Abstract
Food type can affect all functional aspects of an insect’s life. We investigated the effects of different diet regimes on life history parameters of the ladybird beetle Harmonia axyridis. Furthermore, we tested the importance of elytral color, sex, and diet on chemical and immune defense in this species. We also compared hemolymph from cohorts of H. axyridis and Coccinella septempunctata (Coleoptera: Coccinellidae) fed different diets to examine effects on the 2-isopropyl-3-methoxypyrazine (IPMP) content in these beetles. No effects of diet on the duration of larval development and on adult weight were found. We detected, however, significantly higher fecundity and oviposition rates when female H. axyridis were reared on pea aphids than when reared on eggs of Ephestia kuehniella. Males and females did not differ in their immune response. Elytral color affected both immune defense and chemical defense. The antimicrobial activity of the hemolymph differed only when morphotypes were tested against E. coli. Moreover, we observed an effect of elytral pigmentation on IPMP content. The succinea 2 type (orange without dots) had the lowest IPMP content in two out of three feeding regimes compared to the succinea 1 (orange with dots) type. Depending on diet, IPMP contents differed in both species leading to higher contents either in H. axyridis or C. septempunctata. Furthermore, aphid species ingested during larval development significantly affected IPMP content in adult beetles. These results implicate new aspects for risk assessment of H. axyridis in viticulture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ladybird beetles (Coleoptera: Coccinellidae) have a broad prey range that includes aphids, psyllids, scale insects, lepidopteran eggs, and chrysomelids (Hodek and Honek, 1996). At high densities, when prey is scarce or of poor quality, cannibalism is common for some species (Osawa, 1993; Snyder et al., 2000). Diet has, nevertheless, a significant impact on the performance of coccinellids (Specty et al., 2003; Lanzoni et al., 2004, Soares et al., 2005; Berkvens et al., 2008; Jalali et al., 2009). Despite their polyphagy, coccinellids often are specific with regard to their essential food (Hodek, 1993). The aphid species preyed upon and the plant species on which these prey aphids feed can affect larval developmental time, adult longevity, body weight, and fecundity (Hukusima and Kamei, 1970; Fukunaga and Akimoto, 2007).
The Multicolored Asian ladybird beetle, Harmonia axyridis, is native to northeast Asia. It has been introduced to different regions of the world (i.e., France or USA) as a biocontrol agent (Tedders and Schaefer, 1994; Ferran et al., 1996) and became widely established in Europe and America (Koch, 2003). In the fall and prior to hibernation, it feeds on sugar-containing fruits, especially grapes (Galvan et al., 2008). In contrast, the seven-spot ladybird beetle Coccinella septempunctata, a coccinellid beetle native to Europe and invasive to the USA, is a more aphid-specific predator (Hodek and Honek, 1996).
Harmonia axyridis is a polymorphic species, and some phenotypes differ in their fitness. The variable pigmentation of the elytra in H. axyridis results in more than 100 recognizable color morphs (Soares et al., 2003). Phenotypes can be divided into five groups: non-melanic succinea (red with 0–19 black dots), melanic conspicua (black with two red dots), spectabilis (black with four red dots), axyridis (more than four red dots), and entirely black nigra morphs (Soares et al., 2001). This striking feature has led to a number of studies that have examined differences in mating and predatory behavior (Osawa and Nishida, 1992; Seo et al., 2008; Su et al., 2009; Sloggett, 2010) and physiology (Soares et al., 2001, 2005; Bezzerides et al., 2007; Berkvens et al., 2008) for the different phenotypes of this coccinellid species. Moreover, a positive relationship of elytral redness with the content of an alkaloid defense compound (harmonine) in the beetles was reported by Bezzerides et al. (2007). Contrary to chemical defense against predators and parasitoids, immune defense in Coleoptera has received less attention (Bulet et al., 1991; Gross et al., 2008; Haine et al., 2008). Armitage et al. (2003) found no evidence for cuticular melanin content and costs of immune defense in meal worm beetles (Tenebrionidae). For other insects, namely Lepidoptera, some studies have shown a correlation between the amount of cuticle melanization and immune function, while others have reported a lack of such correlation (Wilson et al., 2001; Lee et al., 2008; Karl et al., 2010). To date, no study has related elytral color to immune defense in H. axyridis.
The hemolymph of coccinellid beetles contains defensive substances against natural enemies (Klausnitzer and Klausnitzer, 1997). Additionally, some of these substances may act as aggregation pheromones (Al Abassi et al., 1998) or belong to the beetle’s immune system. For H. axyridis, Gross et al. (2010) observed stronger antimicrobial activity in their hemolymph than in C. septempunctata. The main defensive chemicals identified in the hemolymph of H. axyridis and C. septempunctata are methoxypyrazines (and therein mainly 2-isopropyl-3-methoxypyrazine (IPMP)) (Al Abassi et al., 1998; Pickering et al., 2004; Cai et al., 2007; Kögel et al., 2012b). During grape harvesting and processing, beetles often get crushed or reflex bleed. This causes a specific off-flavor in wine called “ladybird taint” (Pickering et al., 2004, 2005; Galvan et al., 2007; Ross et al., 2010; Kögel et al., 2012a). The influence of food on chemical defense compounds in coccinellid beetles’ hemolymph is currently unknown. Thus, this study examined the effect of diet on the content of 2-isopropyl-3-methoxypyrazine and antimicrobial compounds in the hemolymph of H. axyridis and C. septempunctata.
The performance and IPMP content of H. axyridis and C. septempunctata fed on eggs of Ephestia kuehniella were compared to beetles reared on pea aphids (Acyrthosiphon pisum). For this purpose, we evaluated different fitness parameters between the beetles fed on either of the two diets in the laboratory according to Gross et al. (2004a). Additionally, we investigated the influence of several other aphid species for H. axyridis on IPMP content in the wild. Finally, we controlled for correlation of elytral pigmentation and antimicrobial activity in the hemolymph of H. axyridis by comparing the phenotypes prevailing in our laboratory colony against three different model microorganisms and an insect pathogen.
