Abstract
Apple replant is a widespread agricultural problem documented in all of the major fruit-growing regions of the world. In order to better understand the phytotoxic mechanisms induced by allelochemicals involved with this problem, Malus prunifolia plants were grown hydroponically to the six-leaf-stage in the presence of phthalic acid (0 or 1 mM) for 5, 10, or 15 days. Apple plants were evaluated for: shoot and root length, fresh and dry weight, malondialdehyde (MDA) content, hydrogen peroxide (H2O2) content, superoxide radical (O2 ·−) generation rate, and antioxidant enzyme activities. Shoot and root lengths and fresh and dry weights of M. prunifolia decreased in plants exposed to phthalic acid. MDA and H2O2 content increased in phthalic acid-treated plants as did the generation rate of O2 ·− in M. prunifolia roots. The activities of superoxide dismutase (EC 1.15.1.1), peroxidase (EC 1.11.1.7), catalase (EC 1.11.1.6), ascorbate peroxidase (EC 1.11.1.11), glutathione reductase (EC 1.6.4.2), dehydroascorbate reductase (EC 1.8.5.1), and monodehydroascorbate reductase (EC 1.6.5.4) increased in phthalic acid-stressed roots compared with control roots. These results suggest that activation of the antioxidant system by phthalic acid led to the formation of reactive oxygen species that resulted in cellular damage and the decrease of M. prunifolia growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Allelopathy is widely reported in agroecosystem and silviculture, and is implicated in problems such as exotic plant invasion, replant problems, and soil sickness (Lee et al. 2006; Hao et al. 2007; Fernandez et al. 2008). Allelopathic plants exert detrimental effects via the release of plant compounds (allelochemicals) through leaching, root exudation, volatilization, and/or decomposition of plant materials (Weir et al. 2004) and can interfere with the metabolism of other plants. If the effect of such compounds is harmful to plant growth and development, it becomes a biotic or allelochemical stress (Romero-Romero et al. 2005).
Under normal growth conditions, a dynamic equilibrium exists between the production and detoxification of free radicals in cell organelles. Biotic and abiotic stresses may cause the formation of reactive oxygen species (ROS) such as superoxide radical (O2 ·−), hydroxyl radical (OH·−), and/or hydrogen peroxide (H2O2) that are commonly generated and accumulated in cells (Cho and Seo 2005). Enhanced ROS production can affect membrane permeability, damage DNA and proteins, induce lipid peroxidation, and ultimately lead to programmed cellular death (Ding et al. 2007). Plants have evolved mechanisms that protect cell and subcellular systems from the effects of ROS by using antioxidant systems such as superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR), dehydroascorbate reductase (DHAR), and monodehydroascorbate reductase (MDHAR). Tolerance to allelochemicals by various crop plants has been associated with an increase in the activity of antioxidant enzymes (Yu and Matsui 1997).
The apple replant problem is widespread. Studies have shown that allelochemicals in root exudates or decomposition of residues may play a role in this agricultural problem (Borner 1959; Zhang et al. 2007; Bai et al. 2009). However, the mechanisms involved have not been investigated extensively. Reports suggest that allelochemicals cause increased membrane leakage, and enhance H2O2 and MDA levels in the target plant tissues. For instance, cinnamic acid enhanced the generation of free radicals, increased lipid peroxidation, and oxidative membrane damage in cucumber and figleaf gourd plants (Ding et al. 2007). Similarly, 2-benzoxazolinone inhibited lettuce growth, caused membrane damage, and increased MDA and H2O2 production (Sanchez-Moreiras and Reigosa 2005). Most studies in this regard focused on vegetables and agronomic crops (Baziramakenga et al. 1995; Yu et al. 2003; Ye et al. 2006). However, it is not known whether the inhibitory effects were mediated through production of ROS in apple roots under allelochemical stress. Phthalic acid is a potent allelochemical and inhibits the growth of a number of plants such as Lactuca sativa L. (Lee et al. 2006), Zea mays L. (Chai and Feng 2007), and Malus prunifolia (Bai et al. 2009). Accordingly, its effect on plant growth, ROS generation rate, and ROS-antioxidant enzyme activity were investigated in M. prunifolia seedlings.
