Abstract
Arabidopsis thaliana was used as an experimental model plant to investigate a tritrophic interaction between the plant, a specialist aphid herbivore, Brevicoryne brassicae, and its natural enemy, the parasitoid Diaeretiella rapae. The A. thaliana ecotype Col-5 was transformed with a functional 2-oxoglutarate dependent dioxygenase (BniGSL-ALK) that converts 3-methylsulfinylpropylglucosinolate and 4-methylsulfinylbutylglucosinolate to 2-propenylglucosinolate and 3-butenylglucosinolate, respectively. This transformation results in a change in the glucosinolate hydrolysis profile where 3-butenylisothiocyanate, 2-propenylisothiocyanate and 5-vinyloxazolidine-2-thione are produced in contrast to the wild-type plant where 4-methylsulfinylbutylisothiocyanate is the main product. Performance of B. brassicae was affected negatively by transforming Col-5 with BniGSL-ALK in terms of mean relative growth rates. In a series of behavioral bioassays, naïve D. rapae females were able to discriminate between B. brassicae infested and uninfested Col-5 plants transformed with BniGSL-ALK, with parasitoids showing a preference for B. brassicae infested plants. By contrast, naïve D. rapae females were unable to discriminate between aphid infested and uninfested Col-5 plants. Subsequent air entrainments of B. brassicae infested Col-5 plants transformed with BniGSL-ALK further confirmed the presence of 3-butenylisothiocyanate in the headspace. By contrast, no glucosinolate hydrolysis products were recorded from similarly infested Col-5 plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants can defend themselves against insect herbivores by actively releasing volatile compounds following attack, resulting in the attraction of beneficial natural enemies. There is currently considerable interest in modifying the secondary metabolism of plants to increase the attraction of natural enemies. Arabidopsis thaliana provides a useful model plant with which to study the relevance of specific secondary metabolites and their role in natural enemy attraction (van Poecke and Dicke, 2004). Terpenoid metabolism, for example, has been modified in A. thaliana by targeting an introduced sesquiterpene synthase to mitochondria, which resulted in production of two new isoprenoids and enhanced attraction of carnivorous predatory mites (Phytoseiulus persimilis) (Kappers et al., 2005). We have set out to modify the glucosinolate profile in A. thaliana in order to study tritrophic interactions between the plant, an insect pest (Brevicoryne brassicae) and its natural enemy, the braconid endoparasitoid (Diaeretiella rapae). Cruciferous plants (Brassicaceae) characteristically accumulate glucosinolates, a group of amino acid-derived secondary metabolites consisting of a β-thioglucose moiety, a sulfonated oxime, and a variable side chain. Together with endogenous myrosinase (β-thioglucoside glucohydrolase, 3.2.1.147) glucosinolates serve a central role in defense against herbivores and pathogens (Wink, 1988; Jander et al., 2001; Kliebenstein et al., 2005a, b). Tissue damage brings together myrosinase and glucosinolates, which are otherwise spatially separated (Kelly et al., 1998) yielding a variety of toxic hydrolysis products (Fig. 1), such as isothiocyanates, epithionitriles, thiocyanates, and nitriles (Bones and Rossiter, 1996, 2006; Halkier and Gershenzon, 2006).
The myrosinase-glucosinolate system is an effective defense against many generalist insect herbivores. However, specialist crucifer-feeding insects have evolved counter adaptive biochemical mechanisms to overcome this defense. This is achieved, in the larvae of the cabbage white butterfly, Pieris rapae (Wittstock et al., 2004), and the diamondback moth, Plutella xylostella (Ratzka et al., 2002), through different types of detoxification mechanisms. Other insect herbivores, including larvae of the turnip sawfly, Athalia rosae (Muller et al., 2001), the harlequin bug, Murcantia histrionica (Aliabadi et al., 2002), as well as the cabbage aphid, Brevicoryne brassicae, and the turnip aphid, Lipaphis pseudobrassicae (= L. erysimi) (Bridges et al., 2002; Kazana et al., 2007) appear to tolerate glucosinolates, and actually sequester these compounds from their host plants. Sequestration of glucosinolates provides these insects with a defense against potential natural enemies. In the two species of aphid, a myrosinase, distinct from plant myrosinase, has evolved, resulting in a defense that mimics that of their host plants, with the production of isothiocyanates following tissue damage (Jones et al., 2001, 2002; Bridges et al., 2002; Husebye et al., 2005). Isothiocyanates provide both a direct, toxic, defense against potential natural enemies (Francis et al., 2001), and an indirect defense by synergizing the response of aphids to the alarm pheromone, E-β-farnesene (Dawson et al., 1987). Despite this defense, both B. brassicae and L. pseudobrassicae are subject to attack by a range of natural enemies, such as insect parasitoids, which also apparently are adapted to toxic glucosinolate hydrolysis products (Bridges et al., 2002).
Exploitation of the cruciferous plants’ myrosinase-glucosinolate defense by B. brassicae is not restricted to glucosinolate accumulation. Volatile cues, in the form of isothiocyanates are also utilized by the aphid in host-plant location (Nottingham et al., 1991). Similarly, several studies have shown that the braconid wasp, D. rapae, a solitary endoparasitoid of aphids that feed on crucifers, is able to exploit glucosinolate hydrolysis products as olfactory cues in host foraging behavior. These olfactometry studies recorded positive behavioral responses of female parasitoids to several species of Brassica experimentally infested with aphids, including cabbage (Brassica oleracea) (Reed et al., 1995), turnip (Brassica rapa var rapifera) (Blande et al., 2007), and collard (Brassica oleracea) (Read et al., 1970), as well as the model crucifer A. thaliana (Girling et al., 2006). Additional olfactometry experiments, using glucosinolate hydrolysis products in place of aphid infested plants identified isothiocyanates as important signaling chemicals (Read et al., 1970; Reed et al., 1995; Vaughn et al., 1996; Bradburne and Mithen, 2000; Blande et al., 2007). Air entrainments further confirmed that higher levels of isothiocyanates were produced by aphid infested turnip plants compared with uninfested plants, and the ability of D. rapae females to discriminate between these two odor sources (Blande et al., 2007). Interestingly, parasitoids were not able to discriminate between plants infested with the specialist L. pseudobrassicae or the generalist Myzus persicae, despite the fact that once the aphid host is located, D. rapae attacks the crucifer-specialist at a greater rate than the generalist (Blande et al., 2004).
Tritrophic interactions are inherently complex, and the impact of specific compounds is often difficult to discern (Burow et al., 2006; Barker et al., 2007). Plants that differ in their glucosinolate or glucosinolate hydrolysis profile may also differ in other ways, such as the presence of other deterrents, stimulants, or synergists, leaf morphology, or nutritional content (Nielsen et al., 2001). These concerns may be addressed partly through the use of pure compounds and artificial diets, although this approach is less successful in defenses where toxic compounds are formed from inactive precursors, such as in the myrosinase-glucosinolate system (Wittstock et al., 2003).
