis. We then compared the resistant and susceptible branches of the mosaic trees with the corresponding ecotypes of adjacent trees. The monoterpene (P = 0.23), sesquiterpene (P = 0.49), nitrogen (P = 0.85) and FPC (P = 0.08) concentrations of the leaves from resistant branches of the mosaic trees were similar to the concentrations in leaves from neighboring resistant trees. This is clear in the PCA (Fig. 3) that shows the species separately. The REML analysis also indicated that leaves from the susceptible branches on the mosaic trees and from neighboring susceptible trees contain similar concentrations of monoterpenes (P = 0.45), sesquiterpenes (P = 0.45), nitrogen (P = 0.93) and FPCs (P = 0.34), but the PCA indicates far more variability among susceptible ecotypes. For E. sideroxylon, the PCA of monoterpenes indicate that the susceptible leaves from the mosaic tree group with the leaves from two other susceptible trees (S14 and S16). Lying well away and in a tight group are the leaves sampled from the five branches of another susceptible tree (S8). It is this tree, rather than the mosaic tree, that appears chemically different. Compared to other susceptible E. sideroxylon, the leaves of this tree contain low concentrations of monoterpenes, with no detectable phellandrenes, extremely high concentrations of sesquiterpenes, and the highest nitrogen concentration. In E. melliodora, the PCA indicates that the monoterpene profile of the mosaic’s susceptible leaves group with those of the other susceptible E. melliodora (M11). If we superimpose Fig. 3a and b, the resistant ecotypes from the two species form a single group but the susceptible ecotypes do not. Instead, the data from the susceptible ecotype of the mosaic E. melliodora and from M11 form a separate group that lies between most of the susceptible E. sideroxylon and S8, the E. sideroxylon outlier mentioned previously.
Discussion
The key finding of this work is the identification of both qualitative and quantitative differences in the foliar metabolites of the resistant and susceptible branches of mosaic Eucalyptus trees that go far beyond the original findings of Edwards et al. (1990, 1993). Their thesis, for both E. melliodora and for E. sideroxylon, was that monoterpenes, particularly 1,8-cineole, explained the ecological difference they observed: leaves from the resistant ecotype had much higher concentrations of 1,8-cineole compared to the leaves of the susceptible individuals. In addition to this finding, we also show that there are other qualitative and quantitative differences in monoterpenes and sesquiterpenes and higher concentrations of FPCs. In other words, what appeared as a relatively simple chemical difference between ecotypes to Edwards et al. (1990, 1993) is in fact a consistent but much more complex difference. It answers our third hypothesis by showing that leaves from resistant and susceptible branches differ substantially in the three groups of chemicals we studied.
Perhaps most important, however, was demonstrating that the chemotypes of the leaves of the resistant and susceptible branches of the mosaic trees do not differ chemically from the corresponding chemotypes of adjacent non-mosaic con-specifics. This answered our fourth hypothesis. Therefore, there is nothing novel about the chemical profiles of the resistant and susceptible parts of mosaic trees – they both occur in the surrounding population. If there is an outstanding tree, chemically, then it is not one of the mosaics but instead one of the non-mosaic susceptible E. sideroxylon we identified during this study. The leaves of tree S8 differed chemically from all other susceptible foliage, particularly in its extremely high concentrations of sesquiterpenes and very low concentration of phellandrenes. This suggests that while there appears just one way to be resistant – high concentrations of monoterpenes and FPCs and low concentrations of sesquiterpenes, there is more than one way to be susceptible. Because monoterpenes, sesquiterpenes, and FPCs come from different but related metabolic pathways (Külheim et al., 2011), we argue that the most likely cause of the resistant branches is a mutation at a higher point – one that influences several pathways. If so, these mosaic trees provide a unique opportunity to investigate the genetic basis of chemical variation in eucalypts.
Our first hypothesis considered the chemical similarities of E. melliodora and E. sideroxylon, which although closely related, show substantial morphological differences. For example, E. melliodora lacks the heavy kino-impregnated bark of E. sideroxylon, has smaller flowers, and tends to flower in summer rather than winter. In contrast, the chemical profiles of the leaves of the two species were mostly similar and were indicative of the ecotype regardless of whether they came from a resistant or susceptible tree or a specific branch on a mosaic tree. This suggests that common factors select for chemical traits, and that Australia’s unpredictable environment with sporadic outbreaks of herbivores, such as Christmas beetles, enable susceptible trees to persist. We cannot explain the main difference we observed between the species – a high concentration of phellandrenes in susceptible E. sideroxylon (except S8), but not in susceptible E. melliodora.
