Abstract
Larvae of nine species of sawflies (Symphyta) were fed with the foliage of three birch species, after which the larval hemolymph composition was studied by HPLC–DAD and HPLC–ESI–MS. The hemolymph of sawfly larvae contained high concentrations of flavonol oligoglycosides (tri-, tetra-, penta-, and hexaglycosides) that could not be found in the larval foliar diet. In addition, there were significant between-sawfly species differences in both flavonoid composition and concentration (from 0.6 to 12.3 mg/ml) of the hemolymph. This suggested that the studied species have different biosynthetic activities for the synthesis of flavonoid oligoglycosides. Variation in the foliar diets did not cause differences in the hemolymph composition. Our hypothesis is that sawflies use foliar flavonoid monoglycosides rather than flavonoid aglycones to produce these new types of oligoglycosides. These findings open up new possibilities for understanding the more holistic role of flavonoids in insect biochemistry and complex interactions between plants and herbivores.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flavonoids are a well-known group of phenolic compounds due to their ubiquitous presence in nature and potential health–promoting effects on humans (Iwashina, 2000; Ross and Kasum, 2002). The role of flavonoids in plant-herbivore interactions is complex, as they can function both as feeding stimulants and anti-feedants (Simmonds, 2001, 2003; Cipollini et al., 2008). In addition, some insects have developed methods to utilize flavonoids to increase their fitness. Some butterfly species can e.g., sequester flavonoids into their wing pigments that can help in mate recognition (Geuder et al., 1997; Burghardt et al., 2000).
Earlier studies with birch-feeding insect herbivores have shown that the lipophilic flavonoid aglycones of the epidermal layer of birch leaves act as defensive compounds e.g., against the larvae of autumnal moth (Epirrita autumnata) (Lahtinen et al., 2004). However, insects also have developed methods to avoid the harmful effects of these defenses. Epirrita autumnata and other birch-feeding insects—such as sawfly larvae—were shown to partially detoxify flavonoid aglycones by glycosylation (Salminen et al., 2004; Lahtinen et al., 2005); addition of a single sugar unit into the lipophilic flavonoid aglycone enables the excretion of the formed water-soluble flavonoid monoglycoside into the feces of larvae. However, not all flavonoid aglycones were glycosylated. Only 22% of the ingested flavonoid aglycones were glycosylated by E. autumnata larvae (Salminen et al., 2004), and with sawfly larvae the glycosylation percentages varied from 35 to 44%, depending on the species (Lahtinen et al., 2005). It has remained unclear what the metabolic fate for the remaining portion of ingested flavonoid aglycones is, since only traces were found excreted as such in the feces, and the new fecal monoglycosides did not represent even one half of the ingested amounts of aglycones.
The purpose of this study was to clarify whether any of the missing portions of flavonoid aglycones could be found in larval hemolymph. We had not studied larval hemolymph composition earlier. Therefore we first did some preliminary HPLC-analyses of hemolymph with several species of insect herbivores. We found no flavonoids in the hemolymph of lepidopteran larvae (E. autumnata and Agriopis aurantiaria), possibly because of the high polyphenol oxidase activities in their hemolymph (Ruuhola and Salminen, unpublished data). On the contrary, hemolymph of sawfly larvae contained high levels of flavonoids. To study this phenomenon further, we collected a diverse set of larvae from nine species of birch-feeding sawflies that grew in the northern part of Finland. Each larval species was fed with the foliage of three birch species. These birches were known to differ in their flavonoid aglycone composition and content (Valkama et al., 2003, 2004). Our hypothesis was that different flavonoid aglycone compositions of the three types of foliar diets would be seen directly as differential flavonoid composition of the hemolymph samples.
Methods and Materials
Insect Bioassay and Collection of the Hemolymph
Field experiments were conducted at the Kevo Subarctic Research Station in Utsjoki, northern Finland (69°45’N, 27°00’E). The forest of the study area is dominated by mountain birch (Betula pubescens subsp. Czerepanovi (Orlova) Hämet-Ahti) that is a tree line forming species in northern Fennoscandia. The larvae used in the experiment originated from a field grown ‘laboratory stock’ replenished by yearly collections of wild larvae. Each year pairs of adult males and females were enclosed in mesh bags on mountain birch branches, and the hatching larvae were allowed to feed on naturally growing leaves until the end of the last instar. Prepupating larvae were placed in plastic vials containing moist sphagnum moss where the larvae formed cocoon for overwintering. The species selected for the experiment, from the phenologically earliest to the latest, were: Amauronematus amplus Konow, Pristiphora alpestris Konow, Nematus viridescens Cameron, Nematus brevivalvis Thomson, Arge sp. Schrank, Nematus pravus Konow, Trichiosoma scalesii Leach, Nematus viridis Stephens, and Dineura pullior Schmidt. Arge sp. belongs to the family Argidae, Trichiosoma scalesii to the family Cimbicidae, while the rest are members of the subfamily Nematinae in the family Tenthredinidae.
During the summer of 2004 emerging adult females were allowed to feed on honey water, after which they were enclosed in mesh bags on the branches of haphazardly chosen mountain birch trees. Larvae were allowed to feed in the mesh bags until 2nd–3rd instar, after which the bags were cut down and the larvae were taken into the laboratory. The larvae were divided into three groups to be fed with leaves from either mountain birch, dwarf birch (Betula nana L.), or silver birch (Betula pendula Roth). We used the same three mountain and silver birch individuals throughout the experiment. Since dwarf birches are so small in size, we used haphazardly chosen tree individuals for feeding until the last 4 d of the experiment when the larvae were fed with leaves from the chosen three experimental trees. Larvae were grown singly in 48 ml plastic vials and fed with new leaves every 3rd d. When the larvae had reached their last instar, they were preserved in liquid nitrogen and transferred to the University of Turku in southern Finland (Table 1). Hemolymph sampling was done by piercing a small hole with a needle into the integument of larva and then gently pressing out a small droplet of hemolymph.
HPLC and MS Analyses
Hemolymph was analyzed immediately after its collection by HPLC–DAD without any purification steps. Samples were manually injected (5 or 20 μl) because of the small volumes. The HPLC column was LiChroCART Superspher 100 RP-18 (75 × 4 mm I.D., 4 μm, Merck, Germany). The HPLC–DAD system (Merck-Hitachi, Tokyo, Japan) consisted of a manual Rheodyne injector, an L-7100 pump, an L-7455 diode array detector, and a D-7000 interface module. Two solvents were used: 0.05 M H3PO4 (A) and acetonitrile (B). The elution profile was: 0–3 min, 100% A; 3–20 min, 0–25% B in A (linear gradient); 20–23 min, 25–70% B in A (linear gradient); 23–47 min, column wash and stabilization. The flow rate was 1 ml/min. The UV spectra of hemolymph components were acquired between 195 and 450 nm, and detection wavelength was 341 nm. Additional HPLC–ESI–MS analyses were done by a Perkin-Elmer Sciex API 365 triple quadrupole mass spectrometer (Sciex, Toronto, Canada) that has ion spray interface and Analyst Software 1.1 for data handling. The column used and chromatographic conditions were the same as in Salminen et al. (1999). The mass spectrometer was used in the positive ion mode, and mass spectra were obtained between 100 to 1600 atomic mass units.
Flavonoid Quantification
The total flavonoid contents of hemolymph samples were quantified from the HPLC–DAD analyses at 341 nm. First, we detected all such HPLC peaks that had UV spectra characteristic of flavonoids. Then the HPLC peak areas of all the flavonoids were summed up to obtain the total flavonoid content of each sample. Flavonoids were quantified as quercetin equivalents using quercetin (Sigma Chemical Co, St. Louis, MO, USA) as an external standard.
Results
At first, hemolymph samples of nine sawfly species were analyzed by HPLC–DAD. In the HPLC–DAD chromatograms most of the peaks eluted shortly after 12 min, and many of the peaks co-eluted and produced a hump (Fig. 1). The short elution time of peaks suggested that the compounds were hydrophilic and they might contain sugars. The unknown compounds could not be simple flavonoid aglycones or monoglycosides that were known to elute much later in the chromatogram (Ossipov et al., 1995, 1996; Valkama et al., 2003). These data suggested that the unknown compounds might contain at least two sugar units. The UV spectra of the main compounds were characteristic of flavonols (Fig. 1). In flavonol aglycones such as kaempferol, quercetin, and myricetin, the band I UV absorptions vary between 359–370 nm. However, the band I UV absorptions of unknown hemolymph compounds were mainly between 341–349 nm. This kind of hypsochromic shift in band I maxima has been seen associated with the glycosylation of flavonols (Markham and Mabry, 1975).
Hemolymph samples then were analyzed by HPLC–ESI–MS, but there was enough hemolymph material for only seven of the nine sawfly species: Amauronematus amplus, Arge sp., Nematus brevivalvis, Nematus pravus, Nematus viridis, Pristiphora alpestris, and Trichiosoma scalesii. Time range 11–19 min in the HPLC-chromatograms was named as a “flavonoid part” of the chromatogram, and mass spectra were first acquired from this area (Fig. 2). Most of the mass spectra from the “flavonoid part” had an ion at m/z 303, which fit with the flavonol aglycone quercetin (Kite et al., 2007; Veitch et al., 2008). In addition, a clear pattern of ions could be seen in the mass spectra of different sawfly species: quercetin was followed by ions differing by 162 Da from each other: 465, 627, 789, 951, 1113, and 1275. Value 162 Da indicated a glycosidic bond between quercetin and carbohydrate units (glycohexopyranose e.g., glucose or galactose). The ions 465, 627, 789, 951, 1113, or 1275 could derive from molecular ions ([M + H]+) of flavonol monoglycosides or oligoglycosides, or they could be fragments of these ions. For this reason, each peak in the HPLC chromatograms was analyzed separately for its composition using single ion monitoring. This analysis revealed that all main peaks in the chromatograms belonged to individual flavonol oligoglycosides i.e., compounds where several carbohydrates were attached to a flavonol nucleus. The molecular ion of flavonol oligoglycoside was always preceded by its mass fragments, and each fragment represented a loss of one carbohydrate unit from the original flavonol oligoglycoside structure. The largest oligoglycoside was a quercetin hexaglycoside (m/z 1275), but quercetin tri- (m/z 789), tetra- (m/z 951), and pentaglycosides (m/z 1113) also were present (Fig. 3). There were only traces of quercetin mono- (m/z 465) and diglycosides (m/z 627) in the samples. Interestingly, each sawfly species had its own selection of flavonol oligoglycosides in the hemolymph. Quercetin hexa- and pentaglycosides were found in three sawfly species: Arge sp., Nematus viridis, and Nematus brevivalvis, and the other species contained different compositions of quercetin tri- and tetraglycosides (Table 2).
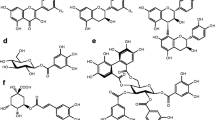
Positive ion mass spectra of flavonol oligoglycosides in the sawfly hemolymph. A: quercetin triglycoside, B: quercetin tetraglycoside, C: quercetin pentaglycoside, D: quercetin hexaglycoside E: quercetin tetraglycoside with one pentose sugar (m/z 921) co-eluting with a quercetin tetraglycoside (m/z 951), F: kaempferol pentaglycoside (m/z 1097) co-eluting with a quercetin hexaglycoside (m/z 1275). Interpretation of the ions: m/z 303 = quercetin; m/z 465 = quercetin with one hexose sugar; m/z 597 = quercetin with one hexose sugar and one pentose sugar; m/z 627 = quercetin with two hexose sugars; m/z 759 = quercetin with two hexose sugars and one pentose sugar; m/z 773 = kaempferol with three hexose sugars; m/z 789 = quercetin with three hexose sugars; m/z 921 = quercetin with three hexose sugars and one pentose sugar; m/z 935 = kaempferol with four hexose sugars; m/z 951 = quercetin with four hexose sugars; m/z 1097 = kaempferol with five hexose sugars; m/z 1113 = quercetin with five hexose sugars; m/z 1275 = quercetin with six hexose sugars; m/z 1577 = [2M + H]+ ion of a quercetin triglycoside. A hexose sugar refers to a glycohexopyranoside and a pentose sugar to a glycopentofuranoside
Many of the mass spectra had ions from the following ion pattern: m/z 597, 759, 921 and 1083. These ions were 30 Da less than the quercetin oligoglycoside ions next to them (represented by m/z 627, 789, 951, and 1113). This indicated that one of the glycohexopyranosides in quercetin oligoglycosides was replaced by a glycopentofuranoside e.g., arabinose sugar that has a molecular mass 30 Da less than glucose or galactose. However, only one of the glycohexopyranosides in flavonol oligoglycosides was replaced by a glycopentofuranoside since there was no mass spectral evidence of flavonol oligoglycosides with two or more glycopentofuranosides. The largest quercetin oligoglycoside with a glycopentofuranoside sugar was a quercetin pentaglycoside in Nematus brevivalvis and Nematus viridis (m/z 1083). This compound had four glycohexopyranosides and one glycopentofuranoside attached to the quercetin backbone (Table 2).
Some of the mass spectra showed yet another ion pattern: m/z 287, 449, 611, 773, 935, and 1097. The ions in the pattern were divided from each other by 162 Da. Again, this indicated a glycosidic bond between a flavonoid and a glycohexopyranoside. The peak at m/z 287 was interpreted as kaempferol (Veitch et al., 2008), and the following ions represented several glycohexopyranosides attached to the kaempferol nucleus. The largest kaempferol oligoglycoside was a kaempferol pentaglycoside (m/z 1097) in Arge sp. (Table 2). Kaempferol oligoglycosides always were less common than quercetin oligoglycosides.
During the feeding experiments, each species of sawfly larvae were divided into three groups that were fed with different birch diets (B. nana, B. pendula, and B. pubescens subsp. Czerepanovi). Different birch diets had no significant effect on the hemolymph composition within the same sawfly species (data not shown). This means that, for instance, quercetin triglycosides were always the main metabolites in the hemolymph of Trichiosoma scalesii although the larvae had eaten different birch diets. The total flavonoid contents of hemolymph samples were quantified from the HPLC–DAD chromatograms (Fig. 4). Total flavonoid contents varied markedly among different sawfly species (from 0.6 to 12.3 mg/ml). Different birch diets did not have significant effects on the total flavonoid contents within the same sawfly species.
Discussion
Hemolymph of sawfly larvae was found to contain high concentrations of flavonol oligoglycosides. These compounds seem to be de novo metabolites i.e., produced by the sawfly larvae themselves since the diet of larvae, birch leaves, are known to contain only flavonol aglycones and monoglygosides (Ossipov et al., 1996; Keinänen and Julkunen-Tiitto, 1998; Lahtinen et al., 2005). Ability to glycosylate flavonoids also has been reported in insects other than sawfly larvae: Larvae of Epirrita autumnata detoxify flavonoid aglycones into flavonoid monoglycosides (Salminen et al., 2004); Dissoteira carolina grasshoppers glycosylate quercetin (Hopkins and Ahmad, 1991); Polyommatus icarus butterflies produce kaempherol 3-O-glucoside from dietary kaempherol (Wiesen et al., 1994); and Bombyx mori silkworms produce quercetin mono-, di-, and triglucosides that are not originally present in their diet (Tamura et al., 2002; Hirayama et al., 2008). Ferreres et al. (2007) studied the larvae of Pieris brassicae L. and found several quercetin and kaempherol derivatives that could be either sequestration or metabolism products of the insect.
This study raises the question of how flavonol oligoglycosides are metabolized by sawfly larvae. The studied birch species (B. nana, B. pendula, and B. pubescens subsp. czerepanovii) have different compositions and concentrations of epidermal flavonoid aglycones (Valkama et al., 2003, 2004), and our original hypothesis stated that different flavonoid aglycone compositions of foliar diets would be seen as differential flavonoid compositions of the hemolymph samples. It could also be possible that sawfly larvae use these epidermal flavonoid aglycones as a starting material for the flavonol oligoglycoside synthesis. However, our results showed that different birch diets did not have significant effects on the compositions of hemolymph samples or concentrations of flavonol oligoglycosides. Also, no trace of epidermal flavonoid aglycones (except kaempferol in kaempherol oligoglycosides) was found in the hemolymph. The main flavonol aglycone part of flavonol oligoglycosides in the sawfly hemolymph was quercetin but this compound seems to be absent from birch leaf epidermal flavonoid aglycones (Valkama et al., 2003; Lahtinen et al., 2006). These findings oppose the idea that sawfly larvae use epidermal flavonoid aglycones for flavonol oligoglycoside synthesis.
