Abstract
Volatile compounds, such as β-cyclocitral, geosmin, and 2-methylisoborneol, from cyanobacteria showed a lytic activity against cyanobacteria. Particularly, β-cyclocitral caused an interesting color change in the culture broth from green to blue during the lysis process. In the present study, the lytic behavior of various cyanobacteria with β-cyclocitral was investigated, and a mechanism for the blue color formation was developed. β-Cyclocitral lysed both the laboratory strains of any genera and bloom samples including many species of cyanobacteria, and caused the characteristic color change from green to blue. β-Cyclocitral provided a characteristic behavior, such that the absorption maxima of chlorophyll-a and β-carotene disappeared, but that of phycocyanin still remained after 12 h, which indicated that β-cyclocitral decomposed chlorophyll-a and β-carotene rapidly, so that the inherent colors from the tolerant water-soluble pigments became observable in the cultured broth. This phenomenon was confirmed by another experiment using Phormidium (NIES-611), which showed a pink color derived from phycoerythrin. β-Cyclocitral was more easily oxidized when compared with similar aldehyde compounds, so that the pH of the solution quickly decreased to 4.5. An oxidation product of β-cyclocitral in water solution was isolated and identified as 2,6,6-trimethylcyclohexene-1-carboxylic acid. This study provides support that β-cyclocitral derived from cyanobacteria plays an important role in the lysis of cyanobacteria and participates in the blue color formation under natural conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Blooms of freshwater cyanobacteria, particularly in the genera Microcystis and Anabaena, have caused increasing problems in recent years. They have frequently resulted in deterioration of water quality, with adverse effects on lake ecology, livestock, the human water supply, and recreational amenity. The most direct way of control involves the application of algicides, but this is potentially damaging to the environment and may cause secondary pollution. Therefore, we have been developing a biological control system that uses microorganisms coexisting in the same ecosystem to decrease the outbreak of cyanobacteria (Sigee et al. 1999; Tsuji et al. 2006). In a previous study, one of the microorganisms collected from Lake Sagami, Japan, Brevibacillus sp., was found to have a lytic activity toward cyanobacteria, but the isolation of active compounds from the cultured broth of this strain was not successful. Instead, the co-culturing of Microcystis with the Brevibacillus sp. enhanced the production of two volatile compounds, β-cyclocitral (Fig. 1) (Jüttner 1984; Jüttner and Höflacher 1985) and 3-methyl-1-butanol (Wright et al. 1991). The former had a characteristic lytic activity (Ozaki et al. 2008). It was confirmed that these volatile compounds were derived from the cyanobacteria themselves. Further, β-ionone (Fig. 1), geosmin, and 2-methylisoborneol (2-MIB) derived from cyanobacteria and similar volatile compounds, i.e., terpenoids, produced by plants, also had lytic activity. Although the minimum inhibition concentration (MIC) values of the cyanobacterial metabolites were estimated to be higher generally than those of compounds from plants, their local concentrations around a cluster of cyanobacteria might reach a sufficient concentration to cause auto-lysis, because they are intrinsic metabolites (Ozaki et al. 2008).
Among these volatile compounds, β-cyclocitral caused a characteristic color change in the culture broth from green to blue during the lysis process (see Supplementary Material Fig. 1). In the past, a similar color change had been found several times in the natural environment and was recently observed in Lake Tsukui (see Supplementary Material Figs. 2, 3, 4). Because it was hypothesized that β-cyclocitral is critical for the regulation of cyanobacteria in a freshwater ecosystem, the blue color formation of cyanobacteria with β-cyclocitral was investigated. This study focuses on the lytic behavior of various cyanobacteria with β-cyclocitral and its mechanism involving the blue color formation.
Methods and Material
Chemicals
β-Cyclocitral was purchased from Wako Pure Chemical Industries (Kyoto, Japan), β-ionone was obtained from Kanto Chemical (Tokyo, Japan), and citral, cinnamaldehyde, perillaldehyde, and vanillin were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). Methanol was purchased from Nacalai Tesque (Kyoto, Japan).