Our remaining hypothesis questioned whether the mosaic trees retain their chemical profiles over periods of years. This is important because much of the discussion about chemical changes in plants concerns induced defenses, whereby plants elicit defenses in response to attack by herbivores or pathogens (Degenhardt and Gershenzon, 2000; Arimura et al., 2004). Furthermore, there is plentiful information about chemical differences within plants due to ontogenetic effects, such as the common sight of juvenile and adult leaves of differing chemical composition on a single eucalypt (Moore et al., 2004). Our finding that we could not discriminate between the chemical profiles of leaves collected from the resistant and susceptible branches in 2005 and those collected from the same branches in 2009 indicates that the chemical profiles of these trees are constitutive. Likewise, we could not detect changes in the chemical profiles of leaves from neighboring trees collected at these times thus providing further evidence for trees having chemical signatures. This conservation combined with the significant variation in leaf chemistry due to “ecotype” and to “tree within species” suggests the influence of genetic rather than environmental factors. Both environmental and genetic factors contribute to variation in defensive chemicals, but most research has considered variation in resource availability most important (Bryant et al., 1983). This may be true over larger areas, such as landscapes, but all branches of a mosaic tree presumably experience a similar environment and have access to the same resources. Furthermore, the similarity of the leaf chemistry in nearby trees provides convincing evidence that environmental factors have only a small influence, and that genetic factors likely determine the patterns we describe for terpenes and FPCs in mosaic trees. The narrow-sense heritability of foliar terpenes and FPCs in a closely related species, E. tricarpa, is about 0.6, indicating a strong genetic component to this trait (Andrew et al., 2007). Furthermore, Andrew et al. (2010) showed that there was only a small genotype by environment effect influencing the concentration of FPCs in the leaves of E. tricarpa.
The fact that herbivores rarely attack the resistant branches of the mosaic trees and that the resistant trees themselves suffer little herbivory could be due to several factors. For instance, there is substantial evidence that nitrogen is a key determinant of insect herbivory in eucalypt forests (Morrow and Fox, 1980). Even the present study suggested nitrogen playing a role. While the total nitrogen concentrations did not differ between the leaves of the E. sideroxylon ecotypes (R = 14.0 mg·g−1 DM; S = 14.3 mg·g−1 DM), the leaves of the susceptible E. melliodora contained more nitrogen (17.0 mg·g−1 DM) than did the leaves of the resistant ecotype (14.2 mg·g−1 DM) (Table 2). Although deserving of further study, especially identifying more susceptible trees, we believe this difference in nitrogen is relatively unimportant compared to the changes in terpenes and FPCs. Further evidence comes from Matsuki et al. (2011), who showed that Christmas beetles, the organisms responsible for the defoliation of these susceptible eucalypts, ate less with increasing concentrations of sideroxylonals and 1,8-cineole. Although nutritional quality is almost certainly important, insects appear to make their initial selection of which leaves to eat based on variation in specialized metabolites. A similar conclusion comes from Gypsy moths, whose feeding and development depended more on the concentration of terpenes and phenols than it did on the nitrogen concentrations of Douglas Fir leaves (Joseph et al., 1993).
Our understanding of the chemical differences of resistant and susceptible leaves on mosaic and non-mosaic trees along with substantial knowledge of the terpene biosynthetic pathway enables us to propose hypotheses to explain the origins of the resistant branches on the mosaic trees. In lieu of RNA sequencing experiments and measurements of enzyme concentrations, we propose that the significant qualitative variation in the profile of foliar monoterpenes between the branches of the mosaic trees points to a factor regulating the terpene synthases. However, our results show that resistance or susceptibility depends on FPCs as well as terpenes. From what we know of terpene and FPC biosynthesis (Külheim et al., 2011), we propose that a simple somatic mutation invoked chemical changes involving several biosynthetic pathways. Support for this thesis comes from Henery et al. (2007), who showed that quantitative trait loci (QTLs) associated with monoterpenes, sesquiterpenes, and FPCs co-locate to the same location on the linkage groups. They claim that this could be a regulatory site governing the transcription of genes from both pathways. Thus, we expect a change in a single regulatory gene, most likely a transcriptional regulator that affects genes in several pathways possibly through this ‘regulatory region’.
Can the Genetic Mosaic Hypothesis (GMH) explain these results? The GMH was proposed to explain intra-individual variation in susceptibility of clonal plants to pests (Whitham and Slobodchikoff, 1981) and extended to explain how long-lived host plants co-evolve with short-lived pests (Gill, 1986). Mosaic Eucalyptus support these ideas and provide an excellent model to study them partly because the biosynthesis of the compounds responsible for the two ecotypes is well-understood and the products have high heritability (Pichersky et al., 2006; Andrew et al., 2007). Eucalypts must defend themselves against various mammalian and insect herbivores, as well as fungi, bacteria and viruses (Edwards et al., 1993; Lawler et al., 1999; Keane et al., 2000; Moore et al., 2005; Crous et al., 2007; Matsuki et al., 2011). Compared to eucalypts, these are all relatively short-lived organisms yet there appears to be a co-evolutionary arms race between them. The mosaic eucalypts explain why this is possible. If a mutation occurs that allows part of the plant to escape predation pressures, then that part gains a selective advantage.