Methods and Materials
Plant Materials and Phthalic Acid Treatment
Seeds of apple rootstock, M. prunifolia, were obtained from Fuping County (34°75′ N, 109°15′ E), Shaanxi Province. Seed sterilization was done according to Zhang et al. (2007) and involved surface-sterilization in 0.3% (v/v) H2O2 for 20 min, followed by several rinses with sterile H2O. The sterilized seeds were stratified at 4°C for 85 days. Sprouted seeds were sown in plastic pots (9 cm in diameter, 12 cm high; three seeds per pot) filled with sterilized sand. All pots were placed in a greenhouse at the College of Horticulture, Northwest A&F University, Yangling (34°20′ N, 108°24′ E). Plants were grown without supplementary illumination with night and day temperatures at 20 to 25°C and relative humidity at 65–80%. Seedlings were watered once a week with Hoagland nutrient solution (Hoagland 1920), pH 6.0 ± 0.2. When the seedlings reached the six-leaf-stage, batches of 45 uniform seedlings were transferred into a hydroponic system (plastic container; 45 × 37 × 22.5 cm) filled with 5 l half Hoagland nutrient solution at pH 6.0 ± 0.2 and electrical conductivity at 1.2 ms/cm, respectively. The containers were placed in a controlled growth room with a L/D regime of 12/12 h, 25/20°C, and a photon flux density of 140–160 μmol m−2 s−1. Seedlings were allowed to acclimate to the hydroponic conditions for 5 days. Phthalic acid (purchased from Yifang S&T Ltd. Tianjin, China), dissolved in ethanol, was added to the nutrient solution to concentrations of 0 or 1 mM. In the preliminary experiment, thinner M. prunifolia leaves and brownish root apices in parallel with a large amount of mucilage was secreted from roots 15 days after application of 1 mM phthalic acid. This indicates that phthalic acid at this concentration is lethal to M. prunifolia seedlings. The final concentration of ethanol in control and treatment solutions was 0.1% (v/v). The nutrient solutions in the containers were aerated continuously by air pumps. The solution in the plastic container was kept at the same level by adding half Hoagland nutrient solution at 24 h intervals. Each treatment was replicated three times in a completely randomized design. Root samples were taken from both control and phthalic acid-treated plants on days 5, 10, and 15 after treatment, and the tissue was frozen in liquid nitrogen and stored at −70°C until analysis. At the end of the experiment (15 days after treatment), root and shoot length and fresh and dry weight of seedlings were measured. The seedlings were dried in an forced-air oven at 60°C until constant mass was reached.
Measurement of MDA, H2O2, and O2 ·− Generation
Lipid peroxidation was followed by measuring MDA accumulation using the method of Baziramakenga et al. (1995) with some modifications. Root samples (0.2 g) were homogenized in 0.1% of trichloroacetic acid in phosphate buffer (5 ml; pH 7.8) and centrifuged at 12,000×g for 15 min. Supernatant (1 ml) was added to 0.5% thiobarbituric acid in 20% trichloroacetic acid (4 ml). The mixture was placed in a water bath at 100°C for 10 min and then quickly cooled in an ice-bath for 15 min. Samples were centrifuged at 12,000×g for 5 min, and then the absorbance of the supernatant was measured at 450, 532, and 600 nm.
H2O2 in the supernatant was measured according to Patterson et al. (1984). Root samples (0.5 g) were homogenized in 5-ml pre-cooled acetone and centrifuged for 10 min at 1,500×g. Titanium chloride (0.1%, w/v) and concentrated ammonia (0.2 ml) were added into the supernatant (1 ml), the mixture was allowed to react (10 min at 25°C) and the reaction mixture was centrifuged at 1,500×g for 10 min. Absorbance at 410 nm was measured, and the H2O2 concentration was calculated according to a standard curve.