The genetic characterization of model plants such as A. thaliana offers a potentially powerful tool with which to assess the importance of specific compounds in both bitrophic and tritrophic interactions. This approach has been applied to investigate the indirect roles of signaling pathways (Mewis et al., 2006) and a novel calmodulin-binding protein (Levy et al., 2005) on insect herbivory through their impacts on glucosinolate levels, while other studies have looked at the effects of elevated glucosinolate levels (Nielsen et al., 2001). A number of studies have focused on glucosinolate hydrolysis and the impact of epithiospecifier protein (ESP) (Jander et al., 2001; Lambrix et al., 2001; Burow et al., 2006) and the associated epithiospecifier modifier1 (ESM1) (Zhang et al., 2006). Other studies have focused on the functional significance of the myrosinase part of this defense (Barth and Jander, 2006).
There have been few studies looking at the impact of modifying the glucosinolate profile by transgenic methodology on plant-herbivore interaction, although recently the effect of overexpressing the dioxygenase AOP2 in Col-0 on a generalist pest was determined (Hansen et al., 2008). Variation in methionine-derived aliphatic glucosinolate side-chain modification in A. thaliana is controlled by the GS-AOP locus, which has three alleles that produce methylsulfinylalkyl (GS-null), alkenyl (GS-ALK), or hydroxyalkyl (GS-OHP) side-chains (Kliebenstein et al., 2001b, 2005a). The functional alleles code for 2-oxoglutarate-dependent dioxygenases that determine the nature of the aliphatic glucosinolate profile by converting the methylsulfinylalkyl functionality to alkenylglucosinolates (AtAOP2) or to hydroxyalkylglucosinolates (AtAOP3). The Columbia (Col) ecotype, however, does not possess a functional GS-AOP, and consequently accumulates 4-methylsulfinylbutylglucosinolate. Thus, by overexpressing a 2-oxoglutarate dependant dioxygenase in Col-5, it is possible to switch the plant from a methylsulfinylalkylglucosinolate producer to one with alkenylglucosinolates (Fig. 2). Here, we present responses of the crucifer specialist, B. brassicae and its natural enemy, D. rapae, to wild-type Col-5 plants and to Col-5 plants transformed with a functional AtAOP2 ortholog from Brassica nigra (BniGSL-ALK). We also compare the response of the parasitoid to the A. thaliana ecotype Ru-0, which produces 2-propenylisothiocyanate.
Methods and Materials
Plant Material and Growth Conditions Seeds of the Arabidopsis thaliana (L.). Heynh. ecotypes Col-5 and Ru-0 were obtained from the Nottingham Arabidopsis Stock Centre (NASC). Seeds were sown on wet soil (Levington F2 compost:vermiculite; 4:1) and cold treated for 2 d at 4°C to get homogenous germination. Seedlings were pricked out 2 wk after cold treatment and transferred to individual 40 × 40 × 50 mm plastic plant cells. Plants were grown under short day conditions in a controlled environment chamber (12 h light: 12 h dark regime, 100–150 µmol m−2 s−1 during light period; 22°C during light and 20°C during dark).
Cloning of an Ortholog of the Arabidopsis thaliana 2-oxoglutarate Dependent Dioxygenase 2 (AtAOP2) from Brassica nigra
An ortholog of the A. thaliana gene coding for the 2-oxoglutarate dependent dioxygenase 2 (AtAOP2; At4g03060) was cloned from B. nigra (L.) Koch genomic DNA by PCR using Pwo DNA polymerase (Roche Applied Science) according to the supplier’s instructions. We named this ortholog BniGSL-ALK.
The oligonucleotide primers (MWG Biotech, Ebersberg, Germany) used for amplification of BniGSL-ALK were designed on published sequences from Brassica oleracea: the genomic DNA sequence BoGSL-ALK (AY044425; Li and Quiros, 2003) and the corresponding region of clone B21H13 (AC122543). The sequence of the forward primer was 5’-atc cc atg ggtgcagacacttctcaacttc-3’ (in bold: start codon; underlined: NcoI site; in italics: additional triplet of an EcoRV restriction site) and that of the reverse primer was 5’-gcgcacgtgtttatgctccagagacg-3’ (in bold: stop codon; underlined: PmlI restriction site).
For cloning purposes, the environment of the original translation initiation site, contained in the forward primer, was changed to create an NcoI restriction site, and an additional 5’ATC-triplet of an EcoRV restriction site also was included. A PmlI restriction site was included in the reverse primer. The PCR amplified product was cloned into EcoRV- restricted pBluescript SK + and partially sequenced.
Ectopic Expression of BniGSL-ALK in Arabidopsis thaliana
An NcoI/PmlI-restricted fragment containing BniGSL-ALK was excised from the pBluescript construct, inserted into the binary vector pCAMBIA3301, where it is under the control of a CaMV35S promoter, and the integration sites of the insert were verified by sequencing. The BniGSL-ALK expression construct was transferred into Agrobacterium tumefaciens (strain GV3101) by electroporation (Sambrook et al., 1989). Transformation of A. thaliana with A. tumefaciens was performed according to the “floral dip method” (Clough and Bent, 1998). As the bar gene in the BniGSL-ALK expression construct confers phosphinothricin (Basta) resistance to transformed plants, seeds were sowed on soil wetted with a Basta solution and watered with this Basta solution every second day. Plantlets resisting this treatment were transferred to individual pots, watered subsequently without selection agent, and the presence of the transgene was verified by PCR analysis. For this purpose, DNA was extracted from leaves as described by Edwards et al. (1991).
RNA Extraction and Northern Blot Analysis
Total RNA was extracted from leaves of 5 wk-old A. thaliana plants as described by Logemann et al. (1987). Ten µg of total RNA was fractionated on denaturing 1.5% formaldehyde agarose gels (Sambrook et al., 1989), and equal RNA loading was determined by ethidium bromide staining. The transfer of RNA to Hybond-N nylon membrane (GE Healthcare, Little Chalfont, UK) was done by capillary blotting (Sambrook et al., 1989). A 0.5 kb gel purified (QiaexII Kit, Qiagen, Crawley, UK) fragment generated by PCR amplification on the cloned BniGSL-ALK was used as probe. The labeling of the probe was performed with 32P-dCTP (MP Biomedicals, UK) using the DecaLabel DNA Labeling Kit as indicated by the supplier (Fermentas, St. Leon-Rot, Germany). Hybridization was performed in Church buffer at 65 ºC overnight (approximately 16 h), and washes were carried out once at 65 ºC in 1xSSC, 0.1% SDS for 20 min, and once at 65 ºC in 0.1xSSC, 0.1% SDS for 20 min. The Blot was exposed for a day to a BioMAX MS film (Kodak, Hemel Hempstead, UK).