Cyanobacteria Cultures
Axenic strains NIES-73, -102, -103, -112 and -298 belonged to Microcystis, NIES-611 was a Phormidium, and NIES-808 was an Anabaena. They were obtained from the National Institute for Environmental Studies (NIES), Tsukuba, Japan. PCC6803 was a Synechocystis sp. obtained from the Pasteur Culture Collection of Cyanobacteria (PCC), Paris, France. These strains were cultured in 1 liter Erlenmeyer flasks, each containing a modified MA medium (300 ml) at 25°C for 8 d under a 28 μEm−2s−1 continuous illumination. The MA medium consisted of a mixture of bicine (500 mg); Ca(NO3)2·4H2O (50 mg); KNO3 (100 mg); NaNO3 (50 mg); Na2SO4 (40 mg); MgCl2·6H2O (50 mg); β-Na2glycerophosphate (100 mg); a mixed metal solution (1 ml; composed of 1 mg of Na2EDTA, 0.1 mg of FeCl3·6H2O, 1 mg of MnCl3·4H2O, 0.1 mg of ZnCl2, 1 mg of CoCl2·6H2O, 0.16 mg of Na2MoO4·2H2O and 4 mg of H3BO3 in 200 ml of distilled water), and the resulting solution was adjusted to pH 8.6.
Collection of Cyanobacterial Blooms
A bloom sample was collected from Lake Suwa on August 6, 2007, and another from Lake Tsukui on August 24, 2007 in Japan.
Measurement of Anticyanobacterial Activity
The anticyanobacterial activity also was determined by measuring the absorbance of chlorophyll-a (Uchida et al. 1998). Briefly, the final concentrations (6.5 mM) of the volatile compounds (β-cyclocitral, β-ionone, citral, cinnamaldehyde, perillaldehyde, and vanillin) were added to 100 ml of the cultured cyanobacteria (8 d) and natural blooms, and incubated at 25°C with shaking for 1 and 10 d under a 28 μEm−2s−1 continuous illumination. Water was used as the control. Five ml aliquots of the culture medium were withdrawn according to the color change during the first day, and then once per day until the 10th day. Simultaneously, the culture broth was photographed. The pH of the withdrawn samples was measured with a pH meter Navi F-52 (Horiba Ltd., Kyoto, Japan) and filtered through a GF/A filter (Whatman International, Ltd., Maidstone, England). The cyanobacteria on the filters were soaked in 2.5 ml of methanol, and the resulting mixture was stored at 4°C in the dark overnight. A 200 μl aliquot of the supernatant was placed on a 96-well microplate well (Kartell, Milan, Italy), and the optical density was measured at 665 nm with an MPR-A4iII microplate reader (TOSOH, Tokyo, Japan). The anticyanobacterial activity was determined as a decrease in absorbance (see Supplementary Material Fig. 6).
Acidification of MA medium by the Volatile Compounds
β-Cyclocitral and β-ionone were added to 100 ml of the MA medium or water to the final concentrations (6.5 mM), and incubated at 25°C with shaking for 1 and 20 h under a 28 μEm−2s−1 continuous illumination. Water and ethanol were used as the negative control. Aliquots of 5 ml of the MA medium or water were withdrawn during the first, 0.5, 3, and 18 h. The pH of withdrawn samples was measured with a Navi F-52 pH meter.
Absorption Spectra of Culture Broths
β-Cyclocitral and β-ionone were added to 100 ml of the Microcystis NIES-102 or Phormidium NIES-611 cultured for 8 d at the final concentrations (6.5 mM), and incubated at 25°C with shaking for 1 and 10 d under a 28 μEm−2s−1 continuous illumination. Water was used as the control. Aliquots of 5 ml of the culture medium were withdrawn according to the color change during the first day, and then once per day for 10 d. Absorption spectra were recorded at 800–190 nm using a Jasco V-560 UV/VIS spectrophotometer (Jasco Int. Co., Tokyo, Japan).
