Abstract
Ant–plant interactions often are mediated by extrafloral nectar (EFN) composition that may influence plant visitation by ants. Over a 300 km range in the Indian Western Ghats, we investigated the correlation between the EFN composition of the myrmecophytic ant-plant Humboldtia brunonis (Fabaceae) and the number and species of ants visiting EFN. EFN composition varied among H. brunonis populations and between plant organs (floral bud vs. young leaf EFN). In general, EFN was rich in sugars with small quantities of amino acids, especially essential amino acids, and had moderate invertase activity. In experiments at the study sites with sugar and amino acid solutions and with leaf or floral bud EFN mimics, dominant EFN-feeding ants differentiated between solutions as well as between mimics. The castration parasite Crematogaster dohrni (northern study site) was the least selective and did not exhibit any clear feeding preferences, while the largely trophobiont-tending non-protective Myrmicaria brunnea (middle study site) preferred higher sucrose concentrations and certain essential/non-essential amino acid mixtures. The mutualistic Technomyrmex albipes (southern study site) preferred sucrose over glucose or fructose solutions and consumed the leaf EFN mimic to a greater extent than the floral bud EFN mimic. This young leaf EFN mimic had low sugar concentrations, the lowest viscosity and sugar:amino acid ratio, was rich in essential amino acids, and appeared ideally suited to the digestive physiology of T. albipes. This preference for young leaf EFN may explain the greater protection afforded to young leaves than to floral buds by T. albipes, and may also help to resolve ant–pollinator conflicts. The differential response of dominant ants to sugar, amino acids, or solution viscosity suggests that plants can fine-tune their interactions with local ants via EFN composition. Thus, EFN can mediate local partner-choice mechanisms in ant–plant interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ant-plants provide food and/or housing to their ant partners in exchange for protection from herbivores (Davidson and McKey, 1993; Heil and McKey, 2003) and/or for provision of limiting nutrients such as nitrogen (Wagner and Nicklen, 2010). Food resources offered to ants consist of carbohydrate-rich extrafloral nectar (EFN) as well as protein- and/or lipid-rich food bodies (Heil and McKey, 2003). While food bodies occur mostly in specialized and stable interactions between plants and obligate ants, EFN is offered in both obligate and facultative interactions (Blüthgen et al., 2000; Heil et al., 2005; Rudgers et al., 2010). Extrafloral nectar is usually carbohydrate-rich and nitrogen-poor (Baker et al., 1978; Blüthgen and Fiedler, 2004).
Since arboreal ants differ in their biology, digestive physiology and nutrient requirements (Davidson, 1997; Davidson et al., 2004; Kay, 2004; Cook and Davidson, 2006), ant species differ in their responses to EFN based on its qualitative and quantitative composition (Heil et al., 2005, 2009; González-Teuber and Heil, 2009a, b). Specifically, ant preferences for EFN solutions of different compositions may be dictated by ant responses to sugar and amino acid identity or concentration, or to ratios of sugar and amino acids, or to non-additive interactions between these components (Davidson and Cook, 2008). For example, ants that do not possess the enzyme invertase, which converts sucrose to glucose and fructose, may be averse to sucrose-rich solutions (Heil et al., 2005). Ants that do not have gut symbionts to synthesize essential amino acids (Cook and Davidson, 2006) may be attracted to solutions rich in essential amino acids. Ant species may develop preferences for sugars or amino acids characteristic of familiar sources of nutrition (González-Teuber and Heil, 2009b).
Variation within a plant in quality and quantity of floral nectar (Lanza et al., 1995; Herrera et al., 2006) impacts plant reproductive success through its effect on pollinators. The effects of differences in EFN composition within a plant, especially EFN produced on different plant structures, e.g., young leaves vs. floral buds, have rarely been examined (Keeler, 1977). Extrafloral nectar secretion may interfere with pollination by attracting pollinator-intimidating ants (Ness, 2006), thus increasing the ecological costs of EFN production (Heil and McKey, 2003).
While intraspecific variation in floral nectar has been described (Nicolson, 2007), less is known about variation in EFN (Rudgers and Gardener, 2004; González-Teuber and Heil, 2009b). Geographical variation in the quality and quantity of EFN may influence the type and frequency of ants visiting EFN-bearing plants (Blüthgen et al., 2000; Rudgers et al., 2010), and thus mediate locality-specific interactions between ants and plants (Rudgers and Gardener, 2004; Rudgers et al., 2010). Variation in EFN quality and in ant responses could set up a mosaic of interactions between plants and ants (Chamberlain and Holland, 2008).
In this study, we used the myrmecophytic plant Humboldtia brunonis Wallich (Fabaceae) to examine how geographical variation in EFN chemistry and related physical properties such as viscosity affected EFN consumption by locally available ant species. This allowed us to evaluate whether EFN composition influences the identity and abundance of ants that are attracted to this ant-plant. Ant preferences for nutrients usually have been tested with simple aqueous solutions, often under laboratory conditions (Lanza, 1988; Tinti and Nofre, 2001; Blüthgen and Fiedler, 2004). Therefore, we also tested ant feeding preferences at the study sites using such simple solutions. We further examined ant response to complex mixtures such as natural EFN by using EFN mimics under natural situations because such interactions as well as the impact of EFN viscosity have not been fully explored (Koptur and Truong, 1998; Kost and Heil, 2005; González-Teuber and Heil, 2009b). Humboldtia brunonis is ideal for an investigation into questions related to the impact of EFN composition on ant–plant mutualisms because it is an unspecialized myrmecophyte that is host to a diversity of ant species throughout its geographical range, which spans 300 km within the Indian Western Ghats (Gaume et al., 2005a,b, 2006; Shenoy and Borges, 2010). Using experiments conducted in the natural setting, we asked: 1. What is the sugar:amino acid ratio in H. brunonis EFN, i.e., is EFN carbohydrate-rich and amino acid-poor? 2. Is there geographical variation in EFN composition across the range of this ant-plant? 3. Does the composition of young leaf (YL) EFN differ from that of floral bud (FB) EFN? 4. Are traits of nectar composition (sugar or amino acid concentration, sugar:amino acid ratios, caloric value, viscosity or familiarity) correlated with ant visitation to EFN? Do patterns vary between ant species at the different sites?