The rate of O2 ·− generation was measured as described by Elstner and Heupel (1976) with some modifications. Root tissue (1 g) was homogenized in 65 mM potassium phosphate buffer (3 ml; pH 7.8). The homogenate was centrifuged at (10,000×g for 15 min). The supernatant (0.5 ml) was added to 65 mM potassium phosphate buffer (0.5 ml; pH 7.8) containing 10 mM hydroxylammoniumchloride (0.1 ml) and incubated (25°C for 20 min). Sulphanilic acid (58 mM; 1 ml) and α-naphthyl amine (7 mM; 1 ml) were added to the mixture, and it was allowed to incubate (25°C for 20 min). The final solution was mixed with an equal volume of chloroform and the absorbance of the pink phase was measured at 530 nm.
Extraction and Assay of Enzyme Activities
Antioxidant enzymes (SOD, POD, and CAT) were extracted according to the method of Yu et al. (2003) with some modifications. Root samples (0.5 g) were homogenized in phosphate buffer (8 ml; 0.1 M; pH 7.5) containing 2% (w/v) polyvinylpyrrolidone. The homogenate was centrifuged (12,000×g for 20 min) and the supernatant was used for enzyme analysis. All assays were carried out at 2–4°C.
Superoxide dismutase activity was measured according to Beauchamp and Fridovich (1971) with minor modification. The assay medium (3 ml) contained phosphate buffer (50 mM; pH 7.8), EDTA-Na (0.1 mM), L-methionine (12 mM), riboflavin (2 μM), and nitrotetrazolium blue chloride (75 μM). Riboflavin was added last. The tubes were shaken and placed at a photosynthetic photon flux of 50 μmol m−2 s−1 for 15 min. The reaction was initiated and terminated by turning the light on and off, respectively. The A560 was measured on a spectrophotometer and tubes containing the assay mixture, but without the root extract (control), were illuminated to determine maximum A560.
Peroxidase activity was measured according to Sofo et al. (2004) with some modification. The reaction solution (3 ml) contained phosphate buffer (2.9 ml, 50 mM; pH 7.0), guaiacol (50 μl; 10 mM), H2O2 (10 μl; 40 mM), and crude enzyme extract (40 μl). The Increase in A470nm due to the oxidation of guaiacol was measured at 20°C.
Catalase activity was assayed by monitoring the decrease in A240nm (Aebi 1984). The reaction mixture contained phosphate buffer (50 mM; pH 7.0) and H2O2 (30% w/v) and was started by adding the reaction solution to crude extract (10 μl).
AsA-related enzymes were extracted according to Nakano and Asada (1981). Generally, each 0.5 g of roots material was homogenized in KH2PO4-KOH (6 ml, 50 mM; pH 7.5) containing ethylenediaminetetraacetic acid (0.1 mM), Triton X-100 (0.3% v/v), and insoluble polyvinylpolypyrrolidone (4% w/v). The homogenate was centrifuged (16,000×g for 15 min at 2°C) and the supernatant was used for APX, GR, MDHAR, and DHAR analyses.
Ascorbate peroxidase was measured by monitoring the decrease in A290 nm (Nakano and Asada 1981). The assay mixture (1 ml) contained Hepes–KOH (50 mM; pH 7.6), ethylenediaminetetraacetic acid (0.1 mM), H2O2 (0.2 mM, AsA 0.5 mM), and enzyme extract. The reaction was initiated by adding H2O2.
Glutathione reductase activity was monitored at A340 nm in a 1 ml reaction mixture containing Tris–HCl (100 mM; pH 8.0), ethylenediaminetetraacetic acid (1 mM), oxidized glutathione (GSSG;1 mM), and NADPH (0.2 mM). The reaction was initiated by adding NADPH (Grace and Logan 1996).
Monodehydroascorbate reductase activity was assayed at 340 nm in a 1 ml reaction mixture containing Hepes–KOH (50 mM; pH 7.6), NADH (0.1 mM), AsA (0.25 mM), and AsA oxidase (EC 1.10.3.3; 0.25 U). The reaction was initiated by adding AsA oxidase (Miyake and Asada 1992).