Isolation of an Acidic Compound
β-Cyclocitral was added to water with shaking for 20 h, and the produced acidic compounds were collected by liquid-liquid separation with ether. The main compound was isolated by using a preparative HPLC whose system consisted of a pair of CCPS pumps, an SD-8022 degasser, a CO-8020 column oven, a UV-8020 detector, and a PX-8020 system controller (TOSOH, Tokyo, Japan). The sample was filtered by using an Ultrafree-MC membrane centrifuge filtration unit (hydrophilic PTFE, 0.2 mm, Millipore, Bedford, MA, U.S.A.) and loaded onto a Cosmosil 5C18-AR-300 column (250 × 10 mm ID, particle size 5 mm, TOSOH, Tokyo, Japan). The mobile phase was methanol/water containing 0.1%(v/v) formic acid. The methanol concentration was increased from 60 to 90% for 25 min in the linear gradient mode. The column temperature was 40°C and the flow rate was 3 ml/min.
LC/MS
Five μl of the sample were filtered using an Ultrafree-MC membrane centrifuge filtration unit (hydrophilic PTFE, 0.2 mm, Millipore, Bedford, MA, U.S.A.). The acidic compound analysis was carried out by LC/MS. The LC separation was performed with an Agilent 1100 HPLC system (Agilent Technologies, Palo Alto, CA, U.S.A.). The compound was monitored at 254 nm using a variable wavelength UV detector. The column was a TSK-gel Super-ODS column (100 × 2 mm ID, particle size 2 mm, TOSOH, Tokyo, Japan). The mobile phase was acetonitrile/water containing 0.1% formic acid. The acetonitrile concentration was increased from 10 to 80% for 20 min in the linear gradient mode. The column temperature was 40°C, and the flow rate was 0.2 ml/min. The MS analysis was accomplished with a Finnigan LCQ Deca XP plus mass spectrometer (Thermo Fisher Scientific, San Jose, CA, U.S.A.), equipped with an electrospray ionization (ESI) interface. The ESI conditions in the positive ion mode were as follows: capillary temperature, 350°C; sheath gas flow rate, 40 (arbitrary unit); ESI source voltage, 5000 V; capillary voltage, 46 V; tube lens offset, 30 V.
NMR Spectroscopy
The isolated acidic compound was dissolved in chloroform-d1. The 1H- and 13C-NMR spectra were measured by a JNM-ECA500 spectrometer (JEOL Tokyo, Japan) at 500.16 MHz and 125.77 MHz, respectively. The obtained signals are summarized as follows: δ1H (ppm), 1.14 (6H, s); 1.44 (2H, m); 1.65 (2H, m); 1.77 (3H, s); 2.01 (2H, t). δ13C (ppm), 18. 6; 21.6; 28.3; 31.7; 33.0; 38.6; 134.0; 136.5: 175.5.
Results and Discussion
Observation of the Blue Color Formation with β-Cyclocitral
Figure 2(A) shows the effect of β-cyclocitral (a), β-ionone (b), and water (c) on the lysis of Microcystis NIES-102 after its addition. In contrast to the case with the water control (c), β-cyclocitral (a) and β-ionone (b) caused a lysis of the cyanobacteria as measured by a decrease in chlorophyll-a absorbance. While β-ionone gradually reduced the green color to provide a colorless solution mixed with white precipitates after 10 d, β-cyclocitral began to cause the characteristic color change from green to blue after 6 h, and the resulting blue color continued for about 1 d. The blue color change also was observed in Microcystis NIES-73, -103, -112, and -298, Anabaena NIES-808, and Synechocystis PCC6803 strains with β-cyclocitral. The Phormidium (NIES-611) strain originally provided a dark brown cultivated broth as shown in Fig. 2(B). Whereas the addition of β-ionone induced a gradual change from the original color to a colorless solution mixed with white precipitates via a diluted brown color [Fig. 2(B-b)], β-cyclocitral provided a characteristic pink color after 3 h, and this color continued for 12 h [Fig. 2(B-a)].