Methods and Materials
Insects
Permanent laboratory colonies of H. axyridis and C. septempunctata were maintained under controlled conditions (alternating temperature regime during one week (168 h): 100 h at 25°C, 68 h at 20°C ; 16:8 h L/D, 60 % rh) in a climate chamber at the Julius Kühn-Institute (JKI), Dossenheim, Germany. Groups of about 100 larvae were kept either (1) on a diet of pea aphids (Acyrthosiphon pisum) that were reared on beans (Vicia faba, tannin reduced cultivar “Tattoo”), (2) on A. pisum and grape juice, or (3) on frozen eggs of meal moths (Ephestia kuehniella, provided by Koppert Biological Systems, The Netherlands) until pupation. All cohorts received water by soaked cotton pads ad libitum. In rearing dishes (50 × 40 × 12 cm), the larvae were kept from egg hatching until pupation. Mortality, developmental time, and numbers of pupae were recorded daily. After emergence, the newly hatched adults were sexed and weighed on a balance (MC 210S, Sartorius, Germany). The beetles were kept in rearing cages (40 × 30 × 30 cm) and fed the same diet as during the larval stage until they were used for the analysis of their hemolymph (antimicrobial activity, IPMP content). Preimaginal survival was monitored as the number of adults obtained from the pupae of each cohort. Fecundity and other oviposition parameters were monitored for a total period of 49 d. Fifteen pairs of beetles for each food treatment (aphids or E. kuehniella eggs) were kept individually in Petri dishes (9 cm diam). The number of eggs and egg clutches were recorded daily. Males were replaced when they had died.
For some experiments, adult beetles were divided into three different morphological groups based on elytral coloration: 1) melanic spectabilis morphs: black elytra with two red dots on either elytron, 2) succinea 1 morph with many intensively black dots on orange colored elytra, and 3) succinea 2 morphs with none or very few, faint black markings on orange elytra. These three morphotypes occurred in our laboratory colony and in the field populations found in the vegetation surrounding the facilities of the Julius Kühn-Institut, Dossenheim.
Field-collected Beetles
For IPMP analysis, pupae of H. axyridis were collected in April 2011 in South Germany (Dossenheim, Siebeldingen and surroundings) from leaves of Prunus persica, P. cerasus, P. spinosa, P. domestica, P. mahaleb, Malus domestica, Acer platanoides, Hedera helix, Sambucus nigra, and Corylus avellana. After eclosion, adults were kept without food (experiment 1) or fed the particular aphid species found on this specific host plant that had also served as prey during larval development (experiment 2). Aphids were identified as Myzus varians on P. persica, Brachycaudus helichrysi on P. domestica, Dysaphis plantaginea on M. domestica, Drepanosiphon platanoides on A. platanoides, Aphis hederae on H. helix, and A. sambuci on S. nigra. The aphid species on the remaining plants could not be identified.
In experiment 1, the IPMP contents of the hemolymph of the adult ladybird beetles were analyzed 3 d after emerging without providing any food for the hatched adults. In experiment 2, newly hatched adults were fed for 5 d with aphids obtained from the respective plants where pupae were collected. After this feeding period, the IPMP content of these beetles was measured by GC-MS analyses. Additionally, 30 adults each of H. axyridis and C. septempunctata were collected on grape berries of several Vitis hybrids from a vineyard around Geilweilerhof, Siebeldingen (September 2011) and their IPMP contents were also analyzed.
Microorganisms
Gram positive Bacillus subtilis and B. thuringiensis ssp. tenebrionis (strain 10 BI 256–82), gram negative Escherichia coli (K12/D31), and the yeast Saccharomyces cerevisiae (DSM 70499) were used as model test organisms. All strains were obtained from the collection at the Julius Kühn-Institut, Dossenheim. Prior to the assays, liquid Mueller Hinton Broth was inoculated with test bacteria; yeast was cultivated in Sabouraud Dextrose Broth. Population growth of the overnight cultures (28°C) was monitored through its optical density as absorbance at 600 nm. Prior to the assays, each culture was diluted to a final concentration of 106 CFU.
Determination of Antimicrobial Activity in the Hemolymph of Harmonia axyridis
The antimicrobial activity in the hemolymph of adult H. axyridis was evaluated with broth dilution assays in 96-well microtiter plates (Sarstedt). The assays to determine the minimal inhibitory concentration (MIC) of each hemolymph pool were conducted as described in Gross et al. (2010). Hemolymph was collected from live, unchallenged adults. Insects were used for the assays after they had reached sexual maturity, i.e., after the onset of oviposition in each cohort. Beetles were separated by sex, elytral color, and diet. We prepared 40 μl hemolymph solutions at a concentration of 5 μl hemolymph /ml sterile water. This stock solution was applied to the first well of a serial dilution plate, and then diluted 1:2 with each of the following pipetting steps until the 12th well in each row of the plate. The amount removed from the last well was discarded. Aliquots of 100 μl of bacterial or fungal cultures were added to each of the 96 wells. Depending on the amount of hemolymph obtained from the beetles for each hemolymph pool, two or three rows were filled per test factor and microorganism. All tests were done in four independent replications. In each assay, the growth inhibition of all test organisms was compared to an antibiotic as a positive reference. Starting concentrations of 50 μg gentamycin/ml sterile water and of 50 μg nystatin/ml dimethylsulfoxid were used, respectively, as controls for bacterial and fungal growth inhibition. Test plates were incubated in a shaker (160 rpm) at 28°C. After 20 h, growth inhibition was evaluated visually as clear wells. This visual control was confirmed with a spectrophotometer through changes in absorption at 600 nm (Microplate Reader Fluostar Omega, BMG Labtech, USA). The minimal inhibitory concentration (MIC) is defined as the minimal concentration of hemolymph or antibiotic that causes complete growth inhibition of the microorganism tested (DIN norm 58940). For each sample, the most abundant minimal inhibitory concentration for the rows from each hemolymph pool and the four replications was taken as MIC for the tested factor and microorganism.
Test of Antimicrobial Properties of Methoxypyrazine Compounds
In order to test for potential antimicrobial properties of methoxypyrazine compounds present in the hemolymph of H. axyridis, we used commercially available 2-isobuthyl-3- methoxypyrazine (IBMP), IPMP, and 2-secbuthyl-3-methoxypyrazine (SBMP) (SIGMA Aldrich Nr. 297666, Nr. 243132 and Nr. 243116) at a concentration of 1 μg/ml, as these compounds were previously identified in the hemolymph of H. axyridis (Cai et al., 2007; Cudjoe et al., 2005; Kögel et al., 2012b). The solutions were used in MIC assays in concentrations of 1 μg/ml and 0.01 μg/ml (diluted 1:100 in a water/ethanol (1:1) mixture) against the four microorganisms mentioned above. Four replications were carried out for each substance and concentration.
