Abstract
Mycotoxins, such as aflatoxins and ochratoxins, are widely distributed in nature and are frequently problematic crop contaminants that cause millions of dollars of annual losses in the United States. Insect infestations of crop plants significantly exacerbate mycotoxin contamination. Damage to a variety of nut species by Amyelois transitella Walker (navel orangeworm, NOW) is associated with infection by Aspergillus species and concomitant production of aflatoxins and ochratoxins. Resistance to aflatoxins in this lepidopteran is compared here with the levels of resistance in Helicoverpa zea (corn earworm, CEW), another lepidopteran that routinely encounters aflatoxins in its diet, albeit at lower levels. Measured as the developmental delay caused by aflatoxin B1 (AFB1), it is apparent that the LC50 (defined as the concentration preventing 50% of newly hatched larvae from entering the 2nd instar within 48 h) for AFB1 is 100 times greater for A. transitella than for H. zea. Similarly, A. transitella 1st instars display substantially higher tolerance to ochratoxin A, another mycotoxin contaminant produced by Aspergillus species, than do H. zea. Our studies indicate that A. transitella, although a hostplant generalist, may well be highly specialized for mycotoxin detoxification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The navel orangeworm Amyelois transitella (Walker) (NOW: Lepidoptera: Pyralidae) is a serious crop pest that attacks almonds, pistachio, walnuts, and figs, causing millions of dollars of agricultural damage in the United States (Connell 2001; Campbell et al. 2003; Burks and Brandl 2004). Damage results from consumption of the nutmeat and contamination of the nut with webbing and frass. In addition to causing such direct losses, NOW feeding damage predisposes almonds to infection by Aspergillus spp., which produces toxic aflatoxins and ochratoxins. Particularly susceptible are soft-shelled cultivars with an extended split hull period (Schade et al. 1975; Schatzki and Ong 2000, 2001). The occurrence of fungal contamination in pistachio, almond, and figs also is closely associated with the extent of NOW damage (Widstrom 1979; Schatzki and Ong 2000, 2001; Campbell et al. 2003). Aflatoxin contamination has tremendous trade implications inasmuch as export to Europe is restricted based on minimum levels (Otsuki et al. 2001).
Aflatoxins are synthesized by Aspergillus flavus and Aspergillus parasiticus, with aflatoxins B1, B2, G1, and G2 most commonly produced (Cleveland et al. 2003). Of these, aflatoxin B1 (AFB1) causes a number of serious health problems in humans including acute and chronic toxicity to humans and mammals by directly damaging tissues, mutating important genes, inducing and inhibiting the expression of other genes, and interfering with apoptosis (Eaton and Gallagher 1994; McLean and Dutton 1995; Neal 1995; Guengerich et al. 1998). Economic losses due to aflatoxin contamination are estimated at millions of dollars in the U.S. each year. To minimize the harmful effects of aflatoxins, strict regulations have been established in the US. While crops and finished food products that contain aflatoxins above 20 ppb are not allowed for human consumption, the limitation is set at 5 ppb for milk (Cleveland et al. 2003; Williams et al. 2004). Two other fungi, Aspergillus ochraceus and Penicillium verrucosum, primarily produce ochratoxins. Ochratoxin A (OTA) interferes with immune system function (Neal 1995) and also may be carcinogenic. Because of these properties, commodities contaminated with high concentrations of mycotoxins (e.g., 20 ppb for aflatoxins) are blocked from sale both in the U.S. and overseas (Park and Troxell 2002; Bayman and Baker 2006). Without appropriate monitoring measures, mycotoxin-contaminated food has had serious impacts on human health in many areas, especially in developing countries in Asia and Africa (Williams et al. 2004).
The level of tolerance to mycotoxins in insects varies from several ppb to several ppm and is both species- and sex-specific (Gudauskas et al. 1967; Chinnici et al. 1976; Llewellyn and Chinnici 1978; Chinnici and Llewellyn 1979; Zeng et al. 2006). Drosophila melanogaster larvae, which feed naturally on fermenting fruits, exhibit significantly altered development on diets containing low AFB1 concentrations (i.e., less than 1 ppm); larvae feeding on higher concentrations will die (Kirk et al. 1971; Chinnici et al. 1976; Llewellyn and Chinnici 1978; Chinnici and Llewellyn 1979). The corn earworm, Helicoverpa zea (CEW), another species that encounters mycotoxin-releasing fungi in damaged plants (Widstrom et al. 1976; Widstrom 1979; Archer and Bynum 1994), also is tolerant of aflatoxins. Low concentrations of AFB1 in the diet of 1st instars (less than 100 ng/g) can affect development, causing delayed pupation and reduced pupal weight; higher concentrations of AFB1 (200 ng/g) are lethal (Zeng et al. 2006).