Extraction of Glucosinolates
Fully expanded leaves from 5 wk-old A. thaliana plants were collected and freeze dried. In the case of the analyzed transgenic line Col-5 (+BniGSL-ALK)-4, homozygous plants of the T3 generation were used. Leaf material was ground to a fine powder with a pestle and mortar, and 20 mg were used for the extraction of glucosinolates. The remainder of the extraction process was carried out as previously described (Heaney and Fenwick, 1993).
Analysis of Glucosinolates by HPLC
Samples were analysed by high performance liquid chromatography (HPLC) on an Agilent 1200 series instrument equipped with a Phenomenex Luna 3 micron C18(2) (150 × 2 mm) column. Freeze dried plant tissue was extracted with boiling 80% methanol for 5 min, and the process repeated. At this stage, either 2-propenylglucosinolate or benzylglucosinolate was added as a standard. Combined supernatants were concentrated to dryness with nitrogen gas, and the residue was reconstituted in water (1 ml). A barium/lead acetate (Rossiter et al., 1990) solution (0.1 ml) was added to each sample and allowed to precipitate. After centrifugation, the extract was applied to a DEAE-A25 sephadex column and treated with desulfatase (Rossiter et al., 1990). Desulfoglucosinolates were separated by using a water-acetonitrile gradient (solvent A water, solvent B acetonitrile; 0 – 15 min 25 % B; 15–17 min 70 % B) at a flow rate of 0.2 ml min−1 and monitored at 229 nm. Retention times of known standards were used to identify desulfoglucosinolates, and sample peaks were confirmed by LC-MS. For mass spectrometry, an Applied Biosystems QTrap interfaced with APCI source was used. The source temp was set to 475°C, and the MS was run in the Enhanced Mass Spectrum mode scanning in the range 70–500 amu.
Extraction of Glucosinolate Hydrolysis Products
Leaves of A. thaliana plants (100–200 mg of fresh weight) were crushed and incubated for 10 min at room temperature. The homogenate was extracted with dichloromethane (4 ml), dried with anhydrous MgSO4, centrifuged, and the organic phase concentrated to approximately 200 µl under a flow of nitrogen.
Analysis of Glucosinolate Hydrolysis Profile by GC-MS
The dichloromethane extracted glucosinolate hydrolysis products were analyzed by Gas Chromatography-Mass Spectrometry (GC-MS) on a Hewlett-Packard 6890 gas chromatograph linked to a 5973 mass selective detector. Injections were made onto an HP-5MS 5% Phenylmethylsiloxane (30 m × 0.25 mm × 0.25 µm) column in the pulsed split (20:1) mode using the following temperature program: inlet temp 225°C; initial temperature 40°C, 5 min; 5°C min−1 until 180°C; 10°C min−1 until 280°C; hold for 10 min. All compounds were identified by using standards and by their mass fragmentation patterns (Spencer and Daxenbichler, 1980).
Insect Cultures
The crucifer specialist aphid Brevicoryne brassicae (L.) was maintained at 18°C, with a 16/8 h L/D photoperiod. Aphids were cultured on individual 4 wk-old Brassica nigra plants, each plant being enclosed within a perforated bread bag. Aphids were transferred to fresh plants every 1–2 wk.
The aphid parasitoid Diaeretiella rapae (McIntosh) (obtained from Rothamsted Research, Harpenden, UK) was maintained at 23°C (16 h light) and 18°C (8 h dark). Standardized cohorts of parasitoids were produced by allowing mated 2–3 d-old adult insects to parasitize mixed age B. brassicae in a Petri dish for approximately 2 h. Parasitized aphids then were transferred to a fresh B. nigra plant (unless otherwise stated) on which they continued to feed. Aphid mummies were removed from these plants approximately 8 d later so that on emergence parasitoids were not provided with cues that might influence behavioral responses. Adult male and female parasitoids were kept together, and mating was assumed. Adult parasitoids were provided with a dilute honey solution, and females were used in the bioassays approximately 72 h after emergence.
Aphid Mean Relative Growth Rate Bioassays
Young (<24 h-old) B. brassicae nymphs were collected from B. nigra plants. Each nymph was weighed (MX5, Mettler-Toledo, Greifensee, Switzerland) and transferred individually to a 5 wk-old A. thaliana plant. Each plant then was covered with a perforated bread bag and maintained for 5 d at 18°C with a 16/8 h L/D photoperiod. Each aphid then was reweighed and returned to the same plant. The mean relative growth rates (MRGR) were calculated using the following equation:
where W1 = end weight (mg), W0 = start weight (mg) and d = development time (days) (Castle and Berger, 1993).
Comparisons of MRGR values of B. brassicae reared either on A. thaliana ecotype Col-5 or the Col-5 transgenic line expressing a functional 2-oxoglutarate dependent dioxygenase (BniGSL-ALK) were made. Statistical analysis of the MRGR values was completed using unpaired t-tests (Genstat 6th Edition).
Olfactometry
A Y-tube olfactometer, of the design previously described by Du et al. (1996) was used to record behavioral responses of female D. rapae to A. thaliana plants infested with B. brassicae. A glass Y-tube olfactometer was used, which had 12 mm internal diameter, 100 mm stem and 100 mm arms at 60° angle. Air was pumped through Teflon tubing by a Dymax30 pump (Charles Austen Pumps Ltd., Byfleet, Surrey, UK) through an activated charcoal filter before being regulated by a flowmeter to 800 ml/min. The airflow then was split by a brass T-junction (Swagelok, OH, USA), each flow of 400 ml/min then passing into an airtight glass chamber (T. Westlake — Artistic & Scientific Glassblower, Kidlington, Oxfordshire, UK) into which the volatile source was placed. From the two glass chambers, air flowed through additional Teflon tubing into the arms of the olfactometer via modified glass quick fit sockets. A white cardboard screen was placed around the olfactometer, in order to exclude any visual cues, and lighting was provided by a fluorescent strip held 300 mm above and 100 mm in front of the branches of the olfactometer. A single female D. rapae was introduced into the stem of the olfactometer and then given 5 min to make a choice. During this period, if the parasitoid failed to move more than 50 mm up the stem of the olfactometer, it was excluded from the experiment. Parasitoids were recorded as having selected an odor if the insect moved more than 50 mm up one of the arms and remained beyond this point for more than 30 sec. Insects that moved more than 50 mm up the stem but did not select an arm of the olfactometer were recorded as not having selected an odor. After each individual was tested, the position of the odor sources was swapped in order to account for any directional bias by the parasitoids. These criteria are similar to those previously described (Girling et al., 2006; Blande et al., 2007). Glassware was washed with acetone and distilled water and then baked overnight at 200°C.