β-Cyclocitral and β-ionone were added to natural bloom samples from Lake Tsukui (see Supplementary Material Fig. 5). Generally, bloom samples of cyanobacteria have a sheath outside of the cells (Amemiya and Nakayama 1989; Amemiya et al. 1988) and we were interested in whether similar results were obtained when β-cyclocitral was applied to the bloom samples. Although the collected samples from Lake Tsukui were very dense, they were used without dilution. While the blue color formation was also observed after 4 d due to the addition of β-cyclocitral (see Supplementary Material Fig. 5), such behavior was not found in the case of β-ionone as stated above (see Supplementary Material Fig. 5). Therefore, β-ionone did not affect the chlorophyll-a concentration in the cells, and this behavior was different from that of the laboratory strains [Fig. 2(A-b) and (B-b)]. These results indicate that there was a difference between β-cyclocitral and β-ionone in the lytic activity against bloom samples composed of several cyanobacteria due to the presence of the sheath, and that β-cyclocitral can lyse the laboratory strains of any genera and bloom samples, including many species of cyanobacteria, and can cause the characteristic color change from green to blue.
Visible Spectral Observation of Photosynthetic Pigments in Lysed Cyanobacteria
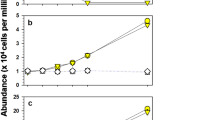
In this experiment, we observed the visible spectra of the cultured broths affected by β-cyclocitral and β-ionone. Figure 3(A-c) shows the visible spectra of Microcystis NIES-102 with water as the negative control, and the absorbances of the characteristic absorption maxima increased with growth of cyanobacterium. These absorption maxima at 420 and 680 nm, 500 nm, and 620 nm can be assigned to those of chlorophyll-a, β-carotene, and phycocyanin, respectively. The addition of β-ionone uniformly reduced these absorption maxima to complete disappearance after 72 h [Fig. 3(A-b)]. In contrast, β-cyclocitral provided characteristic behavior in which the absorption maxima of chlorophyll-a and β-carotene disappeared, but that of phycocyanin remained after 12 h [Fig. 3(A-a)], indicating that the resulting blue color was derived from phycocyanin after the disappearance of the remaining pigments due to β-cyclocitral. This observation corresponded exactly to the phenomena shown in Fig. 2(A). An additional experiment using a Phormidium (NIES-611) strain, whose photosynthetic pigments are chrolophyll-a, β-carotene, and phycoerythrin instead of phycocyanin, which showed a pink color [Fig. 2(B-a)], supported the above conclusion [Fig. 3(B)]. That is, the addition of β-cyclocitral showed behavior similar to that of the absorption maxima disappearance of chrolophyll-a and β-carotene, but that of phycoerythrin at 560 nm still remained after 3 h (data not shown). This was consistent with the behavior shown in Fig. 2(B). These findings indicate that β-cyclocitral rapidly decomposed chlorophyll-a and β-carotene, so that the inherent colors from the tolerant water-soluble pigments became observable in the cultured broth.
The visible spectra of lysed NIES-102 and -611; (A) NIES-102, (B) NIES-611, (a) β-cyclocitral, (b) β-ionone, and (c) water. The circles of (i), (ii), (iii) and (iv) indicate the absorption maxima of chlorophyll-a (420 and 680 nm), β-carotene (500 nm), phycocyanin (620 nm) and phycoerythrin (560 nm), respectively. The absorbances of these characteristic absorption maxima changed with the growth of the cyanobacterium as indicated by the arrows
Acidification of Cultured Broth and Bloom Sample with β-Cyclocitral
The changes in pH of the cultivated broth of NIES-102 and -298 with the addition of β-cyclocitral and β-ionone are shown in Fig. 4. Thirty min after the addition of β-cyclocitral, the pH of both cultured broths decreased quickly to around 6, and it was maintained for several hours. However, such behavior was not observed in the case of β-ionone, in which the pH changed to around 8 over several hours. A dramatic pH change also was found in the bloom samples. As shown in Fig. 5, the pH decreased immediately to 4.5 after the addition of β-cyclocitral, and the pH then was slightly elevated for several hours in the case of the bloom sample from Lake Suwa. The bloom sample from Lake Tsukui showed nearly similar behavior. However, the pH changed to around 7 after the addition of β-ionone (Fig. 5).