References
Andrew, R. L., Wallis, I. R., Harwood, C. E., Henson, M., and Foley, W. J. 2007. Heritable variation in the foliar secondary metabolite sideroxylonal in Eucalyptus confers cross-resistance to herbivores. Oecologia 153:891–901.
Andrew, R. L., Wallis, I. R., Harwood, C. E., and Foley, W. J. 2010. Genetic and environmental contributions to variation and population divergence in a broad-spectrum foliar defence of Eucalyptus tricarpa. Ann. Bot. 105:707–717.
Arimura, G., Ozawa, R., Kugimiya, S., Takabayashi, J., and Bohlmann, J. 2004. Herbivore-induced defense response in a model legume. Two-spotted spider mites induce emission of (E)-beta-ocimene and transcript accumulation of (E)-beta-ocimene synthase in Lotus japonicus. Plant Physiol. 135:1976–1983.
Brooker, M. I. H. 2000. A new classification of the genus Eucalyptus L'Her. (Myrtaceae). Austral. System. Bot. 13:79–148.
Bryant, J., Chapin III, F., and Klein, D. 1983. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 40:357–368.
Butcher, P. A., Doran, J. C., and Slee, M. U. 1994. Intraspecific variation in the leaf oils of Melaleuca alternifolia (Myrtaceae). Biochem. System. Ecol. 22:419–430.
Crous, P. W., Summerell, B. A., Carnegie, A., Mohammed, C., Himaman, W., and Groenewald, J. Z. 2007. Foliicolous Mycosphaerella spp. and their anamorphs on Corymbia and Eucalyptus. Fungal Diversity 26:143–185.
Degenhardt, J. and Gershenzon, J. 2000. Demonstration and characterization of (E)-nerolidol synthase from maize: a herbivore-inducible terpene synthase participating in (3E)-4,8-dimethyl-1,3,7-nonatriene biosynthesis. Planta 210:815–822.
Edwards, P. B., Wanjura, W. J., and Brown, W. V. 1993. Selective herbivory by Christmas beetles in response to intraspecific variation in Eucalyptus terpenoids. Oecologia 95:551–557.
Edwards, P. B., Wanjura, W. J., Brown, W. V., and Dearn, J. M. 1990. Mosaic resistance in plants. Nature 347:434.
Eschler, B. M. and Foley, W. J. 1999. A new sideroxylonal from Eucalyptus melliodora. Austral. J. Chem. 52:157–158.
Gill, D. E. 1986. Individual plants as genetic mosaics: ecological organisms versus evolutionary individuals, pp. 321–343, in M. J. Crawley (ed.), Plant Ecology. Blackwell Scientific Publications, Carlton City.
Halliwell, B. 1999. Oxygen and nitrogen are pro-carcinogens. Damage to DNA by reactive oxygen, chlorine and nitrogen species: measurement, mechanism and the effects of nutrition. Mut. Res. Genet. Toxicol. Environ. Mutagen. 443:37–52.
Henery, M. L., Moran, G. F., Wallis, I. R., and Foley, W. J. 2007. Identification of quantitative trait loci influencing foliar concentrations of terpenes and formylated phloroglucinol compounds in Eucalyptus nitens. New Phytol. 176:82–95.
Herrera, C. M. 2009. Multiplicity in unity: plant subindividual variation and interactions with animals, in J. N. Thompson (ed.), The University of Chicago Press, Chicago.
Joseph, G., Kelsey, R. G., Moldenke, A. F., Miller, J. C., Berry, R. E., and Wernz, J. G. 1993. Effects of nitrogen and Douglas-fir allelochemicals on development of the gypsy moth, Lymantria dispar. J. Chem. Ecol. 19:1245–1263.
Keane, P. J., Kile, G. A., and Podger, F. D. 2000. Diseases and Pathogens of Eucalypts. CSIRO Publishing, Collingwood.
Keszei, A., Brubaker, C. L., and Foley, W. J. 2008. A molecular perspective on terpene variation in Australian Myrtaceae. Austral. J. Bot. 56:197–213.
Kleine, S. and Müller, C. 2011. Intraspecific plant chemical diversity and its relation to herbivory. Oecologia 166:175–186.
Külheim, C., Webb, H., Yeoh, S. H., Wallis, I., Moran, G., and Foley, W. 2011. Using the Eucalyptus genome to understand the evolution of plant secondary metabolites in the Myrtaceae. BMC Proceedings 5(Suppl 7):O11.