Dehydroascorbate rductase activity was measured at 265 nm in a 1 ml assay solution containing Hepes–KOH (100 mM; pH 7.0), ethylenediaminetetraacetic acid (1 mM), GSH (2.5 mM), and DHA (0.2 mM). The reaction was initiated by adding DHA (Dalton et al. 1986).
Statistical Analysis
All data were subjected to analysis of variance, followed by Tukey’s Studentized Range Test (SAS Statistical package, version 8.2). Results are presented as the means ± standard deviation (SD).
Results
Effect on Plant Growth
The toxic effect of 1 mM phthalic acid appeared after 15 days following treatment. The length of shoot and root of M. prunifolia plants had reduced shoot and root length compared to lower than controls (Fig. 1a). Fresh and dry weights were 3.09 and 1.61 g plant−1 in control plants compared to 2.63 and 0.79 g plant−1 for plants treated with 1 mM phthalic acid (Fig. 1b).
Shoot and root length (a) and fresh and dry weight (b) of Malus prunifolia plants grown at 0 (control) or 1 mM phthalic acid. Samples were taken 15 days after treatment. Values are means of three replicates ± standard error (SE). Significant difference (P < 0.05 level) was tested between the control and 1 mM phthalic acid treatment for each dependent variable and indicated by different letters above the bars
Effect on MDA, H2O2, and O2 ·− Generation
MDA concentration in M. prunifolia roots increased significantly after 5, 10, and 15 days of treatment (Fig. 2a). The maximum increase of 125.4% was observed after 15 days. Exposure to phthalic acid also resulted in an increase in the H2O2 content and O2 ·− generation rate, respectively (Fig. 2b, c). The maximum increases of 37.2% and 29.9%, respectively, were observed after 15 days.
MDA (a), H2O2 (b) content, and O2 ·− generation rate (c) in Malus prunifolia plants grown at 0 (control) or 1 mM phthalic acid. Values are means of three replicates ± standard error (SE). Significant difference (P < 0.05 level) was tested between the control and 1 mM phthalic acid treatment at each sampling date separately and indicated by different letters above the bars
Effect on Enzyme Activity
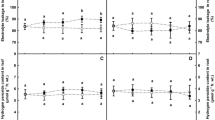
In response to phthalic acid, activities of SOD, POD, and CAT, as well as enzymes in ascorbate-glutathione cycle, e.g., APX, GR, MDHAR, and DHAR, all showed similar trends in the activity of root enzymes in response to phthalic acid. In general, enzyme activities increased progressively during the treatment period (days 5–15) compared to enzymes in the roots of plants not exposed to phthalic acid (Figs. 3 and 4).
SOD (a), POD (b), and CAT (c) activities of Malus prunifolia plants grown at 0 (control) or 1 mM phthalic acid. The values are means of three replicates ± standard error (SE). Significant difference (P < 0.05 level) was tested between the control and 1 mM phthalic acid treatment at each sampling date separately and indicated by different letters above the bars
APX (a), GR (b), MDHAR (c), and DHAR (d) activities of Malus prunifolia plants grown with 0 (control) or 1 mM phthalic acid. The values are means of three replicates ± standard error (SE). Significant difference (P < 0.05 level) was tested between the control and 1 mM phthalic acid treatment at each sampling date separately and indicated by different letters above the bars
Discussion
Phthalic acid treatment significantly decreases the growth of M. prunifolia plants (Fig. 1). This finding is in agreement with studies on L. sativa L. (Lee et al. 2006) and Z. mays L. (Chai and Feng 2007), and consistent with the inhibitory effects of other allelochemicals on plant growth reported previously (Lin and Kao 2000; Asao et al. 2004; Batish et al. 2008).