Comparison of Diet Effects on the Chemical Defense (IPMP content) of H. axyridis and C. septempunctata
Beetles were weighed and sexed prior to the analyses. A single beetle was subsequently crushed in 10 ml of Milli-Q-water (Millipore Corporation, USA) in a mortar. The sample then was transferred to a 20 ml round bottom glass headspace vial. Three g of sodium chloride and 10 ng of an internal standard (2-isopropyl-3-ethoxypyrazine (IPEP)) were added. For each feeding group and beetle species or morphotype, 10 replications were done.
Headspace-sampling and Chemical Analysis
Volatile compounds present in the hemolymph of both ladybird species were extracted using headspace-solid phase microextraction (HS-SPME). Beetle headspace was sampled using a divinylbenzene/carboxen/polydimethylsiloxane fiber (Sigma Aldrich Nr. 57328-U DVB/Carboxen/PDMS) and a manual fiber assembly. The sample vial was heated to 40°C for 20 min in a water bath before fiber exposure to request a subsequent headspace equilibration. After exposure in the headspace for 30 min at a constant temperature of 40°C under continuous stirring, the fiber was thermally desorbed in a GC injection port in splitless mode (Agilent 6890) at 250°C for 2 min and further thermally cleaned for 3 min with 10 ml/min split flow. Coupled gas chromatography–mass spectrometry (GC-MS) was used for analyses. Pyrazines were separated by the following procedure: the GC program was started at 40°C and held for 6 min; then temperature was raised to 100°C at 15°C/min; at 3°C/min to 160°C; at 25°C/min to 200°C; and finally temperature was held at 200°C for 5 min. The column was a DB-Wax (30 m length, 0,250 mm I.D., 0.50 μm film thickness; J&W Scientific). Helium was used as carrier gas. For the first 12 min, an FID was used and afterwards the system was switched to a MS (Agilent 5975B) in SIM Mode. The selected mass channels were m/z 137 and 152 for IPMP and 137 and 151 for IS.
GC-Data Analysis
Data peaks were analyzed using the ChemStation D.02.00.611 software (Agilent Technologies). Identification of IPMP in beetles’ hemolymph was done by comparing mass spectra and retention time with synthetic IPMP as reference substance. Concentrations of the reference samples were similar to those found in target samples. Calibration curves were prepared for five concentrations ranging from 0.5, 1.0, 1.5, 2.0 and 2.5 ng of IPMP and IPEP as internal standard per liter in water solution. Quantification was done by comparing the peak areas of IPMP and internal standard in counts. The standard addition method was used to identify possible matrices effects. Due to equal extraction methods, absolute and relative quantities of IPMP could be calculated. The contents are represented as ng IPMP/g fresh weight of beetles.
Statistical Analysis
The numbers of eggs and egg clutches, adult body weight, and larval development times were statistically compared between different treatments (food) with Mann–Whitney U-tests (Sachs, 1992). The sex ratio and percentage preimaginal survival of beetles reared on different diets were compared by chi 2-tests. Due to normality and homogeneity of data, the comparisons of IPMP contents between different treatments were analyzed by ANOVA followed by post-hoc-tests of Least Significant Difference between means (LSD). Statistical analyses were done with SPSS statistics software 19 (IBM 2011).
Results
Diet Effects on Life History Parameters and Fecundity of Harmonia axyridis
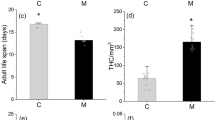
The total duration of the larval developmental time (i.e. four larval stages) was not different for the two diet regimes (Table 1, Mann–Whitney U test, diet 1 N = 104 pupae obtained, diet 2 N = 166 pupae obtained, P > 0.05). Preimaginal survival (χ 2 = 0.09, df = 1, P > 0.05), adult weight (Mann–Whitney U test, N > 30 beetles per sex and diet, P > 0.05), and sex ratio (χ 2 = 0.42, df = 1, P > 0.05) were also similar (Table 1). By contrast, all fecundity parameters differed significantly between aphid and E. kuehniella eggs diets (Table 1). Aphid fed female H. axyridis laid on average more than twice the number of egg clutches (Mann–Whitney U test, N = 15, P < 0.001), and the total number of eggs doubled the eggs laid by E. kuehniella egg fed females (Mann–Whitney U test, N = 15, P < 0.001). Thus, fecundity (eggs/female) and oviposition rate (eggs/female/day) was more than twice as high for aphid fed beetles than for egg fed beetles (Table 1 and Fig. 1, Mann–Whitney U test, P < 0.001). Survival of female beetles in the oviposition trials did not differ between diets.
Oviposition rate depicted as eggs/female/d laid under two different diet regimes, either live Acyrthosiphon pisum aphids or frozen eggs of Ephestia kuehniella, for 15 females of Harmonia axyridis. Eggs were counted for a total period of 49 days. Mann–Whitney U test, P < 0.001. A linear trend line is given
Determination of the Antimicrobial Activity in the Hemolymph
No difference in the antimicrobial activity of the hemolymph between female and male beetles was observed. Thus, data were combined for both sexes in Fig. 2. Independent from the diet ingested, antimicrobial activity in the hemolymph of H. axyridis was most effective against S. cerevisiae, followed by gram negative E. coli, intermediate against gram positive B. subtilis, and least effective against B. t. thuringiensis (Fig. 2). Since differences in MIC values of one dilution step up or down lay within the normal variation of this serial dilution test, only differences in MIC values against the growth of E. coli, spanning various dilution steps, which were observed for succinea 2 morphs, can be regarded as a true effect of diet. No other differences in antimicrobial activity between the two diets or between color morphs were observed. Furthermore, no interactions of elytral color and feeding regime on antimicrobial activity in the hemolymph was found. According to DIN norm sheet 58940, a statistical analysis of MIC values is neither necessary nor possible (Deutsches Institut für Normung, 2010).
Minimal inhibitory concentrations (MIC) in μg/ml found in the hemolymph of live and unchallenged Harmonia axyridis adults. Antibacterial standard gentamycin had a MIC value of 0.195 μg/ml against Escherichia coli and Bacillus subtilis, and of 1.563 μg/ml against Bacillus t. thuringiensis. The antifungal standard nystatin had a MIC value of 0.781 μg/ml against Saccharomyces cerevisiae. MIC values of the standards tested did not differ between diet treatments. Beetles were reared on a diet of live Acyrthosiphon pisum or frozen eggs of Ephestia kuehniella. a) Comparison of the MIC values of three morphotypes (spectabilis, succinea 1 and 2) from each diet regime against three different model microorganisms. b) Comparison of the MIC values of three morphotypes (spectabilis, succinea 1 and 2) for each diet regime against entomopathogenic Bacillus thuringiensis tenebrionis
H. axyridis succinea 2 fed with aphids had lower MIC values against E. coli than succinea 1. In general, we observed more variability between morphs in the measurable growth inhibition for aphid reared beetles than for the beetles reared on E. kuehniella eggs (Fig. 2). Neither of the pyrazine solutions caused a detectable growth inhibition in any of the four microorganisms tested.