Methods and Materials
Study System and Sites Humboldtia brunonis (Fabaceae: Caesalpinioideae) is a dominant understorey ant-plant that houses ants in swollen, hollow internodes called domatia. It is distributed in low elevation, wet evergreen forests (11°10′N to 13°45′N) of the Indian Western Ghats (Ramesh and Pascal, 1997). EFN is produced on the sepals of floral buds, young leaves, reniform stipules, and occasionally from the stalk of the inflorescence, but mostly from young leaves and flower buds. Flowering occurs in the dry season (December–April), as does production of young leaves and maximum ant activity (Basu, 1997). Extrafloral nectar chemistry and ant preferences for it were examined at three sites matched for elevation: (i) Agumbe Reserve Forest (13°31′N, 75°5′E; 633 m asl); (ii) Sampaji Reserve Forest (12°29′N, 75°35′E; 665 m asl); (iii) Solaikolli within Brahmagiri Wildlife Sanctuary (12°4′N, 75°49′E; 651 m asl).
Geographical Variation in EFN Volume, Chemical Composition and Invertase Activity
Extrafloral nectar from bagged (using fine cloth mesh) and ant-excluded (for 24 h) floral bud nectaries of a single inflorescence (FB) or all four leaflets of each young leaf (YL) was pooled to determine volume and composition (Agumbe: N = 10 FBs and 11 YLs; Sampaji: N = 7 and 9; Solaikolli: N = 11 and 8; each FB or YL sample was from a different plant). Inflorescences and young leaves were bagged in the morning between 0900–1200 h. Owing to its high viscosity, EFN was diluted with known volumes of HPLC-grade distilled water for collection and stored with 50 μl of HPLC-grade methanol at −10°C until further analysis. Sugars and amino acids in EFN were derivatized to their trimethyl silylated forms (Kost and Heil, 2005) prior to GC-MS analysis (Agilent-HP GC model 6890N, MS model 5973N) using an HP-5 MS column with the following temperature program: 50°C for 2 min isothermal, 10°C min−1 to 120°C, and 5°C min−1 to 250°C for 5 min isothermal. The GC was operated in the split mode with a split-ratio of 10:1. A mixture containing 1 mg of each of commercial (Sigma-Aldrich, India) sugars (sucrose, glucose, fructose, inositol, galactose, mannose, arabinose, raffinose) and of each of the 20 naturally occurring amino acids was derivatized using 1 ml of N-methyl-N-(trimethyl silyl) trifluoro acetamide in 2 ml pyridine and used as a reference standard for the GC-MS analysis. All sugar and amino acid concentrations are reported in g/100 ml, and all sugar:amino acid ratios are reported as mass ratios. Invertase activity (μg glucose μl −1 min−1) was measured in separately collected samples of FB and YL EFN (Heil et al., 2005). These EFN samples were not diluted with water, to minimize disruption of nectary cells and release of invertase (Pate et al., 1985).
Differential Utilization of Solutions by Ants in Cafeteria Assays
We modified the experimental design of Blüthgen and Fiedler (2004) to test responses of ants to simple standard sugar solutions, to sugars mixed with amino acids, or to complex solutions mimicking EFN compositions (Table 1). At each site we chose 10 H. brunonis trees separated by a distance of at least 8 m to minimize pseudoreplication of ants from the same colony. On these 10 trees, at 1.5 m above the ground, we placed 5 replicate sets of 2 ml plastic vials containing the test solutions. Each tube had a cotton wick that delivered the solution to ants. Vials in each set were randomly ordered to minimize location effect. After setting up the experimental vials between 1100–1200 h, we counted ant workers of the different species that visited the wicks of each solution at five times during the next 24 h (1500 h, 1800 h, 2100 h, next morning 1100 h, and next afternoon 1300 h). We refilled vials at least 1 h before each count, if required. Since EFN is a complex secretion containing sugars (mainly sucrose, glucose, and fructose) and amino acids (Baker et al., 1978), we used sugars and amino acids in our experiments in ranges normally available to ants from plant secretions or honeydew. Plain water solutions were not offered in these experiments since the EFN of H. brunonis is the only major source of extrafloral liquids for ants at these sites in the dry season.
Cafeteria Assays Using Standard Solutions of Sugars and Amino Acids
We explored the preference of ants for three sugars at different concentrations, and for different mixtures of essential and non-essential amino acids. In Experiment 1, ants selected between equal concentration solutions of sucrose, glucose, or fructose. Solutions with sugar concentrations ranging from 5% to 50% were tested. In Experiment 2, we presented ants simultaneously with equicaloric solutions of sucrose, glucose, or fructose. We compared mixtures supplying 0.197 kcal/ml (equivalent to 5% w/v sucrose or 10% w/v of the monosaccharides glucose or fructose) to mixtures supplying 0.788 kcal/ml (equivalent to 20% sucrose or 40% w/v glucose or fructose). In Experiment 3, ants selected between several concentrations of a single sugar. Solutions with sugar concentrations ranging from 5% to 50% were tested. In Experiment 4, ants selected between 15% sucrose solutions containing different combinations of essential and non-essential amino acids each at a concentration of 0.7 mg/ml, which is the average single amino acid concentration in EFN. We assumed that the essential amino acids for ants are the same as the essential amino acids for mammals (Davidson et al., 2004). Three essential amino acid solutions were used: E1 (arg, leu, met, thr, and val), E2 (his, isoleu, lys, phe, and try), and E (all 10 essential amino acids). The non-essential amino acid solutions were prepared as N1 (asn, glu, gly, pro, and tyr), N2 (ala, asp, cys, gln, and ser), and N (all 10 non-essential amino acids). Some of these solutions were used as mixtures (E1N1, E2N2, E1N2, and E2N1) to provide functional group diversity. All six solutions E, N, E1N1, E2N2, E1N2, and E2N1 were simultaneously presented to ants at each site.