The toxicity of aflatoxins to insects depends on their metabolism as mediated by cytochrome P450 monooxygenases (P450s, Phase I detoxification enzymes) into bioactive products. One of the most toxic of these is the AFB1-exo-epoxide, which is derived from AFB1. The bioactivated metabolite of AFB1, exo-8,9-epoxide (AFBO), can bind directly to proteins and DNA and cause cell death (Eaton and Gallagher 1994; McLean and Dutton 1995; Guengerich et al. 1998; Suriawinata and Xu 2004). Glutathione-S-transferases (GSTs, Phase II detoxification enzymes) conjugate glutathione to the lipophilic products of P450-mediated metabolism to form water-soluble metabolites that are more readily excreted (Hayes et al. 2005). In rodent livers, susceptibility to AFB1 is inversely associated with GST activity toward AFBO (Lotlikar et al. 1984; Monroe and Eaton 1988; Buetler et al. 1992; Johnson et al. 1997). Another biotransformation of AFB1 is its direct reduction by NADPH-dependent aflatoxin B1 aldehyde reductases (AFAR) to form aflatoxicol (AFL). However, this product is converted rapidly back to AFB1 by dehydrogenases (Eaton and Gallagher 1994). Alternative biotransformation pathways that generate detoxified metabolites, such as aflatoxicol, aflatoxin M1, and aflatoxin B2a, and that do not generate bioactivated epoxidized metabolites also exist in NOW and contribute to the high aflatoxin tolerance of this species (Lee and Campbell 2000). Characterization of similar biotransformation pathways in CEW indicate that at least one P450 in this species, specifically CYP321A1, is capable of converting AFB1 into polar metabolites, with the demethylated product aflatoxin P1 (AFP1) as the principal metabolite (Niu et al. 2008).
Given the existence of alternative biotransformation pathways in both NOW and CEW, we initiated a series of studies to compare toxicity and tolerance of mycotoxins in these two species. Because NOW is associated regularly with fungus-contaminated hostplant tissue, we hypothesized that its level of tolerance to both aflatoxins and ochratoxins should exceed that of CEW, which only occasionally encounters fungal contamination inasmuch as it feeds on fruits before they fall to the ground.
Methods and Materials
Chemicals
Piperonyl butoxide (PBO) was purchased from Tokyo Kassie Kogyo (Tokyo, Japan). Aflatoxins B1, G1, and ochratoxin A were purchased from Sigma Chemicals (St. Louis, MO, USA). Analytical grade dimethyl sulfoxide (DMSO) was obtained from Fisher Scientific (Pittsburgh, PA, USA). All chemicals were stored at −20°C and dissolved in DMSO to make stock solutions.
Insects
Laboratory colonies of H. zea (originating from specimens collected in Champaign County, IL, USA), and A. transitella (originating on almond trees in Fresno, CA, USA that have been maintained in culture for the past 25 years by the Commodity Protection and Quality Unit, USDA/ARS, Parlier, CA, USA) were kept in an insectary at UIUC at 28 ± 4°C with 16-h/8-h L/D cycle. H. zea larvae were reared individually from egg hatch in 5 oz (142 g) plastic cups containing approximately 5 g control or supplemented diet. A. transitella larvae were mass-reared until pupation in 500 ml plastic containers containing 200 g of wheat bran diet (Tebbets et al. 1978).