We also investigated the pH-lowering effect of a volatile compound with an aldehyde group such as citral, cinnamaldehyde, perillaldehyde, and vanillin from plants, together with β-cyclocitral. Figure 6 shows the pH change due to the addition of these volatile compounds in water (A) and MA medium (B), in which water and ethanol were used as the negative control. The pH of a water solution of perillaldehyde gradually decreased to 4 in 20 h, and that of cinnamaldehyde decreased immediately to 4.5 in a manner similar to that of β-cyclocitral [Fig. 6(A)]. However, this behavior was not observed in the MA medium with buffer action [Fig. 6(B)]. In contrast, the solution decreased promptly to pH 4.5 after the addition of β-cyclocitral as already described.
Conversion of β-Cyclocitral to Corresponding Acidic Compound
Twenty hours after the addition of β-cyclocitral to water, the reaction mixture was separated into the neutral and acidic fractions by liquid-liquid extraction. While a large amount of β-cyclocitral was still present in the former fraction, the acidic fraction contained several components as shown in Fig. 7. Because peaks No. 1 and No. 2 were not stable, it was impossible to isolate them. However, preparative HPLC could separate the main peak (No. 3) in a pure state, and the structural characterization was carried out as follows: the molecular weight was determined to be 168 by LC/MS by using electrospray ionization (ESI), and the 1H- and 13C-NMR spectra were almost identical to those of β-cyclocitral except for an exchangeable proton, which was not observed in CDCl3. From these data, we concluded that β-cyclocitral was converted to the corresponding carboxylic compound (2,6,6-trimethylcyclohexene-1- carboxylic acid) in a water solution. Similar results were obtained in MA medium. As mentioned above, β-cyclocitral showed the remarkable pH change in MA medium, but such behavior was not observed in the case of several other volatile compounds with an aldehyde group as shown in Fig. 6, thus suggesting that the pH-lowering effect was due to oxidation of an aldehyde group to a carboxylic acid. The solubility of the free acid was poor in water. One mg of the sample was not completely soluble in 1 ml of water in a short time period, and it took a few days to change to a clear solution, which resulted in a pH of about 3.3. Huang et al. (2002) reported that the blue color formation of a cyanobacterium (Synechocystis sp. PCC 6308) may be due to acid stress. They used hydrochloric acid for acidification, and the blue color was formed below pH 3.6. Probably, the acid formed from β-cyclocitral contributed to the pH-lowering effect to thus provide the characteristic blue color, although our resulting pH was higher than pH 3.6.
In summary, according to reports by Jüttner (1984), Jüttner and Höflacher (1985), and Watson (2003), β-cyclocitral is derived from β-carotene in Microcystis sp. Recently, it was found that β-carotene in cyanobacteria is cleaved by a specific enzyme (carotenoid cleavage dioxygenase, CCD) to provide retinal and related compounds (Kloer and Schulz 2006; Marasco et al. 2006). Probably, β-cyclocitral is formed together with 3-methyl-1-butanol by CCD in cyanobacteria and shows lytic activity against cyanobacteria. Because β-cyclocitral is more easily oxidized compared to similar aldehyde compounds, it is an interesting question whether β-cyclocitral or its acid contributes to the lytic activity. In a natural environment, cyanobacteria grow intensively during the summer season and gradually disappear in the autumn, and a sudden decline in the cyanobacteria has been observed in some cases (Fallon and Brock 1979). We observed a similar phenomenon together with blue color formation (Supplementary Material Figs. 2, 3, 4) in a lake. The present study provides support that β-cyclocitral plays an important role in this phenomenon. In a subsequent study, the dynamics of β-cyclocitral in cyanobacteria and the environment will be investigated in more detail.