The formation of free radials in cells results in damage to cell membranes due to lipid peroxidation. Thus, the level of MDA, produced during lipid peroxidation, is a good indicator of oxidative damage that could be occurring within cells (Masia 2003). MDA accumulation due to lipid peroxidation has been reported in response to a variety of abiotic and biotic stresses (Dhindsa et al. 1981; Apel and Hirt 2004). In the present study, enhanced MDA (Fig. 2a) suggests that phthalic acid probably induces oxidative stress, and, as a result, disrupts cellular membrane structure, and causes a loss of cellular integrity. Similar results with other allelochemicals have been found (Wu et al. 2002; Batish et al. 2006; Ye et al. 2006). Many studies show that increased MDA content is associated with increased O2 ·− and H2O2 production following biotic and abiotic stresses (Forman et al. 2002; Lara-Nunez et al. 2006). Here, phthalic acid induced O2 ·− and H2O2 production in M. prunifolia roots (Fig. 2b, c), which suggests that phthalic acid could trigger ROS generation and induce oxidative stress in M. prunifolia roots. This observation is consistent with that obtained with mung bean treated with 2-benzoxazolinoen (Batish et al. 2006). Increases in SOD activity (Fig. 3a) also indicate that excessive generation of O2 ·− has been triggered by pthalic acid treatment, and, consequently, that SOD activity was up-regulated to mitigate the oxidative damage. Similar responses have been observed in plants treated with other allelochemicals (Yu et al. 2003; Batish et al. 2006; Wu et al. 2002; Lin et al. 2007).
Accumulation of H2O2 in M. prunifolia roots in response to phthalic acid treatment enhances lipid peroxidation and causes a severe oxidative stress resulting in disruption of metabolic activities in the cell. Enhanced H2O2 levels are removed by the antioxidant enzymes such as CAT, APX, and POD (Blokhina et al. 2003), and glutathione-ascorbate cycle (Nakano and Asada 1981). In the present study, increases in the activities of these antioxidant enzymes paralleled the accumulation of MDA and H2O2 in M. prunifolia root after exposure to phthalic acid (Figs. 2–4). Our observations are consistent with those of Cruz-Ortega et al. (2002), who reported that allelochemical stress causes increases in the level of free radicals and the activity of antioxidant enzymes and suggests that increased induction of these enzymes is necessary to prevent lipid peroxidation (i.e., to counter the higher MDA and H2O2 in roots). Although there were increased levels of antioxidants in roots after exposure to phthalic acid, oxidative stress still occurred. Our data are consistent with those of Batish et al. (2006), who showed that exposure of mung bean to 2-benzoxazolinone increased APX, GR, and CAT activity. An increased POD activity in response to allelochemicals also has been demonstrated in cucumber root (Yu et al. 2003). Oracz et al. (2007) observed an increase in the cell membrane permeability, MDA level, H2O2 concentration, and SOD and CAT activity in mustard treated by sunflower extract. Furthermore, the activity and expression of most antioxidant enzymes is stimulated by ROS accumulation (Apel and Hirt 2004).
In summary, phthalic acid induces oxidative stress in M. prunifolia roots through the generation of ROS and decreases plant growth, despite the concomitant increase in antioxidant enzymes. This might be one of the mechanisms responsible for the apple replant problem. The increase in antioxidant enzymes could reflect a defensive response to the cellular damage provoked by phthalic acid treatment. However, this increase was not strong enough to eliminate all the deleterious effects provoked by phthalic acid, only alleviated the impact of stress.
References
Aebi, H. 1984. Catalase in vitro. Meth. Enzymol. 105:121–126.
Apel, K., and Hirt, H. 2004. Reactive oxygen species: Metabolism, oxidative stress and signal transduction. Annu. Rev. Plant Biol. 55:373–399.
Asao, T., Kitazawa, H., Tomita, K., Suyama, K., Yamamoto, H., Hosoki, T., and Pramanik, M. H. R. 2004. Mitigation of cucumber autotoxicity in hydroponic culture using microbial strain. Sci. Hortic 99:207–214.
Bai, R., Zhao, X., Ma, F. W., and Li, C. Y. 2009. Identification and bioassay of allelopathic substances from the root exudates of Malus prunifolia. Allelopathy J. in press.