Bioassays
AFB1 and OTA stock solutions were prepared in DMSO and stored at −20°C for up to 2 months. Diets containing AFB1 (1, 5, 10, 20, 50, and 100 µg/g) were made from AFB1 stock solutions (1, 100, or 500 µl of 1 mg/ml in 0.5% DMSO) and incorporated into 100 g of diet to make 1 or 5 µg/g of AFB1 diet; 50, 100, 250 and 500 µl of 20 mg/ml of AFB1 stock solution were added to 100 g of the diet to make 10, 20, 50 and 100 µg/g of AFB1 diets in 0.5% DMSO. For low concentrations of AFB1 diets (i.e., 20, 40, 60, 80, 100, 120, or 140 ng/g), AFB1 stock solution in 0.2% DMSO (20, 40, 60, 80, 100,120, 140 µl of 100 µg/ml) was added to 100 g of diet. OTA stock solutions (50 or 250 µl of 2 mg/ml in 0.25% DMSO) were incorporated into 100 g of the OTA–containing diet to make 1 or 5 µg/g of the OTA diet; in addition, OTA (50, 100, or 250 µl of 20 mg/ml in 0.25% DMSO) was added to 100 g of the diet to make 10, 20, or 50 µg/g of the OTA diet. Pilot experiments determined that concentrations of DMSO up to 0.5% of the diet do not affect development or survivorship in either NOW or CEW.
To assess the toxicity of AFB1 to NOW, six concentrations of AFB1 (1, 5, 10, 20, 50, and 100 µg/g) diets were prepared. NOW neonates (20 newly hatched 1st instars) were placed individually into 142-g plastic cups that contained 0.5 g unamended diet or supplemented diet prepared with a final concentration of 0.5% DMSO. Two higher concentrations of AFB1-containing diets (50 and 100 µg/g) were fed to 20 ultimate instars (5th) with 0.5% DMSO as control. Larvae were monitored until pupation, and final pupal weights were measured within 48 h after the final larval molt. Each set of bioassays was replicated three times.
Similarly, OTA toxicity to NOW larvae was tested with a variety of OTA-containing diets (1, 5, 10, 20, or 50 µg/g OTA at a final concentration in 0.25% DMSO); 1st instars (20) in each group were treated. On the 12th day, the proportion of dead larvae was recorded, and each larva was weighed on the 14th day. The pupation rate was measured within 48 h of pupation. To test the toxicity of OTA to CEW, 1st instars were exposed to diets containing 1 or 5 µg/g OTA in DMSO (0.25%) and control diet. The survivorship of larvae at 12 days post-hatch on diets and frequency of 5th instars at 12 days post-hatch were recorded.
To compare the effects of AFB1 on the development of NOW and CEW, 1st instar CEW larvae were fed artificial diets containing 0.2% DMSO and 10, 20, 40, 60, 80, 100, 120, or 140 ng/g AFB1, while 1st instar A. transitella were fed with artificial diets containing 0.5% DMSO, 1, 5, or 10 µg/g AFB1. For each species, the numbers of larvae reaching the 2nd instar were recorded within 48 h.
Midgut Protein Preparations
To obtain sufficient larval midgut tissue, guts were dissected from ultimate instars (at least 100) that had molted within the previous 12 h. Midgut dissections were performed in 0.1 M phosphate buffer and on ice. The midguts were frozen in liquid nitrogen and stored at −80°C until use. For each midgut preparation of ultimate instars, frozen midguts (approx. 40) were ground in a mortar in the presence of liquid nitrogen. The resulting material was transferred into a Eppendorf tube (1.5 ml), suspended in phosphate buffer (1.5 ml; 0.1 M; pH 7.4), EDTA (1 mM), PMSF (0.5 mM), DTT (0.1 mM), and 20% glycerol; the mixture was shaken gently for 5 s. These midgut homogenates were centrifuged (10,000 rpm; 10 min) in an Eppendorf centrifuge at 4°C. The cleared supernatant was transferred into a new 1.5 ml Eppendorf tube for immediate use, or frozen in liquid nitrogen and stored at −80°C for up to 6 months. Protein concentrations for the cleared lysates were determined by using the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA, USA).
In Vitro Metabolism Assays
For all assays, the frozen cleared lysates were taken from the −80°C freezer and thawed on ice (approx. 30 min) before the metabolism assays were initiated. AFB1 and OTA metabolism assays were carried out as described previously in Lee and Campbell (2000) with only slight modification. Stock solutions of AFB1 or OTA at 10 µg/g were prepared in DMSO. Each 250-µl reaction contained AFB1 (1 µl) or OTA stock (10 µg/µl), reduced glutathione (5 mM), protein (1 mg/g), sodium phosphate buffer (0.1 mmol/l; pH 7.4), and EDTA (1 mM). Reactions were initiated with the addition of 50 µl of 3 mM NADPH. After a 60-min incubation period (at 30°C with shaking), 1 ml of ice-cold methanol containing 10 µM AFG1 (internal standard) was added to terminate reactions. The reaction mixtures were centrifuged (10,000 g for 10 min) in an Eppendorf centrifuge, and each supernatant was analyzed by high-pressure liquid chromatography (HPLC). All reactions were run in triplicate and repeated at least twice. To trap the AFB1-exo-epoxide metabolite, mouse liver cytosol (10 μl, 50 mg/ml) was added into reactions containing NOW crude proteins. Reactions were stopped after incubation (30°C for 1 h) and analyzed (10 μl) by HPLC.