Cafeteria Assays Using Solutions Mimicking Young Leaf and Floral Bud EFN
We compared the preferences of ants for solutions that mimicked YL and FB EFN from H. brunonis at the three study sites (Table 1). We prepared EFN-mimicking solutions that represented the inter- and intra-site variability in composition and concentration (high or low concentrations of sugars and amino acids) of FB and YL EFN (Supplemental Table 1, Supplemental Fig. 1). In Experiment 5, we simultaneously offered ants at each site FB EFN mimics corresponding to that site (familiar EFN) and that of other sites (non-familiar EFN) followed by a separate test with familiar and non-familiar YL EFN mimics. In Experiment 6, we offered ants at each site a choice between mimics of FB and YL EFN from that site (familiar EFN only).
We measured the viscosity of all FB and YL EFN mimics at 20, 30, and 40°C with a rotational (stress/strain) rheometer (TA Instruments AR1000N) to determine if viscosity of a solution with a particular chemical composition is a correlate of ant preference for EFN.
Statistical Analyses
The sugar and amino acid compositions of FB and YL EFN types were compared using permutational (non-parametric) multivariate analyses of variance (MANOVA) since the data were not normally distributed. This was done via the Adonis function of the Vegan package in the software R (Anderson, 2001; Oksanen et al., 2011). The Euclidean distance measure and a total of 999 permutations were employed for each comparison. By using this procedure, we examined effects of site and EFN type (FB vs. YL EFN) as well as interaction effects of these variables on EFN composition. Pairwise post-hoc comparisons of these data were performed using the Mann-Whitney U test. EFN volumes and invertase levels were compared between FB and YL EFN types using a non-parametric Kruskal-Wallis ANOVA. All data on ant visitation to simultaneously presented solutions were analyzed for normality using the Shapiro-Wilks test. None of the distributions were normal and could not be rendered normal by any procedure. Therefore, non-parametric Kruskal-Wallis tests followed by Mann-Whitney U tests with appropriate Bonferroni corrections or Wilcoxon matched pairs tests were employed. All univariate non-parametric tests were performed using the software package STATISTICA ’99 Edition, Kernel Release 5.5 A. These tests were performed to statistically identify solutions that were utilized to a greater extent by ants over others presented in the various cafeteria assays. In this paper, although data are not normally distributed, we have elected to depict means and standard errors in the figures for ease of graphical representation.
Results
Geographical Variation in EFN Volume, Chemical Composition and Invertase Activity
There was within- and between-site variation in EFN composition. In all cases, EFN contained more sugar than amino acids, with total sugar:total amino acid mass ratios varying from 37.0 for FB to 86.0 for YL EFN (samples pooled across sites) (Table 2). Across sites, floral buds and young leaves produced the same volume of EFN (5 μl) within 24 h (H 5, 56 =3.524, P = 0.611; Table 2). Twelve sugars were found in EFN (Table 2, Supplemental Table 2). Sucrose, glucose, and fructose were dominant in both types of EFN (Table 2). In general, the concentrations of sucrose and glucose were significantly higher in FB EFN than in YL EFN, pooled across all sites and also within sites (Table 2). In EFN samples collected from all sites, glucose and fructose concentrations were not significantly different from each other (Wilcoxon matched pairs test: Z = 1.17, N = 28 pairs, P = 0.24). Invertase was present in EFN and its activity in FB and YL EFN did not differ across sites (H 5, 29 =6.71, P = 0.243; Table 2).
Essential (E) and non-essential (NE) amino acids were found in EFN (Table 2). In general, the total concentrations of E and NE amino acids in FB and YL EFN were not significantly different from each other across and within sites (Table 2). However, Agumbe FB EFN had the highest concentration of amino acids of all types of EFN examined; this EFN thus had the lowest sugar:amino acid ratio. FB EFN from Sampaji had the highest ratio owing to its low amino acid concentration (Table 2). Among YL EFNs, that from Sampaji also had the highest sugar:amino acid ratio, while that from Solaikolli had the lowest (Table 2). The concentrations of individual amino acids were the same in FB and YL EFN across sites with the exception of alanine which was higher in YL EFN (Supplemental Table 2). Within sites, most essential amino acid concentrations were higher in FB EFN at Agumbe, while those of phenylalanine (E) and alanine (NE) were higher in YL EFN at Solaikolli (Table 2, Supplemental Table 2). Of the 20 normally occurring amino acids, the essential amino acids arginine, lysine and histidine and the non-essential asparagine, cysteine and glutamic acid were not detected (Supplemental Table 2). FB EFN from Agumbe and YL EFN from Solaikolli had amino acid compositions, 7 E + 4 NE and 5 E + 5 NE, respectively, that were more diverse, especially with regard to essential amino acids, than other FB and YL EFNs (Supplemental Table 2).
We evaluated overall differences between nectar types at the various sites by considering sucrose, glucose, and fructose, as well as essential and non-essential amino acids (Table 3). We found a significant effect of site and nectar type as well as a significant interaction effect between site and nectar type (Table 3). A separate multivariate analysis using the three main sugars but not the amino acids gave the same results including the same amount of explained variation (Table 3). There was a significant site, nectar type, and interaction effect with essential amino acids but not with non-essential amino acids (Table 3), indicating that essential amino acids play a significant role in EFN composition at some sites (Table 2). These results taken together indicate that while FB and YL EFNs can be different, the extent and nature of the variation is site-specific. Therefore, locally-specific interactions with ants is possible.