Additionally, this study could not answer our original question about the metabolic fate of birch-derived flavonoid aglycones in sawfly larvae. It seems likely that the larvae use birch leaf flavonol monoglycosides rather than flavonol aglycones for flavonol oligoglycoside synthesis. First, all the birch species in this study contain many vacuolic quercetin monoglycosides (Ossipov et al., 1996; Keinänen and Julkunen-Tiitto, 1998; Graglia et al., 2001; Riipi et al., 2002; Salminen and Lempa, 2002). The concentrations of vacuolic quercetin monoglycosides in birch leaves also are generally higher than the concentrations of other flavonol monoglycosides (kaempherol or myricetin). This would give a partial explanation of why the majority of flavonol oligoglycosides in sawfly hemolymph were quercetin oligoglycosides and why kaempferol oligoglycosides were less common. Birch leaves contain also myricetin monoglycosides, but the hemolymph samples did not show clear evidence of myricetin oligoglycosides. This can mean that the sawfly species of this study may not have the ability to synthesize myricetin oligoglycosides.
The exact structures of flavonol oligoglycosides are unknown. At least one of the sugar units has to be attached directly to a flavonol nucleus, and the remaining sugars will be attached to other hydroxyl groups in flavonol nucleus, or the sugar units may chain to each other. Examples of plant derived flavonol oligoglycosides (e.g., Kite et al., 2007; Taylor et al., 2007; Veitch et al., 2008) give insight about the possible conformations of sawfly flavonol oligoglycosides. To fully solve the structures, one needs to isolate individual flavonol oligoglycosides and determine their structures, e.g., by NMR techniques. However, one sawfly larva contains only a few microliters of hemolymph, and it would require substantial amounts of larval material (several hundreds of individuals) to produce enough hemolymph for the isolation process. With the sparsely populated birch-feeding sawfly larvae, it was beyond the scope of this study to collect such large amounts of hemolymph.
Flavonoid compositions and concentrations within one sawfly species were approximately the same among feeding experiments with different birch species. Small differences could be detected in some of the replicates: e.g., the larvae of Nematus viridis with one birch diet contained quercetin hexaglycosides but the same larvae with another birch diet contained only quercetin pentaglycosides. Species to species differences in total flavonoid concentrations and flavonol oligoglycoside compositions were more obvious (Fig. 4. and Table 2). Some sawfly species contained a series of quercetin oligoglycosides up to hexaglycosides, while other species produced only quercetin triglycosides. This suggested that the sawfly species have different biosynthetic abilities to produce flavonol oligoglycosides.
The total flavonoid contents of hemolymph samples were estimated with quercetin as an external standard. However, the use of quercetin as a standard for flavonol oligoglycosides poses a problem. With quercetin, the whole molecule exhibits UV absorption but the situation is different with flavonol oligoglycosides: in this case, only the flavonol part of the molecule shows UV absorption, and the sugar stays “invisible” to UV detection. This is due to different chemical structural properties of sugars and flavonols. The UV-absorbing chromophore of quercetin oligoglycosides is quercetin (302 Da). With quercetin triglycoside (788 Da), for instance, 302/788 = 38% of molecular mass of the molecule belongs to the UV-absorbing quercetin while 62% of the molecule stays invisible to UV detection. This means that the total flavonoid contents of hemolymph samples (0.6 to 12.3 mg/ml, Fig. 4) are underestimated when only a quercetin standard is used. To get more accurate results one needs an authentic flavonol oligoglycoside standard that is isolated from sawfly larvae. However, it was beyond the scope of this study to collect such large quantities of birch sawfly larvae. Our goal in future studies is to isolate flavonol oligoglycoside standards from larval hemolymph, and these standards will allow us to make more accurate estimations of the flavonol oligoglycoside concentrations in hemolymph samples.
The biological significance of flavonol oligoglycosides for sawfly larvae remains unknown. Glycosylation of flavonoid aglycones has been seen as a detoxification mechanism for the larvae of Epirrita autumnata (autumnal moth) (Salminen et al., 2004). Another study showed that sawfly larvae are capable of glycosylating birch leaf flavonoid aglycones and excreting them as flavonoid monoglycosides into their feces (Lahtinen et al., 2005). A simple hypothesis could propose flavonol oligoglycosides as detoxification products of sawfly metabolism. However, as shown by Salminen et al. (2004), mere flavonoid monoglycosides are hydrophilic enough to be excreted into feces. Why would sawfly larvae need to spend extra sugar units and energy for this oligoglycosylation process? Perhaps flavonol oligoglycosides have some other function for the sawfly larvae. One possibility is that larvae utilize plant secondary chemicals to promote their own defense strategies againts their natural enemies e.g., ants, parasites, or birds. Many Tenthredinidae (Hymenoptera) sawfly species exhibit a defensive strategy termed “easy bleeding”, which means the release of hemolymph through the integument as a result of even slight mechanical pressure or disturbance (Müller et al., 2001; Boevé and Schaffner, 2003; Prieto et al., 2007). We have observed easy bleeding in many of our study species too, but the role of flavonoid metabolism in this respect remains hypothetical. The biological role of flavonol oligoglycosides in sawfly hemolymph needs to be examined in more detail in future studies.