AFB1 and its metabolites were separated by HPLC using a reverse-phase Supercosil LC-18 column (250 × 4.6 mm), a flow rate of 1 ml/min, and were detected at 362 nm with photodiode array UV detector (Waters Novapak). A ternary solvent system consisting of water/methanol/acetonitrile (60:20:20) was used to separate AFG1 (eluted at 10.6 min) and AFB1 (eluted at 14.6 min). Separation of OTA was achieved with an acetic acid/methanol/acetonitrile (1/49/49) solvent system, at a flow rate of 1 ml, and monitoring at 333 nm. OTA eluted at 10.4 min.
Statistics
All data were analyzed by using a one-way ANOVA with a post-hoc Tukey test at a significance level of P < 0.05.
Results
Aflatoxin B1 And Ochratoxin A Toxicity to NOW Larvae Amyelois transitella larvae tolerated high dietary levels of AFB1, with concentrations of 1, 5, 10, and 20 µg/g AFB1 having no apparent toxic effect on 1st instars (Table 1). In diets containing 10 and 20 µg/g AFB1, pupation rates were 77% and 78%, respectively. These did not differ significantly from pupation rates on control DMSO diet, and there was no significant difference in pupal weights between diets containing 10 and 20 µg/g AFB1 and control diets. On the diet containing 50 µg/g AFB1, larval development was delayed and only 55% of the larvae successfully pupated, a rate significantly lower than the rate with the control diet (P < 0.05). At the extremely high AFB1 concentration of 100 µg/g, development of 1st instar A. transitella was severely inhibited; however, 30% of the treated larvae survived for at least 3 weeks. Exposure of later stage larvae to diets containing varying concentrations of AFB1 showed that their tolerance to AFB1 increased with developmental stage; ultimate instars tolerated 100 µg/g AFB1 with no significant decreases in pupation rate compared to control larvae fed diets containing 0.5% DMSO (Table 2).
Similarly, A. transitella 1st instars also were tolerant of OTA, with concentrations less than 10 µg/g having no detectable toxic effect (Table 3). At higher OTA concentrations (20 µg/g), there were no significant differences in mortality rates or larval weight after 14 days. Compared with control larvae fed diets with 0.25% DMSO, A. transitella fed with the highest concentration of OTA (50 µg/g) experienced slight effects on development, as evidenced by lower larval weights; larval weights after 14 days were significantly lower than those fed control diet (P = 0.047). The highest concentration of OTA (50 µg/g) did not lower the pupation rate (Table 3).
Comparison of OTA and AFB1 Toxicity of to CEW and NOW
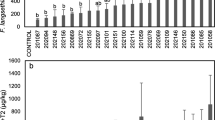
The toxicity of AFB1 in A. transitella and H. zea was measured to compare the effect of AFB1 on 1st instars. The LC50 for AFB1 A. transitella (defined as the concentration preventing 50% of newly hatched larvae from molting to 2nd instar within 48 h) is 100 times greater for than for H. zea (Fig. 1). After 48 h, 60 ng/g AFB1 allowed 50% of 1st instar H. zea to develop into 2nd instars, while 10 µg/g AFB1 allowed 80% of 1st instar A. transitella to develop to 2nd instars—a greater than 100-fold difference in tolerance.
Developmental effects of AFB1 on 1st instar Helicoverpa zea and Amyelois transitella. a Percentage of H. zea larvae molting to 2nd instar after 48 h on diets containing increasing concentrations of AFB1 and a final concentration of 0.2% DMSO. b Percentage of A. transitella larvae molting to 2nd instar after 48 h on diets containing increasing concentrations of AFB1 and a final concentration of 0.5% DMSO
Comparisons of the toxicity of OTA to A. transitella and H. zea also revealed considerably greater resistance to OTA in A. transitella. Development of 1st instar A. transitella was not affected by concentrations as high as 1 and 5 µg/g OTA. These concentrations significantly inhibited the development of 1st instar H. zea compared with control larvae, with 10% or no 5th instars appearing after 10 days on 1 or 5 µg/g OTA-supplemented diets, and 40% 5th instars appearing on the 0.25% DMSO control diet (P = 0.032 or 0.015, respectively) (Fig. 2).