In the following two experiments, two 5 wk-old (3 wk after pricking out) A. thaliana plants were presented as odor sources. Responses of naïve D. rapae females to A. thaliana ecotypes Ru-0 and Col-5 and the transgenic line Col-5 (+BniGSL-ALK)-4 were recorded. In both experiments, responses of naïve female parasitoids were recorded to a B. brassicae infested plant and an uninfested plant of the same ecotype/transgenic line. In each experiment, responses were recorded from two ecotypes/transgenic lines in this way. A final comparison was completed by taking the aphid infested plant of each ecotype/transgenic line and presenting these opposite each other. In an initial experiment, naïve D. rapae were reared on B. brassicae, which in turn were reared on B. nigra. B. brassicae used to infest Ru-0 or Col-5 plants in this experiment were previously reared on the corresponding ecotype. In a second experiment, naïve D. rapae were reared on B. brassicae, which in turn were reared on Col-5. B. brassicae used to infest Col-5 or transgenic plants in this experiment were previously reared on the corresponding ecotype or transgenic line. For each comparison, plants were replaced after every 10 or 15 replicates. Each plant was either left uninfested or infested with approximately 100 B. brassicae (mixed age) for approximately 72 h before completing the bioassay. Naïve female parasitoids, produced as previously described, were used in bioassays.
Chi-square values were used for statistical analysis of numbers of parasitoids responding to each odor source.
Air Entrainments
For air entrainments of B. brassicae infested A. thaliana plants, plants were grown as described above. However, 2 wk after being sown, seedlings were pricked out into the center of 60 mm diam pots. A plastic sheath (5 mm diam) then was carefully placed around each seedling. Seedlings were allowed to continue to grow under the same conditions for a further 3 wk. Approximately 100 mixed age B. brassicae, previously reared on the corresponding A. thaliana ecotype or transgenic line, were transferred onto a test plant. Three days later, a glass vessel (60 mm diam) was placed around the plant. The vessel was in two parts and was held together with clips, clamping around the plastic sheath that protected the hypocotyl of the plant. Charcoal filtered air was pumped into the vessel at 300 ml min−1 through polytetrafluoroethylene (PTFE) tubing (1.6 mm inner diam). Air was drawn out of the vessel at 200 ml min−1 through a separate outlet, thereby creating a positive pressure within the vessel and preventing atmospheric air from entering. Air entrainments were completed within a growth chamber set to 12 h light, 150 µmol m−2 s−1, 65% relative humidity, and constant 20°C. Volatiles were trapped onto Tenax TA (50 mg, mesh 60–80, Supelco, Bellefonte, PA, USA) held in injector liners by plugs of silanized glass wool. Before use, liners were first washed with 2 ml of redistilled diethyl ether before being thermally desorbed at 200 ºC under a flow of helium. Tenax TA filled liners were fitted to both the inlet (providing an extra layer of filtration of air entering the vessel) and outlet of each glass vessel. Volatiles were trapped onto the Tenax TA for a period of 4 h. The liners were then thermally desorbed by using an Optic 2 programmable injector (Anatune, Cambridge, UK) connected to a Hewlett Packard 6890 Series gas chromatograph fitted with a Hewlett Packard 5973 mass selective detector (GC-MS). Injector conditions were equilibrated for 30 sec and then ramped from 50 to 200 ºC at 16°C s−1. An HP-5MS column (30 m × 0.25 mm × 0.25 µm) was used. The carrier gas was helium (constant flow 30 cm s−1). Initial oven temperature was 50°C held for three min, then ramped to 200 ºC at 10 ºC min−1 and held for a further 2 min.
A comparison of volatile chemicals emitted by the A. thaliana ecotype Col-5, the Col-5 (+BniGSL-ALK)-4 transgenic line, and the ecotype Ru-0 when infested with approximately 100 mixed age B. brassicae (previously reared on the corresponding ecotype or transgenic line) or left uninfested was completed.
Results
Altering the Glucosinolate Profile of the Arabidopsis thaliana Accession Col-5 by Expressing the Dioxygenase BniGSL-ALK
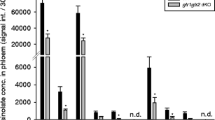
A homolog from Brassica oleracea, called BoGSL-ALK, also has been cloned and characterized previously (Li and Quiros, 2003). We cloned a Brassica nigra gene that encodes a putative polypeptide that shows over 90% and about 60% sequence similarity to BoGSL-ALK and AtAOP2, respectively (data not shown). By analogy to BoGSL-ALK, we called this gene BniGSL-ALK. We expressed it under the control of a CaMV35S promoter in the A. thaliana ecotype Col-5, where the predominant glucosinolate in rosette leaves is 4-methylsulfinylbutylglucosinolate (Table 1). As expected, the glucosinolate content of rosette leaves of the transgenic plants expressing BniGSL-ALK [called hereafter Col-5 (+BniGSL-ALK)] differed qualitatively from the glucosinolate content of the wild-type Col-5 plants. A positive correlation between the transgene expression level and the degree of change of the glucosinolate profile (data not shown) was observed in all characterized independent primary transformants. The glucosinolate profile of rosette leaves of the T3 progeny of the high transgene expression line Col-5 (+BniGSL-ALK)-4 is shown in Table 1. It lacked the short-chain methylsulfinylalkylglucosinolates characteristic of the wild-type Col-5 completely, and contained primarily 3-butenylglucosinolate and 2-hydroxybut-3-enylglucosinolate and to a lesser extent 2-propenylglucosinolate. Transgenic plants maintained at least wild-type levels of the longer chain methylsulfinylalkylglucosinolates (Table 1). Of the two indolylglucosinolates detected in rosette leaves of both the wild-type Col-5 and the transgenic line, only the levels of indol-3-ylmethylglucosinolate were significantly higher in the latter (Table 1) by a factor of 1.6. Aliphatic glucosinolate and total glucosinolate content in rosette leaves were also higher in the transgenic line compared with wild-type Col-5 (Table 1).
As Col-5 does not possess a functional epithiospecifier protein (ESP) (Lambrix et al., 2001; de Torres Zabala et al., 2005) both the wild-type and the Col-5 (+BniGSL-ALK) transgenic lines produce isothiocyanates upon hydrolysis of rosette leaves. Consequently, the hydrolysis profile of the transgenic line Col-5 (+BniGSL-ALK)-4 gave the expected 2-propenylisothiocyanate, 3-butenylisothiocyanate, and 5-vinyloxazolidine-2-thione that correspond to the modified glucosinolate profile. The hydrolysis profiles of both wild-type Col-5 and of the transgenic line Col-5 (+BniGSL-ALK))-4 are shown in Fig. 3A and 3B.