Batish, D. R., Singh, H. P., Setia, N., Kaur, S., and Kohli, R. K. 2006. 2-Benzoxazolinone (BOA) induced oxidative stress, lipid peroxidation and changes in some antioxidant enzyme activities in mung bean (Phaseolus aureus). Plant Physiol. Biochem. 44:819–827.
Batish, D. R., Singh, H. P., Kaur, S., Kohli, R. K., and Yadav, S. S. 2008. Caffeic acid affects early growth, and morphogenetic response of hypocotyl cuttings of mung bean (Phaseolus aureus). J. Plant Physiol 165:297–305.
Baziramakenga, R., Leroux, G. D., and Simard, R. R. 1995. Effects of benzoic and cinnamic acids on membrane permeability of soybean roots. J. Chem. Ecol. 21:1271–1285.
Beauchamp, C., and Fridovich, I. 1971. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem 44:276–287.
Blokhina, O., Virolainen, E., and Fagerstedt, K. V. 2003. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot 91:179–194.
Borner, H. 1959. The apple replant problem. I. The excretion of phlorizin from apple root residues. Contrib. Boyre Thompson Inst 20:39–54.
Chai, Q., and Feng, F. X. 2007. Identification of root exudation of Zea mays L. and allelopathy of 1,2-benzenedicarboxylic acid. J. Gansu Agric. Univ 5:43–48(in Chinese).
Cho, U. H., and Seo, N. H. 2005. Oxidative stress in Arabidopsis thaliana exposed to cadmium is due to hydrogen peroxide accumulation. Plant Sci 168:113–120.
Cruz-Ortega, R., Ayala-Cordero, G., and Anaya, A. L. 2002. Allelochemical stress produced by the aqueous leachate of Callicarpa acuminata: Effects on roots of bean, maize, and tomato. Physiol. Plant 116:20–27.
Dalton, D. A., Russell, S. A., Hanus, F. J., Pascoe, G. A., and Evans, H. J. 1986. Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. PNAS 83:3811–3815.
Dhindsa, R. S., Plumb-Dhindsa, P., and Thorpe, T. A. 1981. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot 32:93–101.
Ding, J., Sun, Y., Xiao, C. L., Yan, K. S., Zhou, H., and Yu, J. Q. 2007. Physiological basis of different allelopathic reactions of cucumber and figleaf gourd plants to cinnamic acid. J. Exp. Bot 58:3765–3773.
Elstner, E. F., and Heupel, A. 1976. Inhibition of nitrite formation from hydroxylammoniumchloride: A simple assay for superoxide dismutase. Anal. Biochem 70:616–620.
Fernandez, C., Voiriot, S., Mevy, J. P., Vila, B., Ormeno, E., Dupouyet, S., and Melou, A. B. 2008. Regeneration failure of Pinus halepensis Mill.: The role of autotoxicity and some abiotic environmental parameters. For. Ecol. Manage 255:2928–2936.
Forman, H. J., Torres, M., and Fukuto, J. 2002. Redox signaling. Mol. Cell. Biochem 234:49–62.
Grace, S. C., and Logan, B. A. 1996. Acclimation of foliar antioxidant systems to growth irradiance in three broad-leaved evergreen species. Plant Physiol 112:1631–1640.
Hao, Z. P., Wang, Q., Christie, P., and Li, X. L. 2007. Allelopathic potential of watermelon tissues and root exudates. Sci. Hortic. 112:315–320.
Hoagland, D. R. 1920. Optimum nutrient solutions for plants. Science 52:562–564.
Lara-Nunez, A., Romero-Romero, T., Ventura, J. L., Blancas, V., Anaya, A. L., and Cruz-Ortega, R. 2006. Allelochemical stress causes inhibition of growth and oxidative damage in Lycopersicon esculentum Mill. Plant Cell Environ 29:2009–2016.