Toxicity of OTA to 1st instar larvae of Helicoverpa zea. a Survivorship of larvae 12 days post-hatch on diets containing increasing concentrations of OTA and a final concentration of 0.25% DMSO. b Frequency of 5th instar larvae 12 days post-hatch on diets containing 0.25% DMSO, 1 µg/g OTA, or 5 µg/g OTA
NOW Metabolism of AFB1 and OTA
To investigate the ability of A. transitella to metabolize AFB1, in vitro metabolism experiments were conducted with cleared midgut lysates quantified based on total protein. In reactions initiated with NADPH (as outlined in Methods and Materials), two polar metabolites of AFB1 (Met1 with Rt = 7.8 min and Met2 with Rt = 8.0 min) were readily detected by HPLC analysis (data not shown). Compared with H. zea midgut lysates, A. transitella midgut lysates have higher turnover rates (32 ± 4 pmol/min/mg total protein vs. 0 pmol/min/mg total protein). In contrast, CEW midguts show no activity toward AFB1 unless induced by prior exposure to coumarin and xanthotoxin (Niu et al. 2008).
In an attempt to trap reactive AFBO intermediates, mouse liver cytosol was added to some in vitro reactions, but no additional metabolite peaks were detected (data not shown). This finding is consistent with the suggestion that AFB1 is detoxified, and not bioactivated, as the result of hydroxylation by midgut proteins. Comparable reactions with OTA showed no OTA metabolism by A. transitella or H. zea lysates either at the level of substrate disappearance or metabolite production. The methods used in this analysis may have been insufficiently sensitive to detect metabolism; alternatively, both of these species may rely on detoxification in tissues other than midgut, or non-enzymatic means, to counter the toxic effects of this compound.
Discussion
Zeng et al. (2006) and Niu et al. (2008) studied the toxicity and metabolism of AFB1 by H. zea, a lepidopteran with tolerance to a broad range of phytochemicals but only limited tolerance to aflatoxins even though it occasionally encounters aflatoxin-releasing fungi. In contrast, A. transitella, a herbivore that specifically locates and feeds on unharvested fruits, is tolerant of extremely high concentrations of mycotoxins in its diet. Our studies indicate that A. transitella, although a hostplant generalist, may well be highly specialized for mycotoxin detoxification. Indeed, recent studies indicate that NOW grows better on fungus-contaminated hostplant tissue and in fact may consume fungal tissue as well (Palumbo et al. 2008; personal observations).
In this study, we demonstrated the extremely high tolerance of A. transitella to concentrations of AFB1 and OTA and showed that this tolerance exceeds that of other mycotoxin-associated species such as H. zea. The concentrations analyzed did not kill A. transitella when administered at early developmental stages and caused only marginal sublethal effects. Comparisons of the toxicological effects in A. transitella and H. zea demonstrated that A. transitella larvae are 100 times more tolerant of AFB1 than H. zea and also are significantly more resistant to OTA. The ecological consequences of this tolerance are apparent in the high survival rates of A. transitella larvae in the mycotoxin-contaminated environments that it exploits as a scavenger. The interaction between NOW and Aspergillus species might be mutualistic in that the fungus may contribute to maintaining a micro-environment that optimizes caterpillar growth and development, and the caterpillar may promote the dispersal and establishment of the fungus by causing damage that allows fungal spores to gain access to fruits (Weis 1982). Aspergillus species can be cultured from adults, and the levels are high in adults emerging from Aspergillus-infested substrate (JS, unpublished data).
Aflatoxin tolerance might be explained by an excess detoxification capacity that generates nontoxic metabolites. Both Lee and Campbell (2000) and this study demonstrated that bioactivated metabolites are not produced in the reactions of NOW proteins. Because AFBO production increases toxicity 100-fold compared to its parent compound (AFB1) (Eaton and Gallagher 1994; McLean and Dutton 1995; Guengerich et al. 1998; Suriawinata and Xu 2004), absence of AFBO production by NOW might be a key feature of AFB1 tolerance. In contrast with AFB1, there are no reports of insect detoxification of OTA. Although we failed to detect metabolites of OTA produced by midgut proteins of either H. zea or A. transitella, bioactivation is involved in its genotoxicity to other organisms (Manderville 2005; Schaut et al. 2008), and the absence of major pathways that produce bioactivated metabolites also may reflect adaptation of these insects to the presence of this mycotoxin in their diet.