Lawler, I. R., Stapley, J., Foley, W. J., and Eschler, B. M. 1999. Ecological example of conditioned flavor aversion in plant–herbivore interactions: effect of terpenes of Eucalyptus leaves on feeding by common ringtail and brushtail possums. J. Chem. Ecol. 25:401–415.
Marcotrigiano, M. 2000. Herbivory could unlock mutations sequestered in stratified shoot apices of genetic mosaics. Am. J. Bot. 87:355–361.
Matsuki, M., Foley, W. J., and Floyd, R. B. 2011. Role of volatile and nonvolatile plant secondary metabolites in host tree selection by Christmas beetles. J. Chem. Ecol. 37:286–300.
Mcgregor, S. E. 1976. Insect Pollination of Cultivated Crop Plants: USDA Agriculture Handbook 496. Washington, D.C.
Moore, B. D., Foley, W. J., Wallis, I. R., Cowling, A., and Handasyde, K. A. 2005. Eucalyptus foliar chemistry explains selective feeding by koalas. Biol. Lett. 1:64–67.
Moore, B. D., Wallis, I. R., Palá-Paúl, J., Brophy, J. J., Willis, R. H., and Foley, W. J. 2004. Antiherbivore chemistry of Eucalyptus - cues and deterrents for marsupial folivores. J. Chem. Ecol. 30:1743–1769.
Morrow, P. A. and Fox, L. R. 1980. Effects of variation in Eucalyptus essential oil yield on insect growth and grazing damage. Oecologia 45:209–219.
Padovan, A., Keszei, A., Koellner, T. G., Degenhardt, J., and Foley, W. J. 2010. The molecular basis of host plant selection in Melaleuca quinquenervia by a successful biological control agent. Phytochemistry 71:1237–1244.
Penfold, A. R. and Morrison, F. R. 1937. The occurrence of a number of varieties of Euclayptus radiata (E. numerosa) as determined by chemical analyses of the essential oils. Part II. J. Roy. Soc. New South Wales 20:375–377.
Pichersky, E., Noel, J. P., and Dudareva, N. 2006. Biosynthesis of plant volatiles: nature’s diversity and ingenuity. Science 311:808–811.
Ramanoelina, P. A. R., Viano, J., Bianchini, J. P., and Gaydou, E. M. 1994. Occurence of various chemotypes in Niaouli (Melalecua quinquenervia) essential oils from Madagascar using multivariate statistical analysis. J. Agr. Food Chem. 42:1177–1182.
Slee, A. V., Brooker, M. I. H., Duffy, S. M., and West, J. G. 2006. EUCLID Eucalypts of Australia, 3rd ed. CSIRO, Canberra.
Southwell, I. A. and Russell, M. F. 2002. Volatile oil comparison of cotyledon leaves of chemotypes of Melaleuca alternifolia. Phytochemistry 59:391–393.
Trilles, B. L., Bombarda, I., Bouraïma-Madjebi, S., Raharivelomanana, P., Bianchini, J.-P., and Gaydou, E. M. 2006. Occurrence of various chemotypes in niaouli [Melaleuca quinquenervia (Cav.) S. T. Blake] essential oil from New Caledonia. Flavour Frag J. 21:677–682.
Wallis, I. R. and Foley, W. J. 2005. The rapid determination of sideroxylonals in Eucalyptus foliage by extraction with sonication followed by HPLC. Phytochem. Anal. 16:49–54.
Wallis, I. R., Keszei, A., Henery, M. L., Moran, G. F., Forrester, R., Maintz, J., Marsh, K. J., Andrew, R. L., and Foley, W. J. 2011. A chemical perspective on the evolution of variation in Eucalyptus globulus. Perspectives in Plant Ecology. Evolut. Syst. 13:305–318.
Whitham, T. G. and Slobodchikoff, C. N. 1981. Evolution by individuals, plant-herbivore interactions, and mosaics of genetic variability: the adaptive significance of somatic mutations in plants. Oecologia 49:287–292.
Acknowledgements
Mosaic trees came to the attention of scientists through the keen observations of Mr Kevin Barker of Yeoval NSW. Dr Penny Edwards and Dr Wolf Wanjura of CSIRO kindly showed us the trees and facilitated our work in many ways. We thank the late Mr Herb Healey, the late Mr Kevin Barker, Mr Bruce Lees, and Mr Simon Dwyer for allowing us access to their properties. We also thank Ms Jessie Au and Ms Hannah Windley for their assistance with nitrogen analysis. This work was supported by a Discovery grant from the Australian Research Council to WJF (DP0877063) and an Australian Geographic Seed Grant to AP.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Padovan, A., Keszei, A., Wallis, I.R. et al. Mosaic Eucalypt Trees Suggest Genetic Control at a Point That Influences Several Metabolic Pathways. J Chem Ecol 38, 914–923 (2012). https://doi.org/10.1007/s10886-012-0149-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-012-0149-z