Lee, J. G., Lee, B. Y., and Lee, H. J. 2006. Accumulation of phytotoxic organic acids in reused nutrient solution during hydroponic cultivation of lettuce (Lactuca sativa L.). Sci. Hortic 110:119–128.
Lin, C. C., and Kao, C. H. 2000. Effect of NaCl stress on H2O2 metabolism in rice leaves. Plant Growth Regul 30:151–155.
Lin, R. Z., Wang, X. R., Luo, Y., Du, W. C., Guo, H. Y., and Yin, D. Q. 2007. Effects of soil cadmium on growth, oxidative stress and antioxidant system in wheat seedlings (Triticum aestivum L.). Chemosphere 69:89–98.
Masia, A. 2003. Physiological effects of oxidative stress in relation to ethylene in post-harvest produce, pp. 165–197, in D. M. Hodges (ed.). Postharvest Oxidative Stress in Horticultural CropsFood Products Press, New York.
Miyake, C., and Asada, K. 1992. Thylakoid-bound ascorbate peroxidase in spinach chloroplasts and photoreduction of its primary oxidation product monodehydroascorbate radicals in thylakoids. Plant Cell Physiol 33:541–553.
Nakano, Y., and Asada, K. 1981. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880.
Oracz, K., Bailly, C., Gniazdowska, A., Come, D., Corbineau, F., and Bogatek, R. 2007. Induction of oxidative stress by sunflower phytotoxins in germinating mustard seeds. J Chem Ecol 33:251–264.
Patterson, B. D., Macrae, E. A., and Ferguson, I. B. 1984. Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal. Biochem 139:487–492.
Romero-Romero, T., Sanchez-Nieto, S., Sanjuan-Badillo, A., Amaua, A. L., and Cruz-Ortega, R. 2005. Comparative effects of allelochemical and water stress in roots of Lycopersicon esculentum Mill. (Solanaceae). Plant Sci 168:1059–1066.
Sanchez-Moreiras, A. M., and Reigosa, M. J. 2005. Whole plant response of lettuce after root exposure to BOA (2(3H)-Benzoxazolinone). J. Chem. Ecol 31:2689–2703.
Sofo, A., Dichio, B., Xiloynnis, C., and Masia, A. 2004. Effects of different irradiance levels on some antioxidant enzymes and on malondialdehyde content during rewatering in olive tree. Plant Sci 166:293–302.
Weir, T. L., Park, S. W., and Vivanco, J. M. 2004. Biochemical and physiological mechanisms mediated by allelochemicals. Curr. Opin. Plant Biol 7:472–479.
Wu, F. Z., Huang, C. H., and Zhao, F. Y. 2002. Effects of phenolic acids on growth and activities of membrane protective enzymes of cucumber seedlings. Agric. Sci. China 35:821–825.
Ye, S. F., Zhou, Y. H., Sun, Y., Zou, L. Y., and Yu, J. Q. 2006. Cinnamic acid causes oxidative stress in cucumber roots, and promotes incidence of Fusarium wilt. Environ. Exp. Bot 56:255–262.
Yu, J. Q., and Matsui, Y. 1997. Effects of root exudates of cucumber (Cucumis sativus) and allelochemicals on ion uptake by cucumber seedlings. J. Chem. Ecol 23:817–827.
Yu, J. Q., Ye, S. F., Zhang, M. F., and Hu, W. H. 2003. Effects of root exudates and aqueous root extracts of cucumber (Cucumis sativus) and allelochemicals, on photosynthesis and antioxidant enzymes in cucumber. Biochem. Syst. Ecol 31:129–139.
Zhang, J. H., Mao, Z. Q., Wang, L. Q., and Shu, H. R. 2007. Bioassay and identification of root exudates of three fruit tree species. J. Integr. Plant. Biol 49:257–261.
Acknowledgements
This work was supported by the Natural Science Fund of Shaanxi province and Talent Support Program of Northwest A&F University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bai, R., Ma, F., Liang, D. et al. Phthalic Acid Induces Oxidative Stress and Alters the Activity of Some Antioxidant Enzymes in Roots of Malus prunifolia . