Abstract
In many insects, mate finding is mediated by volatile sex pheromones, but evidence for this phenomenon in triatomines (Heteroptera: Reduviidae) is still fragmentary. Recently, it was shown that metasternal glands (MGs) are involved in producing signals related to the sexual communication of Triatoma infestans and Rhodnius prolixus. Based on this, we tested whether MG volatiles could be involved in the sexual communication of Triatoma brasiliensis. Odor-mediated orientation responses were studied by using a T-tube olfactometer. These tests showed that males exhibit positive anemotaxis when confronted with adult odor-laden air currents. Moreover, females that had their metasternal glands occluded did not elicit significant orientation by males. Compounds produced by the MGs of T. brasiliensis females were identified by means of SPME, GC-FID, and GC-MS, with achiral and chiral columns. All substances identified were ketones and alcohols, and similar compound profiles were found in the secretions produced by both sexes. The most abundant compounds identified were 3-pentanone, followed by (4R)-methyl-1-heptanol, 3-pentanol, and (2S)-methyl-1-butanol. In addition, GC-EAD recordings showed that the antennae of males responded to several of the main components of female MG secretions. Our results showed that compounds produced by the MGs of T. brasiliensis females are involved in the sexual communication of this species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Triatoma brasiliensis Neiva (Heteroptera: Reduviidae) is a hematophagous insect that feeds fundamentally on endothermic vertebrates. This insect is a vector of the flagellate protozoan Trypanosoma cruzi Chagas, which is the etiological agent of Chagas disease, the principal parasitic infection in Latin America inflicting a socio-economic burden on people of this region (WHO 2005). T. brasiliensis is considered to be the main vector of this disease in the semiarid zones of northeastern Brazil (Alencar 1987). It is an endemic species in sylvan environments, and commonly colonizes human dwellings (Alencar 1987).
After sexual maturation, bugs of the subfamily Triatominae, which includes all vector species that transmit Chagas disease, need to find a mating partner. Given that no orientation responses to sexual pheromones have been clearly described for triatomines, research on this topic is needed. The mating behavior of several triatomine species has been described, and consists of a sequence of behavioral steps performed mainly by males (Rojas et al. 1990; Manrique and Lazzari 1994; Pires et al. 2004; Vitta and Lorenzo 2009). In addition, female receptivity to male copulatory attempts has been shown to affect mating success in Triatoma infestans, Panstrongylus megistus, and T. brasiliensis (Manrique and Lazzari 1994; Pires et al. 2004; Vitta and Lorenzo 2009). Few studies have been conducted on the effect of chemical cues on mating behavior in triatomines. Electrophysiological studies have shown that copulating pairs of T. infestans release odors that elicit responses from the antennae of males (De Brito Sánchez et al. 1995). Several authors have suggested that chemical signals are released by copulating pairs, leading to the aggregation of surrounding males (Baldwin et al. 1971; Manrique and Lazzari 1995).
Triatoma brasiliensis adults have a pair of metasternal glands (MGs) that open to the ventral metathorax (Brindley 1930), but their functions, as well as the composition of their secretion, are unknown for this species. All triatomine species studied to date also possess a pair of Brindley’s glands, opening dorso-laterally between the thorax and abdomen, which secrete isobutyric acid as the most abundant compound; this secretion probably plays a role in alarm and/or defense (Ward 1981; Cruz López et al. 1995; Manrique et al. 2006). Other reports for several triatomine species suggest that the compounds produced by Brindley’s glands are involved in the sexual communication (Fontán et al. 2002; Rojas et al. 2002; Guerenstein and Guerin 2004). Recently, Manrique et al. (2006) and Pontes et al. (2008) suggested that the MG secretion of T. infestans and R. prolixus is involved in sexual communication. These authors identified several highly volatile ketones and alcohols that are produced by MGs of these species, and showed that the contents are emitted by adults during copulation. Moreover, Crespo and Manrique (2007) and Pontes et al. (2008) have shown that MG volatiles are relevant for mating success. However, odor-mediated orientation mechanisms that could promote encounters between adults of triatomines are not known. Therefore, we analyzed the behavior of adult T. brasiliensis in the presence of air currents laden with volatiles emitted by both sexes, searching for oriented responses. Additionally, we tested whether the compounds emitted by the MGs of females are necessary for the expression of orientation responses by males. Moreover, the ability of these compounds to elicit responses from the antennae of males was studied by means of gas chromatography-electroantennographic detection (GC-EAD) assays. Finally, the full identities of 14 of the most abundant volatile compounds produced by the MGs of T. brasiliensis females and males are reported, with special attention to those compounds with GC-EAD activity.
Methods and Materials
Insects
T. brasiliensis were reared in the laboratory at 26 ± 2°C and 60 ± 10% RH, and fed on live chickens. The insects were sorted by sex as fifth instar larvae (Espínola 1966), and maintained apart until the experiments were carried out. Adults were fed weekly and kept in plastic containers, which had a piece of folded filter paper as substrate. Identification of volatiles and behavioral assays were performed with 30 d old virgin adults starved for 2 wk. All insects used for this study were exposed to 12:12 h L/D cycle at least 3 d before experiments.
Olfactometer Bioassays
A two-choice “T” olfactometer was used to test orientation responses of adult insects (Fig. 1). The olfactometer was made of acrylic (two 21.5 cm arms and a 33.5 cm common stem, arm section 24.0 cm2). The apparatus was positioned horizontally in an experimental room under the same temperature conditions as described for rearing insects. All experiments were performed during the first half of scotophase. A piece of filter paper covered the base of the olfactometer in order to allow the insects to walk easily. Filtered air was drawn through the olfactometer by a fan connected to the release chamber. Air was purified by using charcoal filters located before the stimulus chamber of each arm of the olfactometer. The filters consisted of 10-ml glass pipettes filled with granular activated charcoal (4–8 mesh, Sigma, USA). The air-flow was maintained at a speed of 10 cm/s as measured by an anemometer (Testo, Germany) at the end of each arm, which was connected to a small polyacrylic chamber (6 × 6 × 3.5 cm) used for presenting the odor sources.
Filter papers were changed after each test to avoid bias due to chemical contamination left from walking insects. The stimuli associated with each olfactometer arm were alternated after three repetitions to avoid directional bias effects. The olfactometer was washed with distilled water and dried with tissue paper before each experiment.
Bioassay Calibration
Tests were performed to evaluate whether the olfactometer constructed ad hoc presented any intrinsic bias. For this, T. brasiliensis adults were tested under two conditions: a) no air currents associated, and b) both arms associated with clean air currents.
Olfactometer Tests
The olfactometer was used to test whether adult T. brasiliensis showed orientation responses when exposed to air currents associated with volatiles from adult males or females. The orientation of insects of both sexes was tested by offering individual bugs a choice between an arm associated with volatiles from two adult insects of the same gender or another that presented a clean air current. For each series, 30 individuals were tested against odor-laden or clean air currents. A single T. brasiliensis adult was introduced gently into the release chamber and kept there for acclimatization for 10 min. Subsequently, the entrance door of this compartment was opened, and the behavior of the insect was recorded for 15 min. Insects that did not express a choice after 15 min from the beginning of the assay were discounted. Test and stimulus insects were used only once.
To determine preferences, the first choice of each insect, i.e., which arm of the olfactometer that was chosen, was recorded. For this, we adopted the following criterion: an insect made a choice every time that its whole body passed the boundary between the main trunk and the corresponding arm of the olfactometer. Choices were statistically analyzed by means of a binomial test.
The following experimental series were performed to investigate whether male or female T. brasiliensis adults orientate to conspecific odor-laden air currents: a) female bugs exposed to male odor-laden air currents; b) female bugs exposed to female odor-laden air currents; c) male bugs exposed to female odor-laden air currents; d) male bugs exposed to male odor-laden air currents; and e) male bugs exposed to nymph odor-laden air currents.
Because of the positive response of males towards female and male odors, two complementary series of assays were conducted to elucidate further aspects of their behavior. The series tested were: f) male bugs confronted with an air current laden with volatiles from two males with the orifices of their MGs occluded with paraffin; and g) male bugs confronted with an air current laden with volatiles from two females with the orifices of their MGs occluded with paraffin. In order to test whether the response of males was due to the occlusion manipulation, two additional control assay series were performed, as follows: h) male bugs were confronted with an air current laden with volatiles from two sham-treated males to which paraffin was applied without occluding their MG orifices; and i) male bugs were confronted with an air current laden with volatiles from two sham-treated females to which paraffin was applied without occluding their MG orifices. Because of the absence of significant orientation by females in response to volatiles from adults, a positive control was performed to confirm that this apparatus was adequate for testing orientation by female bugs. In this experimental series, the females had to choose between one arm associated with mouse odors from two laboratory-reared Mus musculus newborns vs. a clean air current.
Identification of Compounds Produced by Metasternal Glands
In order to obtain glands for the sampling of volatiles, insects were first kept at −18°C for 7 min to avoid disturbance during the dissection. They were then secured with flour-based modeling dough (Faber-Castell, Brazil), leaving their thorax and abdomen exposed. These preparations were placed into an ice-cold phosphate buffer solution to minimize volatilization of MG secretions. Immediately after mounting the insects, a microsurgical knife was used to remove the MG together with its reservoir and a piece of cuticle surrounding the gland opening (ca. 0.25 mm2). Samples of 10 glands were stored in 2-ml vials closed with Teflon®/silicone-lined caps and kept in an ice bath until the end of the subsequent dissections. To exclude any compounds that were not found exclusively in the MGs, we prepared control samples with pieces of tissue and cuticle from other parts of the thorax. Female and male samples were stored in a freezer at −18ºC for not more than 10 d for subsequent analysis. No change of the chemical profile was observed after storage, compared with freshly prepared samples.
Gland samples were sonicated (40 kHz, Thorton, Inpec Eletrônica, Brazil) for 5 min and then heated at 50°C for 30 min. A solid phase microextraction (SPME) fiber (2 cm, DVB/CAR/PDMS-50/30 µm, SUPELCO, USA) was exposed to the headspace of the samples for 10 min at 50°C prior to analysis by gas chromatography with mass-spectrometric detection (GC-MS Shimadzu 17A coupled to a Shimadzu 5050A). The desorption time in the injection port of the GC was 1 min. Helium was used as carrier gas (30 cm/s), and injections were splitless for 1 min. GC injector and transfer line temperatures were 230°C and 250°C, respectively. The ionization energy was 70 eV. The oven program was 40°C for 5 min, 3°C/min to 120°C, and 15°C/min to 200°C using a SupelcoWax-10 column (30 m × 0.25 mm i.d. × 0.25 µm film; Supelco, USA). Tentative identification of volatiles was based on the comparison of retention index (RI, Kováts 1965) and mass spectra with data from the literature and a spectral library (NIST-02). All tentative identifications were confirmed by peak enhancement in co-injections with authentic synthetic samples (Pontes et al. 2008).