References
Boevé, J.-L., and Schaffner, U. 2003. Why does the larval integument of some sawfly species disrupt so easily? The harmful hemolymph hypothesis. Oecologia 134:104–111.
Burghardt, F., Knüttel, H., Becker, M., and Fiedler, K. 2000. Flavonoid wing pigments increase attractiveness of female common blue (Polyommatus icarus) butterflies to mate-searching males. Naturwissenschaften 87:304–307.
Cipollini, D., Stevenson, R., Enright, S., Eyles, A., and Bonello, P. 2008. Phenolic metabolites in leaves of the invasive shrub, Lonicera maackii, and their potential phytotoxic and anti-herbivore effects. J. Chem. Ecol. 34:144–152.
Ferreres, F., Sousa, C., Valentão, P., Pereira, J. A., Seabra, R. M., and Andrade, P. B. 2007. Tronchuda cabbae flavonoids uptake by Pieris brassicae. Phytochemistry 68:361–367.
Geuder, M., Wray, V., Fiedler, K., and Proksch, P. 1997. Sequestration and metabolism of host-plant flavonoids by the lycaenid butterfly Polyommatus bellargus. J. Chem. Ecol. 23:1361–1372.
Graglia, E., Julkunen-Tiitto, R., Shaver, G. R., Schmidt, I., Jonasson, S., and Michelsen, A. 2001. Environmental control and intersite variations of phenolics in Betula nana in tundra ecosystems. New Phytol. 151:227–236.
Hirayama, C., Ono, H., Tamura, Y., Konno, K., and Nakamura, M. 2008. Regioselective formation of quercetin 5-O-glucoside from orally administered quercetin in the silkworm, Bombyx mori. Phytochemistry 69:1141–1149.
Hopkins, T. L. and Ahmad, S. A. 1991. Flavonoid wing pigments in grasshoppers. Experimentia 47:1089–1091.
Iwashina, T. 2000. The structure and distribution of the flavonoids in plants. J. Plant Res. 113:287–299.
Keinänen, M. and Julkunen-Tiitto, R. 1998. High-performance liquid chromatographic determination of flavonoids in Betula pendula and Betula pubescens leaves. J. Chrom. A. 793:370–377.
Kite, G. C., Stoneham, C. A., and Veitch, N. C. 2007. Flavonol tetraglycosides and other constituents from leaves of Styphnolobium japonicum (Leguminosae) and related taxa. Phytochemistry 68:1407–1416.
Lahtinen, M., Salminen, J.-P., Kapari, L., Lempa, K., Ossipov, V., Sinkkonen, J., Valkama, E., Haukioja, E., and Pihlaja, K. 2004. Defensive effect of surface flavonoid aglycones of Betula pubescens leaves against first instar Epirrita autumnata larvae. J. Chem. Ecol. 30:2257–2268.
Lahtinen, M., Kapari, L., Ossipov, V., Salminen, J.-P., Haukioja, E., and Pihlaja, K. 2005. Biochemical transformation of birch leaf phenolics in larvae of six species of sawflies. Chemoecology 15:153–159.
Lahtinen, M., Lempa, K., Salminen, J.-P., and Pihlaja, K. 2006. HPLC analysis of leaf surface flavonoids for the preliminary classification of birch species. Phytochem. Anal. 17:197–203.
Markham, K. R. and Mabry, T. J. 1975. Ultraviolet-visible and proton magnetic resonance spectroscopy of flavonoids, pp. 45–77, in J. B. Harborne, T. J. Mabry, and H. Mabry (eds.). The Flavonoids. Chapman and Hall Ltd., London, Great Britain.
Müller, C., Agerbirk, N., Olsen, C. E., Boevé, J.-L., Schaffner, U., and Brakefield, P. M. 2001. Sequestration of host plant glucosinolates in the defensive hemolymph of the sawfly Athalia rosae. J. Chem. Ecol. 27:2505–2516.