Differential Utilization of Solutions by Ants in Cafeteria Assays
A total of 36 ant species fed on the different aqueous solutions at the three sites, and these ants differed in their presence and relative abundance across sites (Shenoy and Borges, 2010). Since the most abundant and agressive ant species feeding on these solutions were also the most abundant consumers of EFN of H. brunonis (Shenoy and Borges, 2010), we present the results of differential consumption of various solutions for only these ant species. Additionally, these dominant species were recorded in large enough numbers to provide adequate sample sizes for statistical purposes. These dominant ants were the myrmicines Crematogaster dohrni Mayr (Agumbe) and Myrmicaria brunnea Saunders (Sampaji), and the dolichoderine Technomyrmex albipes Smith (Solaikolli). The results we present for each ant species could be site-specific, and this must be taken into consideration when interpreting them. Throughout this paper, results for C. dohrni are presented only for Agumbe, for M. brunnea only for Sampaji, and for T. albipes only for Solaikolli.
Cafeteria Assays Using Standard Solutions of Sugars and Amino Acids
Overall, the cafeteria choice assays demonstrated that the preferences of ant species for nutrients may depend on the available choices in the experimental system. However, some results were consistent for assays with simple sugar and amino acid solutions and those with EFN mimics; those results are highlighted.
When presented simultaneously with low (5% or 20%) concentrations of sucrose, glucose, or fructose, C. dohrni and T. albipes preferred sucrose compared to glucose or fructose (Experiments 1a–1 d, Table 1; Fig. 1a and c). Myrmicaria brunnea did not discriminate between sugars at any concentrations (Experiments 1a–1 d; Fig. 1b).
Effect of sugar concentration. a-c, Ant visits to vials of different sugars at the same concentration a: Crematogaster dohrni in Agumbe; b: Myrmicaria brunnea in Sampaji; c: Technomyrmex albipes in Solaikolli. d-f, Ant visits to vials of equicaloric low or high sugar concentrations d: Crematogaster dohrni in Agumbe; e: Myrmicaria brunnea in Sampaji; f: Technomyrmex albipes in Solaikolli. Ant visitation was quantified as mean number of ants per sugar solution at each sampling point. Means + SE followed by the same letter within the same experiment are not significantly different (Kruskal-Wallis tests followed by Mann-Whitney U tests after appropriate Bonferroni correction)
The ant species at the three sites differed in their responses to equicaloric solutions of sucrose, glucose, and fructose. Crematogaster dohrni consumed all solutions equally (Experiments 2a–2b; Fig. 1d). Myrmicaria brunnea preferred glucose and fructose solutions over those of sucrose at both caloric levels (Experiments 2a–2b; Fig. 1e). Technomyrmex albipes preferred solutions of glucose over those of sucrose or fructose at low caloric values (Experiment 2a; Fig. 1f). However, T. albipes preferred sugars in the order sucrose > glucose > fructose at high caloric values (Experiment 2b; Fig. 1f). Therefore, C. dohrni appeared equally attracted to all sugars providing the same caloric value (low or high), while M. brunnea when faced with a choice of a disaccharide (sucrose) or a monosaccharide (glucose or fructose) chose the monosaccharide, indicating a general preference for monosaccharides rather than a preference for a particular sugar. Technomyrmex albipes appeared to be more responsive to sugar identity than C. dohrni and M. brunnea by preferring glucose at low caloric values and sucrose at high caloric values.
Ant species differed in their response when simultaneously presented with solutions containing the same sugar at different concentrations (Experiment 3, Table 1). Crematogaster dohrni preferred solutions with higher concentrations of sugars (Experiments 3a–3c; Fig. 2a). Myrmicaria brunnea preferred the highest available concentration of sucrose (Fig. 2b). Technomyrmex albipes consumed only higher concentration solutions of glucose to a significantly greater extent over lower concentrations (Fig. 2c).
Ant visits to vials of different concentrations of the same sugar presented simultaneously a: Crematogaster dohrni in Agumbe; b: Myrmicaria brunnea in Sampaji; c: Technomyrmex albipes in Solaikolli. Ant visitation was quantified by mean number of ants per sugar solution at each sampling point. Means + SE followed by the same letter within the same experiment are not significantly different (Kruskal-Wallis tests followed by Mann-Whitney U tests after appropriate Bonferroni correction)
In experiments with solutions containing mixtures of sugars and amino acids, C. dohrni consumed all solutions equally (Experiment 4; Fig. 3a), while M. brunnea preferred solutions with amino acid groups E1N1, E1N2, and E2N1 (Fig. 3b). Only T. albipes significantly utilized the solution containing all essential amino acids (solution E) to a greater extent over other solutions (Fig. 3c). Technomyrmex albipes thus exhibited greater responsiveness to essential amino acid contents (which could be their specific dietary targets) compared to C. dohrni and M. brunnea.
Ant visits to vials containing solutions of different combinations of amino acids a: Crematogaster dohrni in Agumbe; b: Myrmicaria brunnea in Sampaji; c: Technomyrmex albipes in Solaikolli. Means + SE followed by the same letter are not significantly different (Kruskal-Wallis tests followed by Mann-Whitney U tests after appropriate Bonferroni correction)
Cafeteria Assays Using Solutions Mimicking Familiar and Non-Familiar Floral Bud EFN
Familiarity did not influence consumption of FB EFN mimics. When presented simultaneously with FB EFN mimics from familiar and non-familiar sites, C. dohrni did not differentiate between mimics of the familiar location (Agumbe) and unfamiliar locations (Sampaji, Solaikolli) (Fig. 4a). Similarly, M. brunnea did not differentiate between mimics of familiar (Sampaji) and unfamiliar (Agumbe, Solaikolli) locations (Fig. 4b) (Experiment 5a). Technomyrmex albipes preferred the non-familiar mimics of low concentration FB EFN from Agumbe or Sampaji over the familiar mimics of FB EFN from Solaikolli (Fig. 4c). The mimics of low concentration FB EFN that were preferred by T. albipes had neither the highest amino acid concentration of the FB EFN mimics nor the lowest sugar:amino acid ratio (Supplemental Table 1) but had the lowest viscosity (Fig. 5a). Furthermore, the mimic of Sampaji FB EFN preferred by T. albipes did not contain any amino acids, but had a low sugar content similar to that of the low concentration Agumbe FB EFN (Supplemental Table 1, Fig. 4c). These results are consistent with those from Experiments 1 and 2 (Fig. 1c, f) in which sugar concentrations up to 20% were preferred by this ant.
Ant visits to vials containing solutions that mimic EFN and caloric content and amino acid content for each tested solution. a-c, floral bud EFN mimics a: Crematogaster dohrni; b: Myrmicaria brunnea; c: Technomyrmex albipes. d-f, young leaf EFN mimics d: Crematogaster dohrni; e: Myrmicaria brunnea; f: Technomyrmex albipes. Means + SE showing the number of ants per vial followed by the same letter are not significantly different (Kruskal-Wallis tests followed by Mann-Whitney U tests after appropriate Bonferroni correction)
Cafeteria Assays Using Solutions Mimicking Familiar and Non-Familiar Young Leaf EFN
Familiarity did not influence consumption of YL EFN mimics. When presented simultaneously with mimics of YL EFN from familiar and non-familiar sites, all three ant species preferred the mimic of high concentration YL EFN from Solaikolli over all others (Experiment 5b; Fig. 4d, e, f). Technomyrmex albipes clearly did not prefer the non-familiar mimic of high concentration YL EFN from Sampaji (Fig. 4f). This EFN mimic had the highest sugar concentration and viscosity among YL EFN mimics (Figs. 4f, 5b). The mimic of high concentration YL EFN from Solaikolli that was preferred by all three ant species had the lowest ratio of sugars to amino acids of all YL EFN mimics (Supplemental Table 1) and also the lowest viscosity (Fig. 5b).
Cafeteria Assays Using Solutions Mimicking Familiar Floral Bud and Young Leaf EFN
In a choice between solutions mimicking FB or YL EFN from their native site, C. dohrni utilized all solutions equally (Experiment 6; Fig. 6a). Myrmicaria brunnea preferred FB mimics over high concentration YL EFNs (Fig. 6b). These FB mimics had sugar concentrations in the 40% range and no amino acids (Supplemental Table 1). These results are consistent with those of Experiment 3 in which M. brunnea preferred 35% and 50% sucrose and glucose solutions (Fig. 2b). Technomyrmex albipes preferred mimics of either high or low concentration YL EFN over FB EFN mimics (Fig. 6c). These YL EFNs had lower sugar levels than those of FB EFNs (Supplemental Table 1). These results are also consistent with those from Experiments 1 and 2 (Fig. 1c, f) in which sugar concentrations up to 20% were most utilized by this ant. Among the four solutions presented in this test, only the high concentration YL EFN from Solaikolli had amino acids (Supplemental Table 1).
Ant visits to vials containing solutions that mimic floral bud and young leaf EFN from each site a: Crematogaster dohrni in Agumbe; b: Myrmicaria brunnea in Sampaji; c: Technomyrmex albipes in Solaikolli. Means + SE showing the number of ants per vial followed by the same letter are not significantly different (Kruskal-Wallis tests followed by Mann-Whitney U tests after appropriate Bonferroni correction)
Differences in Viscosity of the EFN Mimics
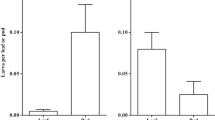
The viscosity of the solution mimicking high concentration FB EFN from Agumbe (1065 mPa∙s) was 400- to 600-fold higher than that of the other FB EFN mimics (Fig. 5a). The viscosity of the solution mimicking high concentration YL EFN from Sampaji (270 mPa∙s) was 50- to 200-fold higher than that of other YL EFN mimics (Fig. 5b). The high viscosities are a result of high sugar concentrations (Table 2, Supplemental Table 1) resulting in crystalline EFN in some cases. For comparison, note that glycerin has a viscosity of about 1500 mPa∙s while the viscosity of motor oils varies between 100–500 mPa∙s.
Discussion
EFN Chemistry in H. brunonis Relative to Other Plants
Overall, the sugar composition of H. brunonis EFN was similar to that of other plants (Baker et al., 1978; Koptur, 1992). However, the total amino acid concentration in some sites (Table 2) was at the higher end of the EFN range reported in the literature (3 × 10−5 to 2.67 g/100 ml) with most other plant species having EFN amino acid concentrations in the range of 3 × 10−2 g/100 ml (Koptur, 1979; Inouye and Inouye, 1980; Caldwell and Gerhardt, 1986; Heil et al., 2000; Blüthgen et al., 2004). Consequently, the sugar:amino acid ratios in H. brunonis EFN were lower than in EFNs of most plants (Davidson and Cook, 2008; Ness et al., 2009). This could be because H. brunonis is a legume and is not nitrogen limited (McKey, 1994), or because it is a myrmecophyte that does not produce food bodies but provides amino acids to its resident ants via EFN thus improving their nutritional balance and ensuring their fidelity. The invertase activity of H. brunonis EFN (Table 2) is similar to that of the EFN produced by generalist (0.01–0.09 μg glucose μl −1 min−1) as compared to specialized (0.73–1.52 μg glucose μl −1 min−1) ant-Acacia plant species (Heil et al., 2005), suggesting a generalist interaction between H. brunonis and its EFN-consuming ants.