The configuration of chiral compounds was determined by GC with flame-ionization detection (FID; Shimadzu 17A) analysis and GC-MS analysis (Shimadzu 17A coupled to a Shimadzu 5050A). Gland samples were heated at 50°C for 30 min. An SPME fiber (2 cm, DVB/CAR/PDMS-50/30 µm) was exposed to the headspace of the glands for a varying time interval depending on the abundance of compounds. The desorption time in the injection port of the GC was 1 min. For the GC-FID, the following settings were used: helium was used as the carrier gas (31 cm/s), and injections were splitless for 1 min. Injector and detector temperatures were 225°C. All enantioselective GC was performed isothermally using a CYCLOSILB column (30 m × 0.25 mm i.d. × 0.25 µm film, J & W Scientific, USA). The column temperature varied from 30°C for 2-butanol to 110°C for 1-phenylethanol. Because of the occurrence of overlapping peaks of different compounds when using the enantioselective GC-FID set-up described above, additional enantioselective GC-MS analysis was carried out according to the protocol for achiral analysis, but with a GammaDex 225 column (30 m × 0.25 mm i.d. × 0.25 µm film, Supelco USA). As for the CYCLOSILB column, isothermal conditions were applied, with column temperatures varying from 30°C to 110°C. The retention times were compared with synthetic standards, and co-injections with peak enhancement were carried out to confirm the configuration of all chiral compounds.
Chemicals
2-butanone, 3-pentanone, (2R)-butanol, (2S)-butanol, 2-methyl-1-propanol, 3-pentanol, (2S)-pentanol, 3-hexanol, (2S)-methyl-1-butanol, 1-heptanol, and (1R)-phenylethanol were purchased from Sigma-Aldrich (Sweden and Brazil). The remaining compounds were synthesized as described below:
For all synthesized compounds 1H-NMR and 13C-NMR spectra of CDCl3 solutions were recorded at 500 MHz and 125 MHz, using a Varian Unity spectrometer. Chemical shifts were expressed in ppm in relation to tetramethylsilane, multiplicity (s, singlet; d, doublet; t, triplet; and m, multiplet), coupling constants (Hz) and number of protons. The starting materials employed were obtained from commercial suppliers and used without further purification. Mass spectra (70 eV, EI) of synthetic compounds were obtained with an HP 6890 GC interfaced to an HP 5973 mass selective detector (Hewlett Packard, Palo Alto, CA, USA), using helium as the carrier gas. The GC was equipped with a BPX-70 column (30 m × 0.25 mm, ID 0.25 μm, SGE, Australia). Enantioselective GC was performed with an HP 5890 GC (Hewlett Packard, Palo Alto, CA, USA) fitted with a CYCLOSILB column (30 m × 0.25 mm, ID 0.25 μm, J & W Scientific, USA). Chromatography on silica gel was carried out using a medium pressure liquid chromatography (Baeckström et al. 1987). All chemicals used as starting materials in the syntheses were used as delivered from Sigma-Aldrich (Sweden) and Alfa Aesar (Germany). Anhydrous solvents were used, and reactions were carried out under nitrogen when appropriate.
(2R)-Pentanol
This chiral alcohol was obtained by resolution of racemic 2-pentanol with the use of Candida antarctica B lipase.
Racemic 2-pentanol (20 g, 0.23 mol), vinyl acetate (70 g, 0.81 mol), and Candida antarctica B lipase (300 mg) were added to dichloromethane (ca. 150 ml). After 160 min, the enzymes were removed by filtration, the solvent removed in vacuo, and pentane was added. The unreacted (2 S)-pentanol was separated from the formed (2R)-pentyl acetate by chromatography. A fraction containing 1.66 g of the acetate was isolated. A solution of (2R)-pentyl acetate (1.50 g, 11.5 mmol), KOH (1.0 g, 18 mmol) and MeOH/water (20 and 3 ml, resp.) was stirred at room temperature (RT) over night. Most of the methanol was removed in vacuo, water was added, and the product was extracted ×3 with diethyl ether, washed with brine, dried over MgSO4, and the diethyl ether was removed on an ice bath in vacuo to yield 710 mg (8.1 mmol) of a slightly yellow product. Chemical and enantiomeric purities were 99%.