Glucosinolate hydrolysis products from rosette leaves (5 wk-old) of; a wild-type Col-5, peak 1 = methylthiobutylisothiocyanate, 2 = 4-methylsulfinylbutylisothiocyanate b transgenic line Col-5 (+BniGSL-ALK)-4, peak 3 = 2-propenylisothiothiocyanate, peak 4 = 3-butenylisothiocyanate, peak 5 = 5-vinyloxazolidine-2-thione, analysed by GC-MS. TIC = total ion current
Aphid Mean Relative Growth Rate Bioassays
The mean relative growth rate (MRGR) of B. brassicae (Fig. 4) was significantly lower when feeding on Col-5 plants transformed with a gene encoding a functional 2-oxoglutarate dependent dioxygenase (BniGSL-ALK), compared with wild-type Col-5 plants (t = 2.44, P = 0.025). This difference corresponds to an increased weight gain of approximately 0.03 mg for aphids feeding on wild-type Col-5 plants compared to aphids feeding on the transgenic Col-5 (+BniGSL-ALK)-4 plants during the five-day period of this experiment. It was subsequently recorded that aphids took approximately 0.5 days longer to complete development when feeding on transgenic Col-5 (+BniGSL-ALK)-4 plants compared to Col-5 wild-type plants.
Air Entrainments
Col-5 plants infested with B. brassicae produced no detectable quantities of glucosinolate-derived volatile chemicals (data not shown). However, from air entrainments of plants of the transgenic line Col-5 (+BniGSL-ALK)-4 infested with B. brassicae, relatively large amounts of 3-butenylisothiocyanate were identified (Fig. 5A, chromatogram a) with a trace of 2-propenylisothiocyanate. In the uninfested line, only a trace amount of 3-butenylisothiocyanate was detected (Fig. 5A, chromatogram b). The Arabidopsis ecotype Ru-0 infested with B. brassicae produced 2-propenylisothiocyanate (Fig. 5B, chromatogram c), while the uninfested plant produced no detectable isothiocyanate (Fig. 5B, chromatogram d).
Air entrainments of (A) transgenic Col-5 (+BniGSL-ALK)-4 plants infested with Brassica brassicae (trace a) and uninfested (trace b); (B) ecotype Ru-0 infested with Brevicoryne brassicae (trace c) and uninfested (trace d). Plants (5 week old) were exposed to 100 aphids and volatiles were collected 72 h post infestation. Glucosinolate hydrolysis products were analysed by GC-MS. Peak 1 = 2-propenylisothiothiocyanate, peak 2 = 3-butenylisothiocyanate. TIC = total ion current
Olfactometry
Two experiments were carried out to evaluate the response of the parasitoid D. rapae towards B. brassicae infested and uninfested plants with different glucosinolate profiles. Results from these experiments indicate that naïve female parasitoids discriminate between aphid infested and uninfested Ru-0 or the transgenic Col-5 (+BniGSL-ALK)-4 plants, with D. rapae females showing a preference for aphid infested plants. By contrast, D. rapae females did not discriminate between aphid infested and uninfested Col-5 plants (Figs. 6A and 6B). When both a B. brassicae infested Ru-0 and Col-5 plant were presented as an odor source, parasitoids were again able to discriminate between the odor sources (Fig. 6A). However, naïve D. rapae females did not discriminate between aphid infested Col-5 and Col-5 (+BniGSL-ALK)-4 plants (Fig. 6B).
(A) Responses of naïve Diaeretiella rapae females in a Y-tube olfactometer to Ru-0 or Col-5 plants either infested with Brevicoryne brassicae or uninfested using D. rapae reared on B. nigra (n = 30); (B) Responses of naïve Diaeretiella rapae females in a Y-tube olfactometer to Col-5 or the transgenic line Col-5 (+BniGSL-ALK)-4 either infested with B. brassicae or uninfested using D. rapae reared on Col-5 (n = 30); INF = infested, UNINF = uninfested, Col-5 (+BniGSL-ALK) = transgenic line expressing BniGSL-ALK
Discussion
In order to examine the effect of differing glucosinolate profiles and/or glucosinolate hydrolysis profiles on the crucifer specialist, B. brassicae, and its natural enemy, the parasitoid, D. rapae, we created transgenic A. thaliana lines that overexpress a gene from B. nigra encoding a dioxygenase. B. nigra accumulates 2-propenylglucosinolate (Cole, 1976) indicating the presence of a functional AtAOP2 ortholog whose expression in the A. thaliana Col-5 background would be expected to result in a glucosinolate profile predominantly composed of 3-butenylglucosinolate. The glucosinolate profile of rosette leaves of transgenic plants that express BniGSL-ALK, the AtAOP2 ortholog we cloned from B. nigra, differed from that of the wild-type Col-5 plants and contained the alkenylglucosinolates 3-butenylglucosinolate, 2-hydroxybut-3-enylglucosinolate, and 2-propenylglucosinolate (Table 1). This shows that the BniGSL-ALK gene we cloned and transformed into the plant is functional and responsible for converting 4-methylsulfinylbutylglucosinolate and 3-methylsulfinylpropylglucosinolate into the respective alkenylglucosinolates. The transgenic Col-5 (+BniGSL-ALK) plants maintain concentrations of the longer chain methylsulfinylalkylglucosinolates (Table 1) suggesting that BniGSL-ALK is unable to catalyze the conversion of these glucosinolates.
The glucosinolate profile changes that we observed in the transgenic Col-5 (+BniGSL-ALK)-4 line are in accordance with an earlier report where the authors transformed the Columbia ecotype with BoGSL-ALK, a Brassica oleracea ortholog of AtAOP2, and observed the appearance of the same glucosinolates as we described above (Li and Quiros, 2003). The appearance of 2-hydroxybut-3-enyl glucosinolate in Col-5 (+BniGSL-ALK)-4 plants is probably not due to the enzymatic activity of the introduced dioxygenase but to that of another, recently identified dioxygenase (Hansen et al., 2008). The absence of 2-hydroxybut-3-enylglucosinolate in the wild-type Col-5 is due to the absence of substrate (i.e., 3-butenylglucosinolate) as a consequence of the lack of a functional AtAOP2.
The fact that indol-3-ylmethylglucosinolate levels were slightly higher in the rosette leaves of the Col-5 (+BniGSL-ALK)-4 plants than in the wild-type Col-5 was unexpected. AtAOP2 is involved in the side chain modifications of aliphatic glucosinolates, and no role in indolylglucosinolate biosynthesis has been reported (Kliebenstein et al., 2001b). Our results are, however, in accordance with a recent report describing a higher total amount of foliar indolylglucosinolate in A. thaliana lines that express BoGSL-ALK (Wentzell et al., 2007). In addition, we measured a higher total amount of glucosinolates in rosette leaves of the transgenic plants, which was largely due to an increase in aliphatic glucosinolates. In a survey of glucosinolate contents in 39 A. thaliana ecotypes, the GS-AOP locus (or closely linked loci) was revealed to control 61% of the variation in leaf aliphatic glucosinolates, and ecotypes with GS-AOP null presented four times lower aliphatic glucosinolate concentrations than ecotypes with GS-ALK (Kliebenstein et al., 2001a). Wentzell et al. (2007) describe a doubling of total foliar aliphatic glucosinolate content in A. thaliana lines that express BoGSL-ALK. Expressing the B. nigra ortholog BniGSL-ALK in Col-5 seems to have a similar effect, although the increase in total foliar glucosinolate levels in our transgenic plants was less pronounced than in the above-mentioned study.