There was between- and within-site variation in EFN composition and significant interaction effects between site and EFN type. The causes for such variation may include microhabitat heterogeneity in soil, humidity, and exposure to microbes that produce invertase and other enzymes that alter EFN composition (Inouye and Inouye, 1980; Koptur, 1994; Heil et al., 2005). The highly viscous EFN at Agumbe, for example, may result from the low canopy cover and resulting evaporation.
Partner-Choice Mechanisms: Role of EFN Composition in Maintaining Ant–Plant Associations
Humboldtia brunonis produces EFN only during the dry season when ant activity is high (Basu, 1997). Such a brief period of EFN availability may preclude long-term specialization by ants (Schemske, 1982) on H. brunonis EFN. In this system, therefore, the short-term partners are likely to be those ant species that discriminate between plants or plant parts through the quality of the seasonal EFN they provide. Our results indicate that in these species, familiarity with the composition of seasonally available EFNs did not influence their utilisation by local ants, unlike some other ant species that are specialized on nectar sources such as honeydew (Völkl et al., 1999; Tinti and Nofre, 2001) or EFN (González-Teuber and Heil, 2009b). We found a single dominant ant species at each site feeding on EFN or on experimental solutions. Of these, C. dohrni at Agumbe is a castration parasite of H. brunonis (Gaume et al., 2005a), M. brunnea at Sampaji is largely a tender of homopterans and a non-predatory scavenger (Gaume et al., 2005a), while the dolichoderine T. albipes at Solaikolli is a mutualist and provides protection to H. brunonis from herbivores (Gaume et al., 2005a; 2005b; Shenoy and Borges, 2010).
The biology of the ants was consistent with their particular nectar preferences. The castration parasite C. dohrni appeared least selective in all tests and did not appear to have specific dietary targets or constraints under the experimental conditions. Whether castration parasitism is coupled with nutrient opportunism in ant–plant interactions is unknown. The largely trophobiont-tending non-protective M. brunnea was attracted to solutions with high sucrose concentrations. It is possible that since trophobiont-tending provides access to sufficient quantities of very dilute sugars in honeydew (Woodring et al., 2004; Detrain et al., 2010), M. brunnea values only high sugar concentration EFN sources. The mutualistic dolichoderine T. albipes demonstrated definite feeding preferences as well as constraints. Dolichoderines such as T. albipes, unlike myrmicines, are constrained by their proventricular anatomy to consume more liquids than solids (Davidson, 1997; Davidson et al., 2004). Technomyrmex albipes had a clear preference for dilute solutions with low viscosity, and also those with the lowest sugar:amino acid ratios. Whether the viscosity constraint results from its proventricular anatomy is unknown. Furthermore, T. albipes preferred solutions with essential amino acids. It is possible that T. albipes does not harbor endoysmbionts that synthesize essential amino acids, as occurs in specialized plant-ants such as Dolichoderus (Schroder et al., 1996; Cook and Davidson, 2006). These constraints could explain why T. albipes preferred those EFN mimics that had sugars in the lower concentration range, lower viscosities, and that contained essential amino acids. The YL EFN mimic from Solaikolli that was most strongly preferred by T. albipes was also the one that had the largest number of amino acids of which most were essential amino acids (Supplemental Information Table 1). We suggest that these essential amino acids could be the specific dietary targets (sensu Simpson and Raubenheimer, 1996) that T. albipes seeks in plant-based exudates. The preferential foraging by T. albipes on individual plants with suitable EFN could also result in greater patrolling or nesting on these plants, and may explain the increased fitness of certain individual trees occupied by T. albipes (Gaume et al., 2005b).
Our study also suggests that the chemical composition of EFN can reduce ant–pollinator conflict, at least in the mutualist T. albipes via its decreased utilization of floral bud EFN. Spatial, temporal, and chemical resolution of ant–pollinator conflicts via floral attributes is known (Raine et al., 2002; Nicklen and Wagner, 2006), but the role of the chemical composition of EFN in ant–pollinator conflict is unexplored. Technomyrmex albipes preferred leaf over floral bud EFN mimics suggesting that EFN composition can play a pivotal role in minimizing possible antagonistic interactions between ants and pollinators. Technomyrmex albipes protects young leaves to a greater extent than floral buds resulting in 24.5% reduction of herbivory on patrolled leaves vs. 16% reduction on patrolled floral buds (Shenoy and Borges, 2010). This difference in protection of plant parts may be due to differential utilization of floral and leaf EFN resulting from their chemical differences. Differential protection of foliar compared to reproductive structures was found in one other ant-plant system (Palmer and Brody, 2007) and was attributed to the fact that only leaves afforded the ants with nutrition and/or housing in the form of domatia.
Our study has shown that local variation in dominant ant species coupled with local variation in plant reward attributes may lead to a local and seasonal protection mutualism between ants and plants. We demonstrated: (i) the ability of mutualistic ant species to choose between food sources that differ in quality; (ii) how this ability to choose could lead to selection of trees or of particular plant tissues producing suitable EFN; and (iii) how ant preference for specific EFN compositions could be based on anatomical/physiological constraints, sugar:amino acid ratios or specific dietary targets but not on familiarity of nutrients. These findings improve our understanding of the extent to which EFN composition can influence the identity and abundance of ants that are attracted to EFN and thereby alter the nature of ant–plant interactions.
References
Anderson, M. J. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26:32–46.
Baker, H. G., Opler, P. A., and Baker, I. 1978. A comparison of the amino acid complements of floral and extrafloral nectars. Bot. Gaz. 139:322–332.