3-Methyl-2-hexanol
This alcohol was prepared by a Grignard reaction between 2-bromopentane and acetaldehyde. Since all isomers were present in similar amounts in the glands, no stereospecific synthesis was carried out:
A round-bottomed flask was charged with magnesium (3.5 g, 0.144 mol), iodine (1 crystal), and diethyl ether (50 ml). To the mixture, 2-bromopentane (15.9 g, 0.10 mol, 13.0 ml dropwise addition via syringe) was added, maintaining spontaneous reflux to form the Grignard reagent. Thereafter, the reflux was continued for 20 min. After cooling to 0°C, acetaldehyde (6.16 g, 0.14 mol, 10.0 ml) was added to the mixture by syringe. After reflux for 20 min, the reaction was quenched by addition of ice-water followed by HCl (10%, aq.) until all precipitate was dissolved. The product was extracted ×4 with diethyl ether and washed twice with bicarbonate (sat, aq.) and brine, dried over MgSO4 and concentrated in vacuo to yield 7.73 g of a slightly yellow oil. Some 3-methyl-2-hexanone had been formed, and to remove this impurity, the crude product was treated with sodium borohydride (0.30 g, 8 mmol) dissolved in aqueous ethanol (50%, 25 ml). The crude product (7.73 g) was added dropwise at 0°C while stirring. Stirring was continued at RT over night. Potassium carbonate was added until the mixture became transparent and saturated. The product was extracted ×3 with diethyl ether, washed twice with brine, dried over MgSO4 and solvents were removed in vacuo to yield 5.60 g (48%) of a slightly yellow oil, which was purified by chromatography. A sample of 3.38 g product of 99% purity (diastereomeric ratio ca. 2:3) was isolated. MS: 39(5), 41(11), 42(5), 43(24), 44(9), 45(100), 55(16), 56(4), 57(5), 59(3), 69(4), 70(29), 71(5), 83(6), 98(3), 101(4). 1HNMR: δ: 3.65-3.70 (m, 1 H), 1.0-1.6 (m, 5H), 1.12-115 (d, 3H, J = 6.3 Hz) 0.85-0.92 (m, 6H) (the peaks of two diastereomers overlapped to a large extent and that made the NMRs difficult to interpret.) 13CNMR: δ: 72.0, 71.7, 40.0, 39.7, 35.1, 35.0, 20.6, 20.6, 20.5, 19.5, 14.7, 14.6, 14.3 ppm (one overlapping signal).
6-Methyl-1-heptanol
This achiral alcohol was synthesized from 3-methyl-1-butylbromide via a Grignard reaction with oxetane (Bestmann and Vostrowsky 1974). MS and NMR data corresponded with literature data (Reiter et al. 2003; Tang et al. 1995).
(4R)-Methyl-1-hexanol
The reaction of (3 S)-citronellyl bromide in a modified Lemieux-von Rudloff periodate-permanganate oxidation (Lemieux and Rudloff 1955; Overberger and Kaye 1967; Higashimura et al. 1983; Chen et al. 2008) followed by an lithium aluminum hydride (LAH) reduction yielded the product in 99% enantiomeric purity.
(3 S)-Citronellyl bromide (1.50 g, 6.84 mmol) was dissolved in 50% acetone in water (50 ml). NaIO4 (5.0 g, 20.7 mmol) and KMnO4 (0.2 g, cat) were dissolved in water (50 ml) and added slowly to the solution of (3 S)-citronellyl bromide at 0°C. The mixture was stirred at RT overnight (16 h). After confirming full conversion (GCMS), the product mixture was filtered in vacuo. Most of the acetone was removed in vacuo, and the remaining pink solution was acidified to pH ≈ 2 by HCl (10%, aq.). The acidic solution was stirred at RT for 30 min. The orange solution was extracted ×3 with diethyl ether, washed twice with brine, dried over MgSO4, and concentrated in vacuo to give a copper-colored oil: 1.45 g (101%). The crude (4 S)-6-bromo-4-methylhexanoic acid (1.45 g) was dissolved in diethyl ether (40 ml) and was added dropwise to a solution of LAH (1.9 g, 50 mmol) in diethyl ether (50 ml) at steady reflux. The mixture was refluxed for 1 h, and then quenched by addition of Baeckström’s reagent (sodium sulfate deca-hydrate/celite, Baeckström et al. 1991). When the suspension had changed from grey to white, the solids were removed by filtration in vacuo. The solvents were removed in vacuo to yield a colorless oil of 63% purity (612 mg, 49%). After purification by chromatography a fraction of 99% purity (257 mg, 99% enantiomeric purity) was obtained. Spectroscopic data correlated well with literature data (NIST 2002; Larsson et al. 2001).
(4R)-Methyl-1-heptanol
This chiral alcohol was synthesized from (2R)-methyl-1-pentanol by malonate ester synthesis (Barth and Effenberger 1993; Hedenström et al. 2002; Tai et al. 2002).
Racemic 2-methyl-1-pentanol (16.0 g, 0.16 mol) and vinyl acetate (56.0 g, 0.65 mol) were dissolved in dichloromethane (250 ml) at RT. Amano PS-D lipase (800 mg) was added while stirring. The reaction was monitored hourly by enantioselective GC. When the (2R)-methyl-1-pentanol remaining in the mixture had an e.e. of 96% (at ca. 75% conversion), the lipase was filtered off. The mixture was concentrated in vacuo, and the alcohol (4.0 g, 50%) was isolated by chromatography.
(2R)-Methyl-1-pentanol (3.40 g, 33.3 mmol) was dissolved in a mixture of p-toluene sulfonyl chloride (6.8 g, 35.7 mmol) in dry pyridine (20 ml). The mixture was stirred at RT for 6 h and then quenched by pouring into ice-water (40 ml). The product was extracted ×3 with diethyl ether, the organic phase was washed with copper sulfate solution (10%, aq.), bicarbonate (sat., aq.), and brine, dried over MgSO4, and concentrated in vacuo to yield a pale yellow oil of 79% purity (6.74 g). MS: 39(6), 41(11), 42(5), 43(19), 55(11), 56(28), 63(4), 65(23), 69(28), 71(4), 77(4), 83(4), 84(100), 85(8), 89(8), 90(5), 91(93), 92(27), 93(4), 107(8), 108(5), 139(5), 155(100), 156(18), 157(21), 172(13), 173(43), 174(4).