References
Archer, T. L., and Bynum, Jr. E.D. 1994. Corn earworm (Lepidoptera: Noctuidae) biology on food corn on the High Plains. Environ. Entomol. 23:343–348.
Bayman, P., and Baker, J. L. 2006. Ochratoxins: a global perspective. Mycopathologia 162:215–23.
Buetler, T. M, Slone, D., and Eaton, D. L. 1992. Comparison of the aflatoxin Bl-8,9-epoxide conjugating activity of two bacterially expressed alpha class glutathione S-transferase isozymes from mouse and rat. Biochem. Biophys. Res. Commun. 188:597–603.
Burks, C. S., and Brandl, D. G. 2004. Seasonal abundance of the navel orangeworm, Amyelois transitella, in figs and the effect of peripheral aerosol dispensers on sexual communication. J. Insect. Sci. 4:40–47.
Campbell, B. C., Molyneux, R. J., and Schatzki, T. F. 2003. Current research on reducing pre- and post-harvest aflatoxin contamination of US almond, pistachio, and walnut. J. Toxicol.:Toxin Reviews 22:225–266.
Chinnici, P. J., and Llewellyn, C. G., 1979. Reduced aflatoxin toxicity in hybrid crosses of aflatoxin B, sensitive and resistant strains of Drosophila melanogaster (Diptera). J. Invert. Pathol., 27:255–258.
Chinnici, J.P., Booker M.A., Llewellyn G. C., 1976. Effect of aflatoxin B1 on viability, growth, fertility, and crossing over in Drosophila melanogaster (Diptera). J. Invert. Pathol., 27:255–258.
Cleveland, T.E., Dowd, P.F., Desjardins, A.E., Bhatnagar, D., and Cotty, P.J. 2003. United States Department of Agriculture-Agricultural Research Service research on pre-harvest prevention of mycotoxins and mycotoxigenic fungi in US crops. Pest Manag. Sci. 59:629–642.
Connell, J. H. 2001. Leading edge of plant protection for almond. Hort. Technology 12:619–622.
Eaton, L.D., and Gallagher, P. E. 1994. Mechanisms of aflatoxin carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 34:135–172.
Gudauskas, R. T., Davis, N. D., Diener, U. L., 1967. Sensitivity of Heliothis virescens larvae to aflatoxin in ad libitum feeding. J. Invert. Pathol., 9:132–133.
Guengerich, F. P., Johnson, W. W., Shimada, T., Ueng, Y. F., Yamazaki, H., and Langouet, S. 1998. Activation and detoxication of aflatoxin B1. Mutat. Res. 402:121–128.
Hayes, J. D., Flanagan, J. U., and Jowsey, I. R. 2005. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 45:51–88.
Johnson, W. W., Ueng, Y. F., Widersten, M., Mannervik, B., Hayes, J. D., Sherratt, P. J., Ketterer, B., and Guengerich, F. P. 1997. Conjugation of highly reactive aflatoxin B1 exo-8,9-epoxide catalyzed by rat and human glutathione transferases: estimation of kinetic parameters. Biochemistry 36:3056–3060.
Lee, S.E., and Campbell, B. C. 2000. In vitro metabolism of aflatoxin B1 by larvae of navel orangeworm, Amyelois transitella (Walker) (Insecta, Lepidoptera, Pyralidae) and codling moth, Cydia pomonella (L.) (Insecta, Lepidoptera, Tortricidae). Arch. Insect Biochem. Physiol. 45:166–174.
Llewellyn, C.G., and Chinnici, P.J.,1978. Variation in sensitivity to aflatoxin B, among several strains of Drosophila melanogaster (Diptera). J. Invert. Pathol. 31: 37–40.
Lotlikar, P. D., Jhee, E. C., Insetta, S. M., and Clearfield, M. S. 1984. Modulation of microsome-mediated aflatoxin Bl binding to exogenous and endogenous DNA by cytosolic glutathione S-transferases in rat and hamster livers. Carcinogenesis 5:269–276.
Manderville, R. A. 2005. A case for the genotoxicity of ochratoxin A by bioactivation and covalent DNA adduction. Chem. Res. Toxicol. 18:1091–7.