Differences in IPMP Contents between C. septempunctata and Morphotypes of H. axyridis within One Feeding Group
The contents of IPMP differed significantly between the different morphotypes of H. axyridis: H. axyridis succinea 2 and H. axyridis spectabilis contained less IPMP than succinea 1 when fed with A. pisum or on E. kuehniella eggs (LSD; P < 0.05; Fig. 3). C. septempunctata contained significantly less IPMP than H. axyridis when fed on A. pisum. When fed additionally on grape juice, the IPMP content of C. septempunctata increased significantly (Fig. 3), and was higher than in all morphotypes of H. axyridis. When fed on E. kuehniella, no differences could be observed between C. septempunctata and H. axyridis succinea 1 and H. axyridis spectabilis (Fig. 3). No differences in IPMP contents between males and females of the same feeding group and with the same elytral color were detected (LSD; P > 0.05). Likewise, no differences in IPMP contents could be measured between H. axyridis and C. septempunctata adults collected from grapes in September 2011 (Mann–Whitney-U test; P > 0.05).
Mean IPMP contents (ng/g fresh weight) +/− SE under different feeding conditions (Acyrthosiphon pisum, A. pisum+grape juice or Ephestia kuehniella eggs) of three morphotypes of Harmonia axyridis (H. axyridis succinea 1 (dark grey), succinea 2 (grey), and H. axyridis spectabilis (light grey) and Coccinella septempunctata (white). Small letters indicate significant differences between morphotypes of H. axyridis and C. septempunctata within one feeding group (P < 0.05). Capital letters indicate significant differences of the two species between the feeding groups (P < 0.05). 10 replications were done in every treatments
Differences in IPMP Contents of Morphotypes of H. axyridis and C. septempunctata between the Feeding Groups and on Different Host Plants
In beetles reared in the laboratory on A. pisum, A. pisum+grape juice and E. kuehniella eggs, significant differences in IPMP contents could be measured (Fig. 3). IPMP contents of all morphotypes of H. axyridis fed with E. kuehniella were higher than when fed on ‘A. pisum’ or ‘A. pisum+grape juice’ (LSD; P < 0.05; Fig. 3). A diet of A. pisum+grape juice increased the IPMP contents of C. septempunctata in comparison to other diet treatments (LSD; A. pisum: P = 0.03; E. kuehniella: P = 0.016).
Significant differences in IPMP contents could be detected in adults that emerged from pupae of H. axyridis previously collected in the field on host plants with specific aphid prey: Larvae fed with aphids collected on P. domestica (Brachicaudus helichrysi), P. mahaleb (unknown aphid species), C. avellana (unknown aphid species), and S. nigra (Aphis sambuci) contained significantly less IPMP than larvae fed with aphids from P. persica (Myzus varians) (LSD; P < 0.05; Fig. 4). In addition, larvae fed with A. sambuci (S. nigra) and aphids from C. avellana had lower IPMP contents than larvae fed on D. platanoides (A. platanoides) (LSD; P < 0.05; Fig. 4).
Mean IPMP contents (ng/g fresh weight) +/– SE of three morphotypes of Harmonia axyridis 3 d after eclosion from field collected pupae on the respective plant species infested by given aphid species (Myzus varians on Prunus persica (N = 5); Brachycaudus helichrysi on Prunus domestica (N = 7); Dysaphis plantaginea on Malus domestica (N = 9); Drepanosiphon platanoides on Acer platanoides (N = 5); Aphis hederae on Hedera helix (N = 5); Aphis sambuci on Sambucus nigra (N = 5); Unknown aphids on Prunus spinosa, Prunus mahaleb, Prunus cerasus and Corylus avellana (N = 5)
No differences were found in IPMP contents when adults were fed on the respective host aphids for another five days after eclosion (Mann–Whitney-U-test; P > 0.05).
Discussion
Under prevailing conditions in our climate chamber, we found a significant effect of diet on the fecundity of H. axyridis females. Fecundity of H. axyridis was higher under a diet of pea aphids compared to E. kuehniella eggs. Contrary to the results reported by Berkvens et al. (2008), we observed no differences in developmental parameters between the cohorts reared on either diet. Those authors concluded from their laboratory data that E. kuehniella eggs were a better food than live A. pisum aphids. Moreover, they related the observed differences to the distinct morphotypes. We did not compare morphotypes in this part of our study. While the preimaginal survival and fecundity was higher in our study than reported by Lanzoni et al. (2004), the oviposition rates were similar, and no differences in sex ratio were found in our experiments. A previous experiment by Specty et al. (2003) reported higher adult weight for female beetles after ingestion of E. kuehniella eggs compared to a diet of live A. pisum. The authors attributed their results to different amino acid and lipid contents in the food. Contrary to these authors, we observed no weight differences in the newly emerged adult beetles reared on either diet. Stathas et al. (2001) found similar fecundity and oviposition rates for Aphis fabae fed H. axyridis females as we did for E. kuehniella eggs fed beetles. However, the fecundity observed for female beetles fed on pea aphids was twice as high in our study. McClure (1987) obtained a similar number of eggs laid per female when the beetles were reared permanently at 27°C with a diet of A. pisum as we did by alternating rearing temperature between 20 and 25°C. Thus, our data confirm the observation previously reported by Stathas et al. (2001) that temperature seems to affect mainly the duration of the preovipositional period.
To date, we do not know what contributes to the observed significant differences in fecundity between the diet regimes we tested. Specty et al. (2003) detected higher amounts of lipids and amino acids in meal moth eggs than in aphids, but higher glycogen content in live aphids than in the eggs. The authors also found differences in mortality and weight based on diet, with E. kuehniella eggs being the better diet. Likewise, they also found no effect of diet on larval development time. Interestingly, like Berkvens et al. (2008), they observed significantly higher fecundity of the beetles reared with E. kuehniella eggs. Our results contradict both of those previous studies. In comparison to our experiment, however, the study by Specty et al. (2003) evaluated the fecundity of H. axyridis females only for a period of 10 days, whereas we followed the females for a total period of 49 days. For both treatments, egg laying activity showed strong daily variation (Fig. 1) until the end of the observational period. It is striking, that in H. axyridis fed a diet of A. pisum the average number of eggs laid per female and day was much higher in the beginning of the egg laying period compared to a diet of E. kuehniella, and was then followed by a gradual decrease of egg numbers laid per female and day until the end of the observational period. By contrast, under a diet of E. kuehniella, the number of eggs laid per female and day started out at a low level and was followed by a constant increase in egg numbers. Since we have no data on the hatching rate of these eggs, we cannot conclude how the age of the beetles and egg quality might be related for either diet. However, in terms of the number of eggs laid during the course of our study, A. pisum can clearly be considered a better diet for H. axyridis females.
Little is known on the influence of host plant secondary compounds experienced by H. axyridis through prey aphids (Francis et al., 2000; Alhmedi et al., 2008). As presented in this paper, larvae fed on Aphis sambuci had lower IPMP concentrations than larvae fed on other aphid species. A. sambuci was shown to be toxic for ladybird beetles as C. septempunctata (Nedved and Salvucci, 2008), but was consumed as non-optimum food by H. axyridis (Ungerova et al., 2010). Further, it was shown that H. axyridis can survive on a diet of A. sambuci contrary to C. septempunctata (Francis et al., 2000). The ability to detoxify plant allelochemicals like the cyanogenic and phenolic glycosides produced by Sambucus nigra (D’Abrosca et al., 2011) and sequestered by the aphids might be responsible for this difference in the diet spectrum of both coccinellid species. Prunus mahaleb is known for its high levels of coumarins. Those secondary compounds are supposed to cause feeding deterrence against Japanese beetles (Popillia japonica) (Patton et al., 1997). Corylus avellanae contains high concentrations of phenolic compounds (Amaral et al., 2010) that remain to be tested for physiological effects on aphids and their predators.
On the plants mentioned above, we collected H. axyridis pupae that supposedly developed as larvae with a diet of aphids feeding on the respective host plants. Thus, we conclude that lower IPMP content in the beetles emerged from those pupae compared to beetles fed on aphids from other host plants (Fig. 4) could indicate an interaction of host plant compounds sequestered in the aphids and ingested by the predatory beetles with autogenously produced methoxypyrazines. Myzus persica fed on leaves of P. persica was shown to be the most suitable food for H. axyridis, which preferred this aphid over A. fabae, Macrosiphum albifrons, M. pseudorosae, and M. euphorbiae (Soares et al., 2004; Finlayson et al., 2010). Alyokhin and Francis (2005) described a significant reduction of M. persicae following the establishment of H. axyridis. In the present study, the IPMP contents in the hemolymph of H. axyridis were significantly higher when fed a closely related aphid species (M. varians) than when fed other species. We hypothesize that H. axyridis may detoxify allomones, but when feeding on chemically defended prey, it can not invest in high methoxypyrazine contents. Allelochemicals can thus influence the herbivore – predator relationship and the outcome of the biological control attempt or the prevalence of a predatory species on a certain host. Further studies on the interactions between chemically defended plants, sequestering insects and ladybird beetles are needed.
Adults fed for five days on the same prey as the larvae showed no higher IPMP contents than adults that were not fed after eclosion. Thus, in our study, larval diet seems to be the decisive factor for IPMP contents detectable in adult beetles. Under field conditions, larval mortality has been reported as the most important factor for the population dynamic of H. axyridis (Osawa 1993). Higher plasticity of larval IPMP content might be interpreted with regard to those field observations.
The results of our assays on the MIC values of the hemolymph of H. axyridis adults show evidence for an impact of beetle diet on the antimicrobial activity of their hemolymph. Similar to a previous study (Gross et al., 2010), we observed the highest susceptibility against the antimicrobial compounds in the hemolymph of H. axyridis for E. coli and S. cerevisiae. We found no differences in the antimicrobial activity of the hemolymph between male and female beetles, regardless of diet regime and elytral color. From our data, we cannot conclude that the three morphotypes of H. axyridis tested differ in the antimicrobial activity of their hemolymph. We found evidence, however, that a diet of pea aphids resulted in stronger growth inhibition of the most susceptible bacterium tested, the gram negative E. coli, when compared to the hemolymph activity of beetles fed on frozen E. kuehniella eggs. These results are congruent with the diet effects on fitness parameters observed here.
Within aphid fed beetles, we also observed higher activity against the growth of E. coli for the succinea 1 morphs than for the less abundant succinea 2 morphs. The spectabilis morphs had an intermediate growth inhibitory effect on this microorganism. Barnes and Siva-Jothy (2000) described a positive correlation of cuticle melanization with parasite resistance for Tenebrio molitor beetles. Since immune-function and melanin production use the same enzymatic pathway, increased melanization might interfere with immune defense (Carton and Nappi, 1997). Our results, however, do not provide evidence for this hypothesis. Based on our data, we cannot conclude that the rare melanic spectabilis morphs were less defended against microorganisms than the prevalent, less melanic succinea 1 morphs of H. axyridis. In our assays, a growth inhibition of B. t. tenebrionis was only visible with the highest concentrations of hemolymph applied.
Furthermore, we found no evidence for antimicrobial properties of methoxypyrazines in our MIC tests. Thus, we conclude that the observed growth inhibition through the hemolymph from H. axyridis must be caused by other substances that remain to be identified. Pyrazines have long been described as major compounds in defensive odors emitted by different insect groups (Rothschild, 1961; Moore and Brown, 1981). Cudjoe et al. (2005) reviewed that H. axyridis contained a hundred times higher concentration of IPMP than C. septempunctata, which was described as the main compound of a so-called ladybird taint and can alter the taste of wine (Pickering et al., 2004; Kögel et al., 2012a). This hypothesizes that H. axyridis could cause greater damage to viticulture than C. septempunctata. Recent studies have refuted this hypothesis: by using gaschromatography and olfactometer tests, it was found that C. septempunctata had the same amount of IPMP in its hemolymph as H. axyridis. Additionally, GC-nitrogen-phosphor detector analyses revealed nearly the same peak areas of IPMP in chromatograms of hemolymph headspace for both species (Kögel et al., 2012b). Additionally, Botezatu and Pickering (2010) described similar IPMP concentrations in wine produced after the addition of H. axyridis or C. septempunctata into crushed grapes. The differences in the concentrations of IPMP measured by Cudjoe et al. (2005), Botezatu and Pickering (2010), and our study might be due to the use of live beetles in our and Pickering’s trials, while Cudjoe et al. (2005) analyzed frozen beetles.