Basu, P. 1997. Seasonal and spatial patterns in ground foraging ants in a rain forest in the Western Ghats, India. Biotropica 29:489–500.
Blüthgen, N., and Fiedler, K. 2004. Preferences for sugars and amino acids and their conditionality in a diverse nectar-feeding ant community. J. Anim. Ecol. 73:155–166.
Blüthgen, N., Gottsberger, G., and Fiedler, K. 2004. Sugar and amino acid composition of ant-attended nectar and honeydew sources from an Australian rainforest. Austral Ecol. 29:418–429.
Blüthgen, N., Verhaagh, M., Goitía, W., Jaffé, K., Morawetz, W., and Barthlott, W. 2000. How plants shape the ant community in the Amazonian rainforest canopy: the key role of extrafloral nectaries and homopteran honeydew. Oecologia 125:229–240.
Caldwell, D. I., and Gerhardt, K. O. 1986. Chemical analysis of peach extrafloral nectary exudate. Phytochemistry 25:41–43.
Chamberlain, S. A., and Holland, J. N. 2008. Density-mediated context-dependent consumer-resource interactions between ants and extrafloral nectar plants. Ecology 89:1364–1374.
Cook, S. C., and Davidson, D. W. 2006. Nutritional and functional biology of exudate-feeding ants. Entomol. Exp. Appl. 118:1–10.
Davidson, D. W. 1997. The role of resource imbalances in the evolutionary ecology of tropical arboreal ants. Biol. J. Linn.Soc. 61:153–181.
Davidson, D. W., and Cook, S. C. 2008. Tropical arboreal ants: linking nutrition to roles in rainforest ecosystems. pp. 334–348 in W. CARSON and S. SCHNITZER (eds.). Tropical Forest Community Ecology. Wiley-Blackwell, UK.
Davidson, D. W., and McKey, D. B. 1993. The evolutionary ecology of symbiotic ant-plant relationships. J. Hymen. Res. 2:13–83.
Davidson, D. W., Cook, S. C., and Snelling, R. R. 2004. Liquid-feeding performances of ants (Formicidae): ecological and evolutionary implications. Oecologia 139:255–266.
Detrain, C., Verheggen, F. J., Diez, L., Wathelet, B., and Haubruge, E. 2010. Aphid–ant mutualism: how honeydew sugars influence the behaviour of ant scouts. Physiol. Entomol. 35:168–174.
Gaume, L., Zacharias, M., and Borges, R. M. 2005a. Ant–plant conflicts and a novel case of castration parasitism in a myrmecophyte. Evol. Ecol. Res. 7:435–452.
Gaume, L., Zacharias, M., Grosbois, V., and Borges, R. M. 2005b. The fitness consequences of bearing domatia and having the right ant partner: experiments with protective and non-protective ants in a semi-myrmecophyte. Oecologia 145:76–86.
Gaume, L., Shenoy, M., Zacharias, M., and Borges, R. M. 2006. Co-existence of ants and an arboreal earthworm in a myrmecophyte of the Indian Western Ghats: anti-predation effect of the earthworm mucus. J. Trop. Ecol. 22:1–4.
González-Teuber, M., and Heil, M. 2009a. Nectar chemistry is tailored for both attraction of mutualists and protection from exploiters. Plant Sign. Behav. 4:1–5.
González-Teuber, M., and Heil M. 2009b. The role of extrafloral nectar amino acids for the references of facultative and obligate ant mutualists. J. Chem. Ecol. 35:459–468.
Heil, M., and McKey, D. B. 2003. Protective ant–plant interactions as model systems in ecological and evolutionary research. Annu. Rev. Ecol. Evol. Syst. 34:425–453.
Heil, M., Fiala, B., Baumann, B., and Linsenmair, K. E. 2000. Temporal, spatial and biotic variations in extrafloral nectar secretion by Macaranga tanarius. Func. Ecol. 14:749–757.
Heil, M., Rattke, J., and Boland, W. 2005. Postsecretory hydrolysis of nectar sucrose and specialization in ant/plant mutualism. Science 308:560–563.
Heil, M., González-Teuber, M., Clement, L. W., Kautz, S., Verhaagh, M., and Silva-Bueno, J. C. 2009. Divergent investment strategies of Acacia myrmecophytes and the coexistence of mutualists and exploiters. Proc. Natl. Acad. Sci. USA 43:18091–18096.
Herrera, C.M., Pérez, R., and Alonso, C. 2006. Extreme intraplant variation in nectar sugar composition in an insect-pollinated perennial herb. Am. J. Bot. 93:575–581.
Inouye, D. W., and Inouye R. S. 1980. The amino acids of extrafloral nectar from Helianthella quinquenervis (Asteraceae). Am. J. Bot. 67:1394–1396.
Kay, A. 2004. The relative availabilities of complementary resources affect the feeding preferences of ant colonies. Behav. Ecol. 15:63–70.
Keeler, K. H. 1977. The extrafloral nectaries of Ipomoea carnea (Convolvulaceae). Am. J. Bot. 64:1182–1188.
Koptur, S. 1979. Facultative mutualism between weedy vetches bearing extrafloral nectaries and weedy ants in California. Am. J. Bot. 66:1016–1020.
Koptur, S. 1992. Extrafloral nectary-mediated interactions between insects and plants. pp. 81–129 in E. A. BERNAYS (ed.). Insect-Plant Interactions. CRC Press, Boca Raton.
Koptur, S. 1994. Floral and extrafloral nectars of Costa Rican Inga trees: a comparison of their constituents and composition. Biotropica 26:276–284.
Koptur, S., and Truong, N. 1998. Facultative ant–plant interactions: nectar sugar preferences of introduced pest ant species in south Florida. Biotropica 30:179–189.
Kost, C., and Heil, M. 2005. Increased availability of extrafloral nectar reduces herbivory in Lima bean plants (Phaseolus lunatus, Fabaceae). Basic Appl. Ecol. 6:237–248.