The crude (2R)-methyl-1-pentyl tosylate (6.6 g, 25.8 mmol) was added to a solution of lithium bromide (12.7 g, 146 mmol) in acetone (100 ml). The mixture was refluxed overnight and then concentrated. Water was added, and the mixture was extracted ×4 with pentane, the organic phases washed twice with water and dried over MgSO4. The solvent was removed in vacuo to yield a pale yellow oil of 57% purity (6.6 g, 89%). After chromatography, a fraction of 2.89 g was used as such in the next step. MS: 39(13), 41(35), 42(12), 43(7), 55(16), 56(10), 57(9), 69(12), 71(21), 84(8), 85(100), 86(10), 121(7).
Diethyl malonate (2.84 g, 17.7 mmol) was added to a solution of sodium ethoxide (1.15 g in 10 ml ethanol), and the mixture was refluxed for 15 min. 2-Methylpentyl bromide (2.80 g, 17.1 mmol) was added dropwise at RT while stirring, whereafter the mixture was refluxed for 1 h. The solution was acidified at RT by acetic acid (3 drops), and then diluted by water (10 ml) and diethyl ether (10 ml). The organic phase was separated, and the water phase was extracted ×4 with diethyl ether. The organic phases were washed twice with brine, dried over MgSO4, and the diethyl ether was removed in vacuo. The copper-colored residue (1.76 g) was added to a solution of potassium hydroxide in ethanol (30 ml, 0.14 g/ml), and the mixture was refluxed for 4 h. After removal of most of the ethanol in vacuo, water was added, and the solution was washed twice with diethyl ether, acidified to pH ≈ 1 (HCl, conc.), extracted ×3 with diethyl ether, washed with water and brine, concentrated in vacuo, and heated at 180°C for 2 h. A solution of sodium carbonate (sat., aq.) was added at RT, and the aqueous solution was washed with diethyl ether, acidified (HCl, conc.), extracted with diethyl ether, washed with water and brine, dried over MgSO4, and concentrated in vacuo to yield a pale brown oil (203 mg). The carboxylic acid intermediate (203 mg, 1.4 mmol) was added in diethyl ether (5 ml) by syringe to a solution of LAH (65 mg, 1.7 mmol) in diethyl ether (5 ml). The mixture was stirred for 1 h, whereafter Baeckström’s reagent was added. When the reaction mixture turned white, the precipitate was removed by filtration in vacuo and the solvent removed in vacuo. The yield of a colorless oil of 99% purity was 141 mg (6%, 98% enantiomeric purity). Spectroscopic data correlated well with literature data (NIST 2002; Tai et al. 2002). MS: 39(10), 40(2), 41(46), 42(17), 43(44), 44(3), 45(3), 53(4), 54(1), 55(44), 56(56), 57(12), 60(1), 67(5), 68(5), 69(100), 70(54), 71(16), 82(3), 83(13), 84(81), 85(6), 87(3), 97(5), 112(1). 1HNMR: δ: 3.63 (m, 2H), 1.1-1.6 (m, 9H), 0.87 (m, 6H), 13CNMR: δ: 63.7, 39.5, 33.1, 32.6, 30.6, 20.3, 19.8, 14.6 ppm. Total yield from (2R)-methyl-1-pentanol was 4%.
Gas Chromatography Coupled to Electroantennographic Detection
Headspace collections were made over 8 excised MGs of females by means of SPME, using the same fiber type as described for the chemical analyses. The volatiles collected were analyzed by GC-EAD using the same GC-setup as for GC-FID analyses, fitted with a SupelcoWax-10 column (30 m × 0.25 mm ID; 0.25 μm film), and coupled to an electroantennographic setup (Syntech, Hilversum, The Netherlands). Recordings of responses to volatiles eluting from the GC column were obtained from the antennae of 8 different males in separate experiments. An antenna was isolated from the head by cutting at the antennal base. The proximal end of the isolated antenna was inserted into a borosilicate glass micropipette containing 0.1 M KCl solution, serving as the reference electrode. The distal end of the antenna was inserted into another glass micropipette filled with 0.1 M KCl solution after the tip of the segment was cut off. Ag-AgCl wire was used to maintain electrical continuity between the electrodes, and a high input impedance headstage preamplifier (Syntech, Kirchzarten, Germany). The EAG signals through the preamplifier were further amplified and processed with a PC-based signal processing system (Syntech, Kirchzarten, Germany). The digitized signals from the FID and antenna were analyzed by means of the EAD2000 software (ver. 2.3, Syntech, Hilversum, The Netherlands).
Results
Olfactometer Bioassays
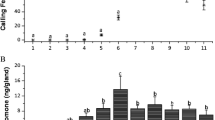
In the negative control experiments, neither male nor female insects showed significant orientation to either arm of the olfactometer when no air current or two clean air currents were presented. Female insects did not show a significant preference for male or female odor-laden air currents (Fig. 2) but were significantly attracted to the odor emitted by mice (Fig. 2, P < 0.001) in the positive control experiments, showing that the apparatus is capable of evincing orientation responses by these bugs. T. brasiliensis males, in contrast to females, significantly preferred the arm of the olfactometer associated with male or female odors (Fig. 3, P < 0.01 and P < 0.001, respectively). When the MGs of the female insects used as stimuli were occluded, males did not show a significant orientation (Fig. 4). However, sham-treated females promoted the orientation of males in the olfactometer (Fig. 4, P < 0.01). The occlusion of the MGs of males used as sources of stimuli did not promote a change in the orientation of males in the olfactometer (Fig. 4, P < 0.01).