The crucifer specialist B. brassicae has developed a defense mechanism that allows it to sequester plant-derived glucosinolates (Francis et al., 2001; Kazana et al., 2007), but shows a reduced MRGR when fed on the transgenic Col-5 (+BniGSL-ALK)-4 plants compared to the wild-type Col-5. This could be a direct effect of the BniGSL-ALK expression, and can be explained by one or a combination of the glucosinolate related changes i.e., change in profile from methylsulfinylalkylglucosinolates to alkenylglucosinolates, increased amounts of indol-3-ylmethylglucosinolate, or increase in the total content of glucosinolates. Previous work with an A. thaliana mutant (atr1D) that overproduces indolylglucosinolates (Kim et al., 2008) has shown that these plants are toxic to Myzus persicae. However, the increase in indolylglucosinolates in our transgenic plants was small in comparison to that of the atr1D mutant. The subsequent changes in hydrolysis products (i.e., production of alkenylisothiocyanates instead of methylsulfinylalkylisothiocyanates, increased amount of hydrolysis products released) could also contribute, although the impact of glucosinolate hydrolysis products produced during attack on these aphids is not clear (Barth and Jander, 2006). However, of significance is the presence of 5-vinyloxazolidine-2-thione in the hydrolysis profile. It is possible that this degradation product has an adverse effect on aphid performance, and this requires further investigation. In addition, plant-aphid interactions are not static, and several, although sometimes conflicting, studies have shown that aphid attacks affect gene expression and glucosinolate levels (Mewis et al., 2005, 2006; de Vos et al., 2007; Kusnierczyk et al., 2007), and such results may contribute to what we observed.
Air entrainment results presented here suggest that the higher volatility of 2-propenylisothiocyanate and 3-butenylisothiocyanate compared with that of 4-methylsulfinylbutylisothiocyanate may be an important determining factor in olfactometry results with naïve D. rapae females. Ecotype Ru-0 produced 2-propenylisothiocyanate when infested with B. brassicae, but this compound was not detected from uninfested plants. Similarly, the transgenic line Col-5 (+BniGSL-ALK)-4 produced 3-butenylisothiocyanate when infested with aphids, while only trace amounts of this compound were detected from uninfested plants. In both cases, naïve D. rapae females were able to discriminate between B. brassicae infested and uninfested plants. By contrast, for ecotype Col-5 no detectable quantities of glucosinolate-derived volatile chemicals were detected in air entrainments, and naïve female parasitoids were unable to discriminate between aphid infested and uninfested plants. Similarly, it was found (Girling et al., 2006) that naïve D. rapae females were unable to discriminate between a Col-gl plant infested with M. persicae and an uninfested plant. However, parasitoids were able to discriminate between these two odor sources if they were first provided with oviposition experience; thereby suggesting that the volatile profiles of aphid infested and uninfested plants did in fact differ.
By exploiting the wild-type Col-5 and the transgenic line Col-5 (+BniGSL-ALK)-4, we were able to record behavioral responses of D. rapae females to near isogenic A. thaliana plants with contrasting glucosinolate hydrolysis profiles. Interestingly, although more parasitoids responded to aphid infested Col-5 (+BniGSL-ALK)-4 plants when presented as odor sources against aphid infested Col-5 plants, the difference was not significant. This suggests that although naïve D. rapae females did not discriminate between aphid infested and uninfested Col-5 plants, the odor from the wild-type plant was either weakly attractive or in some way disrupted the behavioral response of the parasitoids to the transgenic plants. By contrast, naïve D. rapae females did discriminate between B. brassicae infested Ru-0 plants and similarly infested Col-5 plants. This result provides evidence of discrimination between B. brassicae infested A. thaliana ecotypes by naïve D. rapae females, and may also reflect the importance of glucosinolate cues accumulated as the parasitoid emerges from the aphid mummy (Blande et al., 2004). This is because parasitoids responding to Ru-0 plants were reared on B. brassicae infested B. nigra plants, which like Ru-0 accumulate 2-propenylglucosinolate as the main secondary metabolite. However, although reared on B. brassicae infested Col-5 plants, which accumulate 4-methylsulfinylbutylglucosinolate as opposed to the alkenylglucosinolates produced by the transgenic plants, naïve D. rapae responded to transgenic but not wild-type plants.
Results presented here indicate that transforming Col-5 plants with BniGSL-ALK may increase indirect defense against B. brassicae by increasing apparency to its natural enemy, D. rapae. Coupled with the reduced MRGRs of B. brassicae when feeding on transgenic plants compared with wild-type plants this would suggest that the transformation enhanced both direct and indirect defense against this specialist insect herbivore. This and other work manipulating glucosinolate and hydrolysis profiles by transgenic approaches will lead to a better understanding of the role of secondary metabolites in plant-insect interactions.
References
ALIABADI, A., RENWICK, J. A. A., and WHITMAN, D. W. 2002. Sequestration of glucosinolates by harlequin bug Murgantia histrionica. J. Chem. Ecol. 28:1749–1762.
BARKER, J. E., POPPY, G. M., and PAYNE, C. C. 2007. Suitability of Arabidopsis thaliana as a model for host plant-Plutella xylostella-Cotesia plutellae interactions. Entomol. Exp. Appl. 122:17–26.
BARTH, C. and JANDER, G. 2006. Arabidopsis myrosinases TGG1 and TGG2 have redundant function in glucosinolate breakdown and insect defense. Plant J. 46:549–562.
BLANDE, J. D., PICKETT, J. A., and POPPY, G. M. 2004. Attack rate and success of the parasitoid Diaeretiella rapae on specialist and generalist feeding aphids. J. Chem. Ecol. 30:1781–1795.
BLANDE, J. D., PICKETT, J. A., and POPPY, G. M. 2007. A comparison of semiochemically mediated interactions involving specialist and generalist Brassica-feeding aphids and the braconid parasitoid Diaeretiella rapae. J. Chem. Ecol. 33:767–779.
BONES, A. M. and ROSSITER, J. T. 1996. The myrosinase-glucosinolate system, its organization and biochemistry. Physiol. Plantarum 97:194–208.
BONES, A. M. and ROSSITER, J. T. 2006. The enzymic and chemically induced decomposition of glucosinolates. Phytochemistry. 67:1053–67.
BRADBURNE, R. P. and MITHEN, R. 2000. Glucosinolate genetics and the attraction of the aphid parasitoid Diaeretiella rapae to Brassica. Proc. R. Soc. B-Biol. Sci. 267:89–95.
BRIDGES, M., JONES, A. M. E., BONES, A. M., HODGSON, C., COLE, R., BARTLET, E., WALLSGROVE, R., KARAPAPA, V. K., WATTS, N., and ROSSITER, J. T. 2002. Spatial organization of the glucosinolate-myrosinase system in brassica specialist aphids is similar to that of the host plant. Proc. Royal Soci. London, Series B: Biological Sciences 269:187–191.