In this study, several factors that may influence production of IPMP in ladybird beetles were investigated. IPMP contents of H. axyridis were significantly higher than of C. septempunctata in one out of three feeding treatments (A. pisum) in the laboratory. In one treatment (A. pisum+grape juice), C. septempunctata had higher IPMP contents than the Multicolored Asian ladybird beetle. In beetles fed on E. kuehniella eggs and in field-collected beetles, no significant differences between the two species could be observed. Thus, concerning methoxypyrazine contents, H. axyridis cannot be regarded as more hazardous for viticulture than C. septempunctata. In addition to development time, fresh body mass and fecundity, IPMP content can be used to evaluate food quality (Kalushkov and Hodek, 2004).
Elytral color apparently has an influence on IPMP content. Cai et al. (2007) previously described significant differences between the succinea 1 and succinea 2 morphs. In our study, H. axyridis succinea 1 also contained significantly higher values of IPMP than succinea 2. Differences between red and black color morphs were studied before by Bezzerides et al. (2007). They showed that darker beetles were less defended against predators due to lower alkaloid contents. Slogett (2010) could not measure this, but they showed higher predation of non-melanic morphs by an orb-web spider. In our assays, significant differences in IPMP content were observed in one feeding group between the H. axyridis succinea 1 morphs and H. axyridis spectabilis morphs (Fig. 4). Grill and Moore (1998) suggested elytral coloration was an effect of diet. In our experiments, larvae fed on A. pisum, E. kuehniella, or other aphid species did not differ in variation of elytral pigmentation.
Currently, we have no explanations for the differences detected in methoxypyrazine content in relation to elytral color. Bezzerides et al. (2007) argued that lower defense levels in melanic beetles could be balanced by other advantages, such as increased activity rates through a thermal advantage during periods with lower temperature. The advantages of melanic beetles with respect to body temperature, activity range, and walking speed at lower temperatures have been shown for leaf beetles and ladybird beetles (De Jong et al., 1996; De Jong and Brakefield, 1998; Gross et al., 2004b; Michie et al., 2010).
In summary, our results provide further evidence for the influence of diet on fitness and defense chemistry in coccinellid beetles. It leads us to conclude that the predicted risk of a single specimen of H. axyridis for viticulture, with regard to tainting wine with IPMP, is not higher compared to specimens of other ladybird beetle species. However, H. axyridis will remain a threat for wine production due to huge population densities that may develop during some years, bearing the risk of tainting wine by getting harvested and processed in high numbers.
Our observations on factors influencing the IPMP content in both ladybird species examined show the need of further tritrophic level studies for H. axyridis. Field observations on prey species and the size of local sub-populations of this ladybird species, invasive in many countries, are important to understand the environmental factors that affect its competitive advantage over native ladybird species (Kindlmann et al., 2011).
References
Al Abassi, S., Birkett, M. A., Pettersson, J., Pickett, J. A., and Woodcock, C. M. 1998. Ladybird beetle odour identified and found to be responsible for attraction between adults. Cell. Mol. Life Sci. 54:876–879.
Alhmedi, A., Haubruge, E., and Francis, F. 2008. Role of prey-host associations on Harmonia axyridis and Episyrphus balteatus reproduction and predatory efficiency. Entomol. Exp. Appl. 128:49–56.
Alyokhin, A. and Francis, F. 2005. Density-dependent regulation in populations of potato-colonizing aphids. Popul. Ecol. 47:257–266.
Amaral, J. S., Valentão, P., Andrade, P. B., Martins, R. C., and Seabra, R. M. 2010. Phenolic composition of hazelnut leaves: Influence of cultivar, geographical origin and ripening stage. Sci. Hortic. 126:306–313.
Armitage, S. A. O., Thompson, J. J. W., Rolff, J., and Siva-Jothy, M. T. 2003. Examining costs of induced and constitutive immune investment in Tenebrio molitor. J. Evol. Biol. 16:1038–1044.
Barnes, A. I. and Siva-Jothy, M. T. 2000. Density-dependent prophylaxis in the mealworm beetle Tenebrio molitor L. (Coleoptera: Tenebrionidae): cuticular melanization is an indicator of investment in immunity. Proc. R. Soc. Lond. B 267:177–182.
Berkvens, N., Bonte, J., Berkvens, D., Tirry, L., and De Clercq, P. 2008. Influence of diet and photoperiod on development and reproduction of European populations of Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). BioControl 53:211–221.
Bezzerides, A. L., Mcgraw, K. J., Parker, R. S., and Husseini, J. 2007. Elytral color as a signal of chemical defense in the Asian ladybird beetle Harmonia axyridis. Behav. Ecol. Sociobiol. 61:1401–1408.
Botezatu, A. and Pickering, G. J. 2010. Ladybug (Coccinellidae) taint in wine, in A. G. Reynolds (ed.), Understanding and Managing Wine Quality and Safety. Woodhead Publishing Limited, Cambridge.
Bulet, P., Cocianicich, S., Dimarcq, J.-L., Lambert, J., Reichhart, J.-M., Hoffmann, D., Hetru, C., and Hoffmann, J. A. 1991. Isolation from a coleopteran insect of a novel inducible antibacterial peptide and of new members of the insect defensin family. J. Biol. Chem. 266:24520–24525.
Cai, L., Koziel, J. A., and O’Neal, M. E. 2007. Determination of characteristic odorants from Harmonia axyridis beetles using in vivo solid-phase microextraction and multidimensional gas chromatography–mass spectrometry-olfactometry. J. Chromatogr. A 1147:66–78.
Carton, Y. and Nappi, A. J. 1997. Drosophila cellular immunity against parasitoids. Parasitol. Today 13:218–227.
Cudjoe, E., Wiederkehr, T. B., and Brindle, I. D. 2005. Headspace gas chromatography–mass spectrometry: a fast approach to the identification and determination of 2-alkyl-3-methoxypyrazine pheromones in ladybugs. Analyst 130:152–155.
D’Abrosca, B., Dellagreca, M., Fiorentino, A., Monaco, P., Previtera, L., Simonet, A. M., and Zarelli, A. 2011. Potential allelochemicals from Sambucus nigra. Phytochemistry 58:1073–1081.
De Jong, P. W. and Brakefield, P. M. 1998. Climate and change in clines for melanism in the two-dot ladybird, Adalia bipunctata (Coleoptera: Coccinellidae). Proc. Roy. Soc. Lond. B. Biol. Sci. 265:39–43.