Responses of Triatoma brasiliensis males to different stimuli in an olfactometer. MO = males with occluded metasternal glands (MGs), CM = control males treated with paraffin on a different part of their body surface, FO = females with occluded MGs and, CF = control females treated with paraffin on a different part of their body surface. Significant differences between treatments were determined by a binomial test (*P < 0.01 and **P < 0.001)
Gas chromatogram (FID) and corresponding electroantennogram (EAD) traces from one antenna of a Triatoma brasiliensis male, in response to a headspace collection of metasternal gland (MG) volatiles. Active compounds are represented with numbers: 1) 3-pentanone, 2) unknown, 3) 2-methyl-1-butanol, 4) 4-methyl-1-heptanol, and 5) 1-phenylethanol
Identification of Compounds Produced by MGs
The MGs of T. brasiliensis are the sources of a complex mixture of volatiles. In total, sixteen compounds were identified, with the most abundant compounds being 3-pentanone, 3-pentanol, and (4R)-methyl-1-heptanol (Table 1). The configurations of the chiral compounds varied; 2-methyl-1-butanol was present as the S-enantiomer, 4-methyl-1-hexanol, 4-methyl-1-heptanol, and 1-phenylethanol were present as the R-enantiomers, while 3-hexanol and 3-methyl-2-hexanol were present in all isomeric forms. For 2-butanol and 2-pentanol, problems with co-elution and/or low abundance of the compounds prevented us from confirming the configuration.
Gas Chromatography Coupled to Electroantennographic Detection
Our GC-EAD recordings showed that the antennae of males of T. brasiliensis respond to a series of volatile compounds produced by the MGs of females (Fig. 5). Responses to 3-pentanone were consistently observed in all recordings and were normally the strongest. In addition, 75% of the recordings presented strong responses to 4-methyl-1-heptanol, and responses also were observed for 2-methyl-1-butanol and 1-phenylethanol. It is worth highlighting that one of the compounds reported here as unknown (RI = 1,156) was capable of promoting responses from 3 out of 8 antennae tested (Fig. 5).
Discussion
Orientation in response to odor-laden air currents is a widespread mechanism that mediates sexual encounters between male and female insects. However, no cases of anemotactic orientation to sexually related signals are known to date for triatomine bugs. Our report presents significant evidence showing that T. brasiliensis males express oriented responses to air currents associated with volatiles from conspecific adults. These anemotactic responses are apparently stronger when males are confronted by air currents laden with female volatiles rather than with male odors. This finding suggests that males are attracted by odors emitted by distant females, and exploit air currents to define the direction of their responses. This may represent a putative mechanism to mediate encounters between adults of different gender, and suggests that a sexual pheromone is produced by females.
Our results showed that MGs of females emit a volatile signal that is necessary to mediate the orientation expressed by males. On the other hand, the preference observed when male insects were confronted with male odor was not affected by the occlusion of MGs. Therefore, male to male orientation is apparently not mediated by signals produced by these glands. As females did not show oriented responses to air currents laden with odors from adult bugs, we propose that T. brasiliensis males are the only gender attracted to sexual signals.
The use of antennae of male insects as detectors for electrophysiologically active compounds made it possible to demonstrate that several substances produced by MGs stimulate male receptors. Specifically, 3-pentanone and (4R)-methyl-1-heptanol, elicited strong responses in most preparations, while (2S)-methyl-1-butanol, (1R)-phenylethanol and two unknown compounds (RI = 1,156 and RI = 1,548, Table 1) elicited weaker responses from antennae of males. Whether or not other components of the female MG secretion are capable of stimulating antennal receptors needs to be determined. It is worth mentioning that while the secretions produced in the MGs of females and males were similar according to our GC-MS analyses, the responses observed when males were tested in olfactometers suggest that the products of male and female MGs are different. This is supported by the two occlusion experiments, as the occlusion of male MGs did not affect the orientation of males to the air currents laden with volatiles from other males.
It has been proposed that the secretion of the MGs of triatomines is involved in mediating sexual communication (Manrique et al. 2006; Pontes et al. 2008). The identification of the volatile compounds detected over the headspace of T. brasiliensis MGs corroborated that these glands produce ketones and alcohols in triatomines. The fact that 3-pentanone is the most abundant compound produced by MGs is coincident with the results presented by Manrique et al. (2006) for T. infestans. The relatively less volatile 4-methyl-1-heptanol, that appears to be characteristic of the latter species, is reported here for the first time. Our GC-EAD results suggest that both substances could be involved in mediating behavioral responses of the kind described in this study. Several other compounds reported here (2-butanone, 2-pentanone, (2S)-butanol, 3-methyl-2-butanol, 3-pentanol, (2S)-pentanol, and (3S)-hexanol) were previously detected in the MGs of other blood-sucking bugs (Manrique et al. 2006; Pontes et al. 2008). All compounds found in the MGs except 4-methyl-1-hexanol, 6-methyl-1-heptanol, and 4-methyl-1-heptanol have been reported previously in other insect species (El-Sayed 2008). It is relevant to highlight that capturing volatile compounds by means of SPME allowed us to perform successful GC-EAD recordings, confirming that this odor extraction technique is adequate for the preparation of odor samples for electrophysiological studies.