BUROW, M., MULLER, R., GERSHENZON, J., and WITTSTOCK, U. 2006. Altered glucosinolate hydrolysis in genetically engineered Arabidopsis thaliana and its influence on the larval development of Spodoptera littoralis. J. Chem. Ecol. 32:2333–2349.
CASTLE, S. J. and BERGER, P. H. 1993. Rates of growth and increase of Myzus persicae on virus-infected potatoes according to type of virus-vector relationship. Entomol. Exp. Appl. 69:51–60.
CLOUGH, S. J. and BENT, A. F. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16:735–743.
COLE, R. A. 1976. Isothiocyanates, nitriles and thiocyanates as products of autolysis of glucosinolates in Cruciferae. Phytochemistry 15:759–62.
DAWSON, G. W., GRIFFITHS, D. C., PICKETT, J. A., WADHAMS, L. J., and WOODCOCK, C. M. 1987. Plant-derived synergists of alarm pheromone from turnip aphid, Lipaphis (Hyadaphis) erysimi (Homoptera, Aphididae). J. Chem. Ecol. 13:1663–1671.
DE TORRES ZABALA, M., GRANT, M., BONES, A. M., BENNETT, R., LIM YIN, S., KISSEN, R., and ROSSITER, J. T. 2005. Characterisation of recombinant epithiospecifier protein and its over-expression in Arabidopsis thaliana. Phytochemistry 66:859–67
DE VOS, M., KIM, J. H., and JANDER, G. 2007. Biochemistry and molecular biology of Arabidopsis-aphid interactions. BioEssays 29:871–883
DU, Y. J., POPPY, G. M. ,and POWELL, W. 1996. Relative importance of semiochemicals from first and second trophic levels in host foraging behavior of Aphidius ervi. J. Chem. Ecol. 22:1591–1605.
EDWARDS, K., JOHNSTONE, C. and THOMPSON, C. 1991. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucl. Acids Res. 19:1349–1349.
FRANCIS, F., LOGNAY, G., WATHELET, J. P., and HAUBRUGE, E. 2001. Effects of allelochemicals from first (Brassicaceae) and second (Myzus persicae and Brevicoryne brassicae) trophic levels on Adalia bipunctata. J. Chem. Ecol. 7:243–256.
GIRLING, R. D., HASSALL, M., TURNER, J. G., and POPPY, G. M. 2006. Behavioural responses of the aphid parasitoid Diaeretiella rapae to volatiles from Arabidopsis thaliana induced by Myzus persicae. Entomol. Exp. Appl. 120:1–9.
HALKIER, B. A. and GERSHENZON, J. 2006. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 57:303–333.
HANSEN, B. G., KERWIN, R. E., OBER, J. A., LAMBRIX, V. M., MITCHELL-OLDS, T., GERSHENZON, J., HALKIER, B. A., and KLIEBENSTEIN, D. J. 2008. A novel 2-oxoacid-dependent dioxygenase involved in the formation of the goiterogenic 2-hydroxybut-3-enyl glucosinolate and generalist insect resistance in Arabidopsis. Plant Physiol.148:2096–2108.
HEANEY, R. K. and FENWICK, R. G. 1993. Methods for glucosinolate analysis. Methods in Plant Biochemistry 8 (Alkaloids and Sulphur Compounds): pp. 531–50.
HUSEBYE, H., ARZT, S., BURMEISTER, W. P., HAERTEL, F. V., BRANDT, A., ROSSITER, J. T., and BONES, A. M. 2005. Crystal structure at 1.1 Å. resolution of an insect myrosinase from Brevicoryne brassicae shows its close relationship to β-glucosidases. Insect Biochem. Mol. Biol. 35:1311–1320.
JANDER, G., CUI, J. P., NHAN, B., PIERCE, N. E., and AUSUBEL, F. M. 2001. The TASTY locus on chromosome 1 of Arabidopsis affects feeding of the insect herbivore Trichoplusia ni. Plant Physiol. 126:890–898.
JONES, A. M. E., BRIDGES, M., BONES, A. M., COLE, R., and ROSSITER, J. T. 2001. Purification and characterization of a non-plant myrosinase from the cabbage aphid Brevicoryne brassicae (L.). Insect Biochem. Mol. Biol. 31:1–5.
JONES, A. M. E., WINGE, P., BONES, A. M., COLE, R., and ROSSITER, J. T. 2002. Characterization and evolution of a myrosinase from the cabbage aphid Brevicoryne brassicae. Insect Biochem. Mol. Biol. 32:275–284.
KAPPERS, I. F., AHARONI, A., VAN HERPEN, T., LUCKERHOFF, L. L. P., DICKE, M., and BOUWMEESTER, H. J. 2005. Genetic engineering of terpenoid metabolism attracts bodyguards to Arabidopsis. Science 309:2070–2072
KAZANA, E., POPE, T. W., TIBBLES, L., BRIDGES, M., PICKETT, J. A., BONES, A. M., POWELL, G., and ROSSITER, J. T. 2007. The cabbage aphid: a walking mustard oil bomb. Proc. R. Soc. B-Biol. Sci. 274:2271–2277.
KELLY, P. J., BONES, A., and ROSSITER, J. T. 1998. Sub-cellular immunolocalization of the glucosinolate sinigrin in seedlings of Brassica juncea. Planta 206:370–377.
KIM, J. H., LEE, B. W., SCHROEDER, F. C., and JANDER, G. 2008. Identification of indole glucosinolate breakdown products with antifeedant effects on Myzus persicae (green peach aphid). Plant J. 54:1015–1026.
KLIEBENSTEIN, D. J., KROYMANN, J., BROWN, P., FIGUTH, A., PEDERSEN, D., GERSHENZON, J., and MITCHELL-OLDS, T. 2001a. Genetic control of natural variation in Arabidopsis glucosinolate accumulation. Plant Physiol. 126:811–825.
KLIEBENSTEIN, D. J., LAMBRIX, V. M., REICHELT, M., GERSHENZON, J., and MITCHELL-OLDS, T. 2001b. Gene duplication in the diversification of secondary metabolism: Tandem 2-oxoglutarate-dependent dioxygenases control glucosinolate biosynthesis in Arabidopsis. Plant Cell 13:681–693.
KLIEBENSTEIN, D. J., KROYMANN, J., and MITCHELL-OLDS, T. 2005a. The glucosinolate-myrosinase system in an ecological and evolutionary context. Curr. Opin. Plant Biol. 8:264–271.
KLIEBENSTEIN, D. J., ROWE, H. C. and DENBY, K. J. 2005b. Secondary metabolites influence Arabidopsis/Botrytis interactions: variation in host production and pathogen sensitivity. Plant J. 44:25–36.
KUSNIERCZYK, A., WINGE, P., MIDELFART, H., ARMBRUSTER, W. S., ROSSITER, J. T., and BONES, A. M. 2007. Transcriptional responses of Arabidopsis thaliana ecotypes with different glucosinolate profiles after attack by polyphagous Myzus persicae and oligophagous Brevicoryne brassicae. J. Exp. Bot. 58:2537–2552.