De jong, P. W., Gussekloo, S. W. S., and Brakefield, P. M. 1996. Differences in thermal balance, body temperature and activity between non melanic and melanic two-dot laybird beetles (Adalia bipunctata) under controlled conditions. J. Exp. Biol. 199:2655–2666.
Deutsches Institut Für Normung. 2010. DIN 58940: Susceptibility testing of pathogens to antimicrobial agents—Part 4: Evaluation classes of the minimum inhibitory concentration, pp. 354–60, in DIN-Taschenbuch Medizinische Mikrobiologie und Immunologie, Normen und weitere Unterlagen. Beuth-Verlag, Berlin and Cologne.
Ferran, A., Niknam, H., Kabiri, F., Picart, J. L., Deherve, C., Brun, J., Iperti, G., and Lapchin, L. 1996. The use of Harmonia axyridis larvae (Coleoptera: Coccinellidae) against Macrosiphum rosae (Hemiptera: Sternorrhyncha: Aphididae) on rose bushes. Eur. J. Entomol. 93:59–67.
Finlayson, C., Alyokhin A., Gross S., and Porter E. 2010. Differential consumption of four aphid species by four lady beetle species. J. Insect Sc. 10:10:31 available online: insectscience.org/10.31.
Francis, F., Haubrige, E., and Gaspar, C. 2000. Influence of host plants on specialist/generalist aphids and on the development of Adalia bipunctata (Coleoptera: Coccinellidae). Eur. J. Entomol. 97:481–485.
Fukunaga, Y. and Akimoto, S. 2007. Toxicity of the aphid Aulacorthum magnolia to the predator Harmonia axyridis (Coeloptera: Coccinellidae) and genetic variance in the assimiliation of the toxic aphids in H. axyridis larvae. Entomol. Sci. 10:45–53.
Galvan, T. L., Burkness, E. C., Vickers, Z., Stenberg, P., Mansfield, A. K., and Hutchison, W. D. 2007. Sensory-based action threshold for multicolored Asian lady beetle-related taint in winegrapes. Am. J. Enol. Viticult. 58:518–522.
Galvan, T. L., Koch, R. L., and Hutchinson, W. D. 2008. Impact of fruit feeding on overwintering survival of the multicolored Asian lady beetle, and the ability of this insect and paper wasps to injure wine grape berries. Entomol. Exp. Appl. 128:429–436.
Grill, C. P. and Moore, A. J. 1998. Effects of a larval antipredator response and larval diet on adult phenotype in an aposematic ladybird beetle. Oecologia 114:274–282.
Gross, J., Fatouros, N. E., and Hilker, M. 2004a. The significance of bottom-up effects for host plant specialization in Chrysomela leaf beetles. Oikos 105:368–376.
Gross, J., Schmolz, E., and Hilker, M. 2004b. Thermal adaptations of the leaf beetle Chrysomela lapponica to different climes of Central and Northern Europe. Environ. Entomol. 33:799–806.
Gross, J., Schumacher, K., Schmidtberg, H., and Vilcinskas, A. 2008. Protected by fumigants: beetle perfumes in antimicrobial defense. J. Chem. Ecol. 34:179–188.
Gross, J., Eben, A., Müller, I., and Wensing, A. 2010. A well protected intruder: the effective antimicrobial defense of the invasive ladybird Harmonia axyridis. J. Chem. Ecol. 36:1180–1188.
Haine, E. R., Moret, Y., Siva-Jothy, M. T., and Rolff, J. 2008. Antimicrobial defense and persistent infection in insects. Science 322:1257–1259.
Hodek, I. 1993. Habitat and food specificity in aphidophagous predators. Biocontr. Sci. Technol. 3:91–100.
Hodek, I. and Honek, A. 1996. Ecology of Coccinellidae. Kluwer, Dordrecht. 464.
Hukusima, S. and Kamei, M. 1970. Effects of various species of aphids as food on development, fecundity and longevity of Harmonia axyridis Pallas (Coleoptera: Coccinellidae). Res. Bull. Fac. Agric. Gifu Univ. 29:53–66.
Jalali, M. A., Tirry, L., and De Clercq, P. 2009. Effects of food and temperature on development, fecundity and life-table parameters of Adalia bipunctata (Coleoptera: Coccinellidae). J. Appl. Entomol. 133:615–625.
Kalushkov, P. and Hodek, I. 2004. The effects of thirteen species of aphids on some life history parameters of the ladybird Coccinella septempunctata. Biocontrol 49:21–32.
Karl, I., Hoffmann, K. H., and Fischer, K. 2010. Cuticular melanisation and immune response in a butterfly: local adaptation and lack of correlation. Ecol. Ent. 35:523–528.
Kindlmann, P., Ameixa, O. M. C. C., and Diyon, A. F. G. 2011. Ecological effects of invasive alien species on native communities, with particular emphasis on the interactions between aphids and ladybirds. Biocontrol 56:469–476.
Klausnitzer, B. and Klausnitzer H. 1997. Marienkäfer: Westarp Wissenschaften.
Koch, R. L. 2003. The multicolored Asian lady beetle, Harmonia axyridis: a review of its biology, uses in biological control, and non-target impacts. J. Insect Sci. 3:32–48.
Kögel, S., Gross, J., and Hoffmann, C. 2012a. Sensory detection thresholds of ‘ladybird taint’ in ‘Riesling’ and ‘Pinot noir’ under different fermentation and processing conditions. Vitis 51:27–32.
Kögel, S., Gross, J., Hoffmann, C., and Ulrich, D. 2012b. Diversity and frequencies of methoxypyrazines in hemolymph of Harmonia axyridis and Coccinella septempunctata and their influence on the taste of wine. Eur. Food Res. Technol. 234:399–404.
Lanzoni, A., Accinelli, G., Bazzocchi, G. G., and Burgio, G. 2004. Biological traits and life table of the exotic Harmonia axyridis compared with Hippodamia variegata, and Adalia bipunctata (Coleoptera: Coccinellidae). J. Appl. Entomol. 28:298–306.
Lee, K. P., Simpson, S. J., and Wilson, K. 2008. Dietary protein-quality influences melanization and immune function in an insect. Funct. Ecol. 22:1052–1061.
Mcclure, M. S. 1987. Potential of the Asian predator Harmonia axyridis Pallas (Coleoptera: Coccinellidae), to control Matsucoccus resinosae Bean and Godwin (Homoptera: Margarodidae) in the United States. Environ. Entomol. 16:224–230.