Baldwin et al. (1971), as well as Manrique and Lazzari (1995), have shown that R. prolixus and T. infestans males aggregate around mating pairs, and suggested that this behavior is mediated by chemical signals. In addition, Vitta and Lorenzo (2009) have shown recently that T. brasiliensis males react to the presence of competitors by showing increased mate-guarding responses. Furthermore, these authors described that mating duration can be shortened if males copulate with previously mated females. Whether these changes in male behavior are triggered by chemical signals is not known. Crespo and Manrique (2007) and Pontes et al. (2008) have demonstrated that the occlusion of the MGs of T. infestans and R. prolixus affects mating success in a dramatic manner. In addition, it has been suggested that the secretion from Brindley’s glands is involved in sexual communication of triatomines (Fontán et al. 2002; Rojas et al. 2002; Guerenstein and Guerin 2004). Nevertheless, no behavioral evidence has been reported associating compounds from Brindley’s glands with behavioral responses specifically related to sex.
Baits and traps for triatomine bugs are necessary for the early detection of reinvasion of houses after insecticide treatments, in order to avoid contact between triatomines and humans. Whether the compounds and behavioral responses described here can be exploited for the development of control tools will depend on further work testing synthetic compounds in dose-response electrophysiological and behavioral bioassays.
References
Alencar, J. E. 1987. História natural da doença de Chagas no Estado do Ceará, Fortaleza. Imprensa Universitária da Universidade Federal do Ceará, Fortaleza, Brazil.
Baeckström, P., Stridh, K., Li, L., and Norin, T. 1987. Claisen rearrangements with mesityl oxide dimethyl ketal. Synthesis of ipsdienone, E- and Z-ocimenone, 2,6-dimethyl-2,7-octadien-4-one and 2,6-dimethyl-2,7-octadien-4-ol. Acta Chem. Scand. B 41:442–447.
Baeckström, P., Li, L., Polec, I., Unelius, C. R., and Wimalasiri, W. R. 1991. Convenient method for the synthesis of lineatin, a pheromone component of Trypodendron lineatum. J. Org. Chem. 56:3358-3362.
Baldwin, W. F., Knight, A. G., and Lynn, K. R. 1971. A sex pheromone in the insect Rhodnius prolixus (Hemiptera: Reduviidae). Can. Entomol. 103:18-22.
Barth, S., and Effenberger, F. 1993. Lipase-catalyzed resolution of racemic 2-alkyl substituted 1-alkanols, Tetrahedron Asymmetry. 4:823-833.
Bestmann, H. J., and Vostrowsky, O. 1974. Pheromone III: Eine stereoselektive Synthese von 7,8-z-epoxy-2-methyloctadecan, dem Sexuallockstoff des Schwammspinners (Lymantria dispar, Porthetria dispar, Lepidoptera). Tetrahedron Lett. 15:207-208.
Brindley, M. D. H. 1930. On the metasternal scent glands of certain Heteroptera. Trans. Ent. Soc. Lond. 78:199-208.
Chen, Z., Chen, H., Hu, H., Yu, M., Li, F., Zhang, Q., Zhou, Z., Yi, T., and Huang, C. 2008. Versatile synthesis strategy for carboxylic acid-functionalized upconverting nanophosphors as biological labels. J. Am. Chem. Soc. 130:3023-3029.
Crespo, J., and Manrique, G. 2007. Mating behavior of the hematophagous bug Triatoma infestans: Role of Brindley's and metasternal glands. J. Insect Physiol. 53:708-714.
Cruz-López, L., Morgan, E. D., and Ondarza, R. N. 1995. B rindley’s gland exocrine products of Triatoma infestans. Med. Vet. Entomol. 9:403-406.
De Brito Sánchez, M. G., Manrique, G., and Lazzari, C. R. 1995. Existence of a sex pheromone in Triatoma infestans (Hemiptera: Reduviidae) II. Electrophysiological correlates. Mem. Inst. Oswaldo Cruz 90:649-651.
El-Sayed, A. M. 2008. The pherobase: database of insect pheromones and semiochemicals. http://www.pherobase.com.
Espínola, H. N. 1966. Nota sobre as diferenças sexuais em formas imaturas de Triatominae (Hemiptera: Reduviidae). Rev. Bras. Biol. 26:263-267.
Fontán, A., Audino, P. G., Martinez, A., Alzogaray, R. A., Zerba, E. N., Camps, F., and Cork, A. 2002. Attractant volatiles released by female and male Triatoma infestans (Hemiptera: Reduviidae), a vector of Chagas disease: chemical analysis and behavioral bioassay. J. Med. Entomol. 39:191-197.
Guerenstein, P. G., and Guerin, P. M. 2004. A comparison of volatiles emitted by adults of three triatomine species. Entomol. Exp. Appl. 111:151-155.
Hedenström, E., Nguyen, B. V., and Silks, L. A. 2002. Do enzymes recognise remotely located stereocentres? Highly enantioselective Candida rugosa lipase-catalysed esterification of the 2- to 8-methyldecanoic acids, Tetrahedron Asymmetry. 13:835-844.
Higashimura, T., Sawamoto, M., Hiza, T., Karaiwa, M., Tsuchii, A., and Suzuki, T. 1983. Effect of methyl substitution on microbial degradation of linear styrene dimers by two soil bacteria. Appl. Environ. Microbiol. 46:386-391.