References
Amemiya, Y. and Nakayama, O. 1989. Separation and sugar composition of gelatinous sheath carbohydrates produced by Microcystis. Jpn. J. Phycol. 37: 253–262.
Amemiya, Y., Kato, K., and Nakayama, O. 1988. Extracellular products of Microcystis species: formation of slime layer and DOC pool in surrounding waters. Verh. Internat. Verein. Limnol. 23: 1886–1892.
Fallon, R. D. and Brock, T. D. 1979. Lytic organisms and photoxidative effects: influence on blue-green algae (cyanobacteria) in lake Mendota, Wisconsin. Appl. Environ. Microbiol. 38: 499–505.
Huang, J. J., Kolodnyy, N. H., Redfeam J. T., and Allen, M. M. 2002. The acid stress response of the cyanobacterium Synochocystis sp. strain PCC 6308. Arch. Microbiol. 177: 486–493.
Jüttner, F. 1984. Dynamics of the volatile organic substances associated with cyanobacteria and algae in a eutrophic shallow lake. Appl. Environ. Microbiol. 47: 814–820.
Jüttner, F. and Höflacher, B. 1985. Evidence of β-carotene 7,8 (7’,8’) oxygenase (β-cyclocitral, crocentindial generating) in Microcystis. Arch. Microbiol. 141: 337–343.
Kloer, D. P. and Schulz, G. E. 2006. Structural and biological aspects of carotenoid cleavage. Cell. Mol. Life Sci. 63: 2291–2303.
Marasco, E. K., Vay, K., and Schidt-Dnnert, C. 2006. Identification of carotenoid cleavage dioxygenases from Nostoc sp. PCC 7120 with different cleavage activities. J. Biol. Chem. 281: 31583–31593.
Ozaki, K., Ohta, A., Iwata, C., Tsuji, K., Ito, E., Ikai, Y., and HARADA, K.-I. 2008. Lysis of cyanobacteria with volatile organic compounds. Chemosphere, 71: 1531–1538.
Sigee, D. C. R., Glenn, M. J., Andrews, E. G., Bellinser, R. D., Butler, H. A., Epton S., and Hendry, R. D. 1999. Biological control of cyanobacteria: principle and possibilites. Hydrobiologia 395/396: 161–172.
Tsuji, K., Asakawa, M., Anzai, Y., Sumino, T., and Harada, K.-I. 2006. Degradation of microcystins using immobilized microorganism isolated in an eutrophic lake. Chemosphere 65: 117–124.
Uchida, H., Kouchiwa, T., Watanabe, K., Kawasaki, A., Hodoki, Y., Otani, I., Yamamoto, Y., Suzuki, M., and Harada, K.-I. 1998. A coupled assay system for the lysis of cyanobacteria. Jpn. J. Water Treat. Biol. 34: 67–75.
Watson, S. B., 2003. Cyanobacterial and eukaryotic algal odour compounds: signals or by-products? A review of their biological activity. Phycologia 42: 332–350.
Wright, S. J., Linton, C. J., Edwards, R. A., and Drury, E. 1991. Isoamy alcohol (3-methyl-1-butanol), a volatile anticyanobacterial and phytotoxic product of some Bacillus spp. Lett. Appl. Microbiol. 13: 130–132.
Acknowledgement
The authors thank Dr. Yuriko Nozawa at Taisho Pharmaceutical Co. Ltd., for measurement of NMR spectra.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Harada, KI., Ozaki, K., Tsuzuki, S. et al. Blue Color Formation of Cyanobacteria with β-Cyclocitral. J Chem Ecol 35, 1295–1301 (2009). https://doi.org/10.1007/s10886-009-9706-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-009-9706-5