Ossipov, V., Nurmi, K., Loponen, J. Prokopiev, N., Haukioja, E., and Pihlaja, K. 1995. HPLC Isolation and identification of flavonoids from white birch Betula pubescens leaves. Biochem. Syst. Ecol. 23:213–222.
Ossipov, V., Nurmi, K., Loponen, J., Haukioja, E., and Pihlaja, K. 1996. High-performance liquid chromatographic separation and identification of phenolic compounds from leaves of Betula pubescens and Betula pendula. J. Chrom. A 721:59–68.
Prieto, J. M., Schaffner, U., Barker, A., Braca, A., Siciliano, T., and Boevé, J.-L. 2007. Sequestration of furostanol saponins by Monophadnus sawfly larvae. J. Chem. Ecol. 33:513–524.
Riipi, M., Ossipov, V., Lempa, K., Haukioja, E., Koricheva, J. Ossipova, S., and Pihlaja, K. 2002. Seasonal changes in birch leaf chemistry: are there trade-offs between leaf growth and accumulation of phenolics? Oecologia 130:380–390.
Ross, J. A. and Kasum, C. M. 2002. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 22:19–34.
Salminen, J.-P. and Lempa, K. 2002. Effects of hydrolysable tannins on a herbivorous insect: fate of individual tannins in insect digestive tract. Chemoecology 12:203–211.
Salminen, J.-P., Ossipov., V., Loponen, J., Haukioja, E., and Pihlaja, K. 1999. Characterisation of hydrolysable tannins from leaves of Betula pubescens by high-performance liquid chromatography–mass spectrometry. J. Chrom. A 864:283–291.
Salminen, J.-P., Lahtinen, M., Lempa, K., Kapari, L., Haukioja, E., and Pihlaja, K. 2004. Metabolic modifications of birch leaf phenolics by an herbivorous insect: detoxification of flavonoid aglycones via glycosylation. Z. Naturforsch. 59c:437–444.
Simmonds, M. S. J. 2001. Importance of flavonoids in insect-plant interactions: feeding and oviposition. Phytochemistry 56:245–252.
Simmonds, M. S. J. 2003. Flavonoid-insect interactions: recent advances in our knowledge. Phytochemistry 64:21–30.
Tamura, Y., Nakajima, K., Nagayasu, K., and Takabayashi, C. 2002. Flavonoid 5-glucosides from the cocoon shell of the silkworm, Bombyx mori. Phytochemistry 59:275–278.
Taylor, W. G., Fields, P. G., and Sutherland, D. H. 2007. Fractionation of lentil seeds (Lens culinaris Medik.) for insecticidal and flavonol tetraglycoside components. J. Agric. Food Chem. 55:5491–5498.
Valkama, E., Salminen, J.-P., Koricheva, J., and Pihlaja, K. 2003. Comparative analysis of leaf trichome structure and composition of epicuticular flavonoids in Finnish birch species. Ann. Bot. 91:643–655.
Valkama, E., Salminen, J.-P., Koricheva, J., and Pihlaja, K. 2004. Changes in leaf trichomes and epicuticular flavonoids during leaf development in three birch taxa. Ann. Bot. 94:233–242.
Veitch, N. C., Kite, G. C., and Lewis, G. P. 2008. Flavonol pentaglycosides of Cordyla (Leguminosae: Papilionoideae: Swartzieae): Distribution and taxonomic implications. Phytochemistry 69:2329–2335.
Wiesen, B., Krug, E., Fiedler, K., Wray, V., and Proksch, P. 1994. Sequestration of host-plant-derived flavonoids by lycaenid butterfly. J. Chem. Ecol. 20:2523–2538.
Acknowledgements
We thank Maarit Karonen and Vladimir Ossipov for their comments on the earlier versions of the manuscript. This work was supported by a grant from Magnus Ehrnrooth Foundation and grant no. 119659 from the Academy of Finland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vihakas, M.A., Kapari, L. & Salminen, JP. New Types of Flavonol Oligoglycosides Accumulate in the Hemolymph of Birch-Feeding Sawfly Larvae. J Chem Ecol 36, 864–872 (2010). https://doi.org/10.1007/s10886-010-9822-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-010-9822-2