Kováts, E. 1965. Gas chromatographic characterization of organic substances in the retention index system, pp. 229-247, in J. C. Giddings and R. A. Keller (eds.). Advances in Chromatography, vol. 1. Edward Arnold Ltd., London.
Larsson, M., Nguyen, B. V., Högberg, H. E., and Hedenström, E. 2001. Synthesis of the sixteen stereoisomers of 3,7,11-trimethyl-2-tridecanol, including the (2S,3S,7S,11R) and (2S,3S,7S,11S) stereoisomers identified as pheromone precursors in females of the pine sawfly Microdiprion pallipes (Hymenoptera: Diprionidae). Eur. J. Org. Chem. 2:353-363.
Lemieux, R. U., and Rudloff, E. V. 1955. Periodate-permanganate oxidations. Can. J. Chem. 33:1701-1709.
Manrique, G., and Lazzari, C. R. 1994. Sexual behavior and stridulation during mating in Triatoma infestans (Hemiptera: Reduviidae). Mem. Inst. Oswaldo Cruz 89:629-633.
Manrique, G. and Lazzari, C. R. 1995. Existence of sex pheromone in Triatoma infestans (Hemiptera: Reduviidae): I-Behavioral Evidence. Mem. Inst. Oswaldo Cruz 90:645-648.
Manrique, G., Vitta, A. C., Ferreira, R. A., Zani, C. L., Unelius, C. R., Lazzari, C. R., Diotaiuti, L., and Lorenzo, M. G. 2006. Chemical communication in Chagas disease vectors. Source, identity, and potential function of volatiles released by the metasternal and Brindley's glands of Triatoma infestans adults. J. Chem. Ecol. 32:2035-2052.
NIST 2002. The NIST mass spectral search program for the NIST/EPA/NIH mass spectral library.
Overberger, C. G., and Kaye, H. 1967. The synthesis of some optically active ε-caprolactones. J. Am. Chem. Soc. 89:5640-5645.
Pires, H. H. R., Lorenzo, M. G., Lazzari, C. R., Diotaiuti, L., and Manrique, G. 2004. The sexual behavior of Panstrongylus megistus (Hemiptera, Reduviidae): an experimental study. Mem. Inst. Oswaldo Cruz 99:295-300.
Pontes, G. B., Bohman, B., Unelius, C. R., and Lorenzo, M. G. 2008. Metasternal gland volatiles and sexual communication in the triatomine bug, Rhodnius prolixus. J. Chem. Ecol. 34:450-457.
Reiter, B., Burger, B. V., and Dry, J. 2003. Mammalian exocrine secretions. XVIII: Chemical characterization of interdigital secretion of red hartebeest, Alcelaphus buselaphus caama. J. Chem. Ecol. 29:2235-2252.
Rojas, J. C., Malo, E. A., Gutierrez-Martinez, A., Ondarza, R. A. 1990. Mating behavior of Triatoma mazzottii Usinger (Hemiptera: Reduviidae) under laboratory conditions. Ann. Entomol. Soc. Am. 83:598–602.
Rojas, J. C., Rios-Candelaria, E., Cruz López, L., Santiesteban, A., Bond-Compean, J. G., Brindis, Y., and Malo, E. A. 2002. A reinvestigation of Brindley’s gland exocrine compound of Rhodnius prolixus (Hemiptera: Reduviidae). J. Med. Entomol. 39:256-265.
Tai, A., Syouno, E., Tanaka, K., Fujita, M., Sugimura, T., Higashiura, Y., Kakizaki, M., Hara, H., and Naito, T. 2002. Regio- and stereochemical study of sex pheromone of pine sawfly; Diprion nipponica. Bull. Chem. Soc. Jpn. 75:111-121.
Tang, R., Webster, F. X., and Müller-Schwarze, D. 1995. Neutral compounds from male castoreum of North American beaver, Castor canadensis. J. Chem. Ecol. 21:1745-1762.
Vitta, A. C. R., and Lorenzo, M. G. 2009. Copulation and mate guarding behavior in Triatoma brasiliensis. J. Med. Entomol. 46:789-795.
Ward, J. P. 1981. A comparison of the behavioral responses of the haematophagous bug, Triatoma infestans to synthetic homologues of two naturally occurring chemicals (n- and isobutyric acid). Physiol. Entomol. 6:325-329.
WORLD HEALTH ORGANIZATION. 2005. Report of the Scientific Working Group on Chagas Disease. http://www.who.int/tdr/diseases/chagas/default.htm.
Acknowledgements
The authors are grateful to FAPEMIG, CNPq and FIOCRUZ for financial support. This work was also supported by the Swedish International Development Cooperation Agency (SIDA), and by the University of Kalmar (Sweden). The New Zealand Institute for Plant & Food Research Limited, Lincoln, New Zealand provided some research facilities for BB and CRU. ACRV wishes to thank Mr. Arnaldo Aroeira for his kind support on drawing the olfactometer scheme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vitta, A.C.R., Bohman, B., Unelius, C.R. et al. Behavioral and Electrophysiological Responses of Triatoma brasiliensis Males to Volatiles Produced in the Metasternal Glands of Females. J Chem Ecol 35, 1212–1221 (2009). https://doi.org/10.1007/s10886-009-9709-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-009-9709-2