McLean, M., and Dutton, M. F. 1995. Cellular interactions and metabolism of aflatoxin: an update. Pharmacol. Ther. 65:163–192.
Monroe, D. H., and Eaton, D. L. 1988. Effects of modulation of hepatic glutathione on biotransformation and covalent binding of aflatoxin Bl to DNA in the mouse. Toxicol. Appl. Pharmacol. 94:118–127.
Neal, G. E. 1995. Genetic implications in the metabolism and toxicity of mycotoxins. Toxicol. Lett. 82–83:861–867.
Niu, G., Wen, Z., Rupasinghe, S.G., Zeng, R.S., Berenbaum, M.R., and Schuler M. A. 2008. Aflatoxin B1 detoxification by CYP321A1 in Helicoverpa zea. Arch. Insect Biochem. Physiol. 69:32–45.
Otsuki, T., Wilson, S. J., and Sewadeh, M. 2001. Saving two in a billion: quantifying the trade effect of European food safety standards on African exports. Food Policy 26: 495–514.
Palumbo, J. D., Mahoney N. E., and Light, D. M. 2008. Navel orangeworm (Amyelois transitella) as a vector of Aspergillus flavus on almonds. Phytopathology 98:S119.
Park, D. L., and Troxell, T. C. 2002. U.S. perspective on mycotoxin regulatory issues. Adv. Exp. Med. Biol. 504:277–285.
Schade, J. E., McCreev, Y. K., King, A. D., Mackey, B., and Fuller, G. 1975. Incidence of aflatoxin in California almonds. Appl. Microbiol. 29:48–53.
Schatzki, T. F., and Ong, M. S. 2000. Distribution of aflatoxin in almonds. 2. Distribution in almonds with heavy insect damage. J. Agric. Food Chem. 48:489–492.
Schatzki, T. F., and Ong, M. S. 2001. Dependence of aflatoxin in almonds on the type and amount of insect damage. J. Agric. Food Chem. 49:4513–4519.
Schaut, A., De Saeger, S., Sergent, T., Schneider, Y. J., Larondelle, Y., Pussemier, L., Blank, R., and Van Peteghem, C. 2008. Liquid chromatographic methods for biotransformation studies of ochratoxin A. Biomed. Chromatogr. 22:1013–1020.
Suriawinata, A., and Xu, R. 2004. An update on the molecular genetics of hepatocellular carcinoma. Semin. Liver Dis. 24:77–88.
Tebbets, J.S., Curtis, C.E., and Fries, R.D. 1978. Mortality of immature stages of the navel orangeworm stored at 3.5°C. J. Econ. Entomol. 71:875–876.
Weis, A. E.1982. Use of a symbiotic fungus by the gall maker Asteromyia carbonifera to inhibit attack by the parasitoid Torymus capite. Ecology 63:1602–1604.
Widstrom, N. W. 1979. The role of insects and other plant pests in aflatoxin contamination of corn, cotton, and peanuts—a review. J. Environ. Qual. 8:5–11.
Widstrom, N. W., Lillehoj, B. E., Sparks, N. A., and Kwolek, F. W. 1976. Corn earworm damage and aflatoxin B, on corn ears protected with insecticide. J. Econ. Entomol. 69:677–679.
Williams, J. H., Phillips, T. D., Jolly, P. E., Stiles, J. K., Jolly, C. M., and Aggarwal, D., 2004. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 80:1106–1122.
Zeng, R. S., Niu, G., Wen, Z., Schuler, M. A., and Berenbaum, M. R. 2006. Toxicity of aflatoxin B1 to Helicoverpa zea and bioactivation by cytochrome P450 monooxygenases. J. Chem. Ecol. 32:1459–1471.
Acknowledgments
We thank Dr. Rensen Zeng and Dr. Zhimou Wen for advice and assistance in the design, execution, and analysis of bioassays. Also, we thank Allen Lawrance for assistance with colony maintenance. This project was funded by a grant from the Almond Board of California (ABC-C6480).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niu, G., Siegel, J., Schuler, M.A. et al. Comparative Toxicity of Mycotoxins to Navel Orangeworm (Amyelois transitella) and Corn Earworm (Helicoverpa zea) . J Chem Ecol 35, 951–957 (2009). https://doi.org/10.1007/s10886-009-9675-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-009-9675-8