LAMBRIX, V., REICHELT, M., MITCHELL-OLDS, T., KLIEBENSTEIN, D. J., and GERSHENZON, J. 2001. The Arabidopsis epithiospecifier protein promotes the hydrolysis of glucosinolates to nitriles and influences Trichoplusia ni herbivory. Plant Cell 13:2793–2807.
LEVY, M., WANG, Q. M., KASPI, R., PARRELLA, M. P., and ABEL, S. 2005. Arabidopsis IQD1, a novel calmodulin-binding nuclear protein, stimulates glucosinolate accumulation and plant defense. Plant J. 43:79–96.
LI, G. and QUIROS, C. F. 2003. In planta side-chain glucosinolate modification in Arabidopsis by introduction of dioxygenase Brassica homolog BoGSL-ALK. Theor. Appl. Genet. 106:1116–1121.
LOGEMANN, J., SCHELL, J., and WILLMITZER, L. 1987. Improved method for the isolation of RNA from plant tissues. Anal. Biochem. 163:16–20.
MEWIS, I., APPEL, H. M., HOM, A., RAINA, R., and SCHULTZ, J. C. 2005. Major signaling pathways modulate Arabidopsis glucosinolate accumulation and response to both phloem-feeding and chewing insects. Plant Physiol. 138:1149–1162.
MEWIS, I., TOKUHISA, J. G., SCHULTZ, J. C., APPEL, H. M., ULRICHS, C., and GERSHENZON, J. 2006. Gene expression and glucosinolate accumulation in Arabidopsis thaliana in response to generalist and specialist herbivores of different feeding guilds and the role of defense signaling pathways. Phytochemistry 67:2450–2462.
MULLER, C., AGERBIRK, N., OLSEN, C. E., BOEVE, J. L., SCHAFFNER, U., and BRAKEFIELD, P. M. 2001. Sequestration of host plant glucosinolates in the defensive hemolymph of the sawfly Athalia rosae. J. Chem. Ecol. 27:2505–2516.
NIELSEN, J. K., HANSEN, M. L., AGERBIRK, N., PETERSEN, B. L., and HALKIER, B. A. 2001. Responses of the flea beetles Phyllotreta nemorum and P. cruciferae to metabolically engineered Arabidopsis thaliana with an altered glucosinolate profile. Chemoecology 11:75–83.
NOTTINGHAM, S. F., HARDIE, J., DAWSON, G. W., HICK, A. J., PICKETT, J. A., WADHAMS, L. J., and WOODCOCK, C. M. 1991. Behavioral and electrophysiological responses of aphids to host and nonhost plant volatiles. J. Chem. Ecol.17:1231–1242.
RATZKA, A., VOGEL, H., KLIEBENSTEIN, D. J., MITCHELL-OLDS, T., and KROYMANN, J. 2002. Disarming the mustard oil bomb. Proc. Natl. Acad. Sci. USA 99:11223–11228.
READ, D. P., FEENY, P. P., and ROOT, R. B. 1970. Habitat selection by aphid parasite Diaeretiella rapae (Hymenoptera:Braconidae) and hyperparasite Charips brassicae (Hymenoptera:Cynipidae). Canad. Entomologist 102:1567–1578.
REED, H. C., TAN, S. H., HAAPANEN, K., KILLMON, M., REED, D. K., and ELLIOTT, N. C. 1995. Olfactory responses of the parasitoid Diaeretiella rapae (Hymenoptera: Aphidiidae) to odor of plants, aphids, and plant-aphid complexes. J. Chem. Ecol. 21:407–418.
ROSSITER, J. T., JAMES, D. C. and ATKINS, N. 1990. Biosynthesis of 2-hydroxy-3-butenylglucosinolate and 3-butenylglucosinolate in Brassica napus. Phytochemistry 29:2509–2512.
SAMBROOK, J., FRITSCH, E. F., and MANIATIS, T. 1989. Molecular Cloning, a Laboratory Manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press
SPENCER, G. F. and DAXENBICHLER, M. E. 1980. Gas chromatography-mass spectrometry of nitriles, isothiocyanates and oxazolidinethiones derived from cruciferous glucosinolates. J. Sci. Food Agric. 31:359–367.
VAN POECKE, R. M. P. and DICKE, M. 2004. Indirect defence of plants against herbivores: Using Arabidopsis thaliana as a model plant. Plant Biol. 6:387–401
VAUGHN, T. T., ANTOLIN, M. F., and BJOSTAD, L. B. 1996. Behavioral and physiological responses of Diaeretiella rapae to semiochemicals. Entomol. Exp. Appl. 78:187–196.
WENTZELL, A. M., ROWE, H. C., HANSEN, B. G., TICCONI, C., HALKIER, B. A., and KLIEBENSTEIN, D. J. 2007. Linking metabolic QTLs with network and cis-eQTLs controlling biosynthetic pathways. PloS Genet. 3:1687–1701.
WINK, M. 1988. Plant breeding: importance of plant secondary metabolites for protection against pathogens and herbivores. Theor. Appl. Genet. 75:225–233.
WITTSTOCK, U., AGERBIRK, N., STAUBER, E. J., OLSEN, C. E., HIPPLER, M., MITCHELL-OLDS, T., GERSHENSON, J., and VOGEL, H. 2004. Successful herbivore attack due to metabolic diversion of a plant chemical defense. Proc. Natl. Acad. Sci. USA 101:4859–4864.
WITTSTOCK, U., KLIEBENSTEIN, D. J., LAMBRIX, V., REICHELT, M., and GERSHENZON, J. 2003. Glucosinolate hydrolysis and its impact on generalist and specialist aphid herbivores. pp 101–125 in: Romeo JT, editor. Integrative Phytochemistry: from Ethnobotany to Molecular Ecology. Oxford: Pergamon.
ZHANG, Z. Y., OBER, J. A., and KLIEBENSTEIN, D. J. 2006. The gene controlling the quantitative trait locus EPITHIOSPECIFIER MODIFIER1 alters glucosinolate hydrolysis and insect resistance in Arabidopsis. Plant Cell 18:1524–1536.
Acknowledgments
This work was supported by the BBSRC (Biotechnology and Biological Sciences Research Council) (grant 32/P17268). Rothamsted receives grant-aided support from the BBSRC. We thank Wendy Byrne and Valerie Elliot for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ralph Kissen and Tom W. Pope contributed equally
Rights and permissions
About this article
Cite this article
Kissen, R., Pope, T.W., Grant, M. et al. Modifying the Alkylglucosinolate Profile in Arabidopsis thaliana Alters the Tritrophic Interaction with the Herbivore Brevicoryne brassicae and Parasitoid Diaeretiella rapae . J Chem Ecol 35, 958–969 (2009). https://doi.org/10.1007/s10886-009-9677-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-009-9677-6