Lanza, J. 1988. Ant preferences for Passiflora nectar mimics that contain amino acids. Biotropica 20:341–344.
Lanza, J., Smith, G. C., Sack, S., and Cash, A. 1995. Variation in nectar volume and composition of Impatiens capensis at the individual, plant, and population levels. Oecologia 102:113–119.
McKey, D. B. 1994. Legumes and nitrogen: the evolutionary ecology of a nitrogen-demanding lifestyle. pp. 211–228 in J. I. SPRENT AND D. McKey (eds.). Advances in Legume Systematics, Part 5. Royal Botanic Gardens, Kew, U. K.
Ness, J. H. 2006. A mutualism’s indirect costs: the most aggressive plant bodyguards also deter pollinators. Oikos 113:506–514.
Ness, J. H., Morris, W. F., and Bronstein, J. L. 2009. For ant-protected plants, the best defense is a hungry offense. Ecology 90:2823–2831.
Nicklen, E. F, and Wagner, D. 2006. Conflict resolution in an ant–plant interaction: Acacia constricta traits reduce ant costs to reproduction. Oecologia 148:81–87.
Nicolson, S. W. 2007. Amino acid concentrations in the nectars of Southern African bird-pollinated flowers, especially Aloe and Erythrina. J. Chem. Ecol. 33:1707–1720.
Oksanen, J., Blanchet F. G., Kindt R., Legendre P., O’hara R. B., Simpson G. L., Solymos P., Stevens M. H. H, and Wagner H. 2011. Vegan: community ecology package. Version 1.17-4. http://cran.r-project.org/web/packages/vegan
Palmer, T. M., and Brody, A. K. 2007. Mutualism as reciprocal exploitation: African plant-ants defend foliar but not reproductive structures. Ecology 88:3004–3011.
Pate, J. S., Peoples, M. B., Storer, P. J., and Atkins, C. A. 1985. The extrafloral nectaries of cowpea (Vigna unguiculata (L.) Walp.) II. Nectar composition, origin of solutes, and nectary functioning. Planta 166:28–38.
Raine, N. E., Willmer, P., and Stone, G. N. 2002. Spatial structuring and floral avoidance behavior prevent ant–pollinator conflict in a Mexican ant-acacia. Ecology 83:3086–3096.
Ramesh, B. R., and Pascal, J. P. 1997. Atlas of Endemics of the Western Ghats (India): Distribution of Tree Species in the Evergreen and Semi-evergreen Forests. French Institute of Pondicherry, Pondicherry, India.
Rudgers, J. A., and Gardener, M. C. 2004. Extrafloral nectar as a resource mediating multispecies interactions. Ecology 85:1495–1502.
Rudgers, J. A., Savage, A. M., and Rúa, M. A. 2010. Geographic variation in a facultative mutualism: consequences for local arthropod composition and diversity. Oecologia 163:985–996.
Schemske, D. W. 1982. Ecological correlates of a neotropical mutualism: ant assemblages at Costus extrafloral nectaries. Ecology 63:932–941.
Schroder, D., Deppisch, H., Obermayer, M., Krohne, G., Stackebrandt, E., Hölldobler, B., Goebel, W., and Gross, R. 1996. Intracellular endosymbiotic bacteria of Camponotus species (carpenter ants): systematics, evolution and ultrastructural characterization. Mol. Microbiol. 21:479–489.
Shenoy, M. and Borges, R. M. 2010. Geographical variation in an ant–plant interaction correlates with domatia occupancy, local ant diversity and interlopers. Biol. J. Linn. Soc. 100:538–551.
Simpson S. J., and Raubenheimer, D. 1996. Feeding behaviour, sensory physiology and nutrient feedback: a unifying model. Entomol. Exp. Appl. 80:55–64.
Tinti, J. M., and Nofre, C. 2001. Responses of the ant Lasius niger to various compounds perceived as sweet in humans: a structure-activity relationship study. Chem. Senses 26:231–237.
Völkl, W., Woodring, J., Fischer, M., Lorenz, M. W., and Hoffmann, K. H. 1999. Ant-aphid mutualisms: the impact of honeydew production and honeydew sugar composition on ant preferences. Oecologia 118:483–491.
Wagner, D., and Nicklen, E. F. 2010. Ant nest location, soil nutrients and ant-associated plants: does extrafloral nectar attract ant nests and thereby enhance plant nutrition? J. Ecol. 98:614–624.
Woodring, J., Wiedemann, R., Fischer, M. K., Hoffmann, K. H., and Völkl, W. 2004. Honeydew amino acids in relation to sugars and their role in the establishment of ant-attendance hierarchy in eight species of aphids feeding on tansy (Tanacetum vulgare). Physiol. Entomol. 29:311–319.
Acknowledgements
This work was funded by the Ministry of Environment and Forests, Government of India. We are grateful to T. M. Musthak Ali for ant identification. We thank Anupama, Karthik, Megha, Priya, Shaji, Sivan, Sudheesh, and G. Yathiraj for assistance, P. Balaram and S. Chandrasekaran for help with the GC-MS analysis, and the Karnataka Forest Department for research permissions. We are especially grateful to Yuvaraj Ranganathan for doing the multivariate analyses and for help with the final figures. We thank Judie Bronstein, Doyle McKey, Martin Heil, and an anonymous reviewer for helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 396 kb)
Rights and permissions
About this article
Cite this article
Shenoy, M., Radhika, V., Satish, S. et al. Composition of Extrafloral Nectar Influences Interactions between the Myrmecophyte Humboldtia brunonis and its Ant Associates. J Chem Ecol 38, 88–99 (2012). https://doi.org/10.1007/s10886-011-0052-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-011-0052-z