Michie, L. J., Mallard, F., Majerus, M. E. N., and Jiggins, F. M. 2010. Melanic through nature or nurture: genetic polymorphism and phenotypic plasticity in Harmonia axyridis. J. Evol. Biol. 27:1699–1707.
Moore, B. P. and Brown, W. V. 1981. Identification of warning odour components, bitter principles and antifeedants in an aposematic beetle: Metriorrhynchus rhipidius (Coleoptera: Lycicae). Insect Biochem. 11:493–499.
Nedved, O. and Salvucci, S. 2008. Ladybird Coccinella septempunctata (Coleoptera: Coccinellidae) prefers toxic prey in laboratory choice experiment. Eur. J. Entomol. 105:431–436.
Osawa, N. 1993. Population field studies of the aphidophagous ladybird beetle Harmonia axyridis Pallas (Coleoptera: Coccinellidae): life tables and key factor analysis. Res. Popul. Ecol. 35:335–348.
Osawa, N. and Nishida, T. 1992. Seasonal variation in elytral colour polymorphism in Harmonia axyridis (the ladybird beetle): the role on non-random mating. Heredity 69:297–307.
Patton, C. A., Ranney, T. G., Burton, J. D., and Walgenbach, J. F. 1997. Natural pest resistance of Prunus taxa to feeding by adult Japanese Beetles: role of endogenous allelochemicals in host plant resistance. J. Am. Soc. Hort. Sci. 122(5):668–672.
Pickering, G. J., Lin, J., Riesen, R., Reynolds, A., Brindle, I., and Soleas, G. 2004. Influence of Harmonia axyridis on the sensory properties of white and red wine. Am. J. Enol. Viticult. 55:153–159.
Pickering, G. J., Lin, Y., Reynolds, A., Soleas, G., Riesen, R., and Brindle, I. 2005. The influence of Harmonia axyridis on wine composition and aging. J. Food Sci. 70:128–135.
Ross, C. F., Rosales, M. U., and Fernandez-Plotka, V. C. 2010. Aroma profile of Niagara grape juice contaminated with multicoloured Asian lady beetle taint using gas chromatography/mass spectrometry/olfactometry. Int. J. Food Sci. Technol. 45:789–793.
Rothschild, M. 1961. Defensive odours and Muellerian mimicry among insects. Trans. Roy. Ent. Soc. Lond. 113:101–121.
Sachs, L. 1992. Angewandte Statistik, 7th ed. Springer Verlag, Berlin.
Seo, M. J., Kim, G. H., and Youn, Y. N. 2008. Differences in biological and behavioural characteristics of Harmonia axyridis (Coleoptera: Coccinellidae) according to colour patterns of elytra. J. Appl. Entomol. 132:239–247.
Sloggett, J. J. 2010. Colour pattern polymorphism and chemical defence in Harmonia axyridis. IOBC/wprs Bulletin 58:114–123.
Snyder, W. E., Joseph, S. B., Preziosi, R. F., and Moore, A. J. 2000. Nutritional benefits of cannibalism for the lady beetle Harmonia axyridis (Coleoptera: Coccinellidae) when prey quality is poor. Environ. Ent. 29:1173–1179.
Soares, A. O., Coderre, D., and Schanderl, H. 2001. Fitness of two phenotypes of Harmonia axyridis (Coleoptera: coccinellidae). Eur. J. Entomol. 98:287–293.
Soares, A. O., Coderre, D., and Schanderl, H. 2003. Effect of temperature and intraspecific allometry on predation by two phenotypes of Harmonia axyridis Pallas (Coleoptera: Coccinellidae). Environ. Entomol. 32:939–944.
Soares, A. O., Coderre, D., and Schanderl, H. 2004. Dietary self-selection behaviour by the adults of the aphidophagous ladybeetle Harmonia axyridis (Coleoptera: Coccinellidae) J. Animal Ecol. 73:478–486.
Soares, A. O., Coderre, D., and Schanderl, H. 2005. Influence of prey quality in the finess of two phenotypes of Harmonia axyridis adults. Ent. Exp. Appl. 114:227–232.
Specty, O., Febvay, G., Grenier, S., Delober, B., Piotte, C., Pageaux, J. F., Ferran, A., and Guillaud, J. 2003. Nutritional plasticity of the predatory ladybeetle Harmonia axyridis (Coleoptera: Coccinellidae): comparison between natural and substitution prey. Arch. Insect Biochem. Physiol. 52:81–91.
Stathas, G. J., Eliopoulos, P. A., Kontodimas, D. C., and Giannopapas, J. 2001. Parameters of reproductive activity in females of Harmonia axyridis. Eur. J. Entomol. 98:547–549.
Su, W., Michaud, J. P., Runzhi, Z., Fan, Z., and Shuang, L. 2009. Seasonal cycles of assortative mating and reproductive behaviour in polymorphic populations of Harmonia axyridis in China. Ecol. Entomol. 34:483–494.
Tedders, W. L. and Schaefer, P. 1994. Release and establishment of Harmonia axyridis (Coleoptera, Coccinellidae) in the southeastern United-States. Entomol. News 105:228–243.
Ungerova, D., Kalushkov, P., and Nedved, O. 2010. Suitability of diverse prey species for development of Harmonia axyridis and the effect of container size. IOBC/wprs Bulletin 58:165–174.
Wilson, K., Cotter, S. C., Reeson, A. F., and Pell, J. K. 2001. Melanism and disease resistance in insects. Ecol. Lett. 4:637–649.
Acknowledgments
This work was supported by a grant from the ‘Forschungsring Deutscher Weinbau’ (FDW). The authors thank Svenja Hoferer and Sabine Wetzel (JKI Dossenheim) for technical assistance. We thank the Norddeutsche Pflanzenzucht KG for a gift of untreated seeds of Vicia fabae (cultivar “Tattoo”). We are grateful to M. Paulus and C. Emmerling from FB VI Geography/Biogeography, Trier University (Germany) for cooperation. Special thanks go to Th. Thieme (BioTest Labor, Sagerheide, Germany) who provided expertise on the determination of field collected aphid species, and Eva Gross (Dossenheim, Germany) and Lukasz Stelinski (Lake Alfred, Florida, USA) for linguistic improvements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kögel, S., Eben, A., Hoffmann, C. et al. Influence of Diet on Fecundity, Immune Defense and Content of 2-Isopropyl-3-Methoxypyrazine in Harmonia axyridis Pallas. J Chem Ecol 38, 854–864 (2012). https://doi.org/10.1007/s10886-012-0139-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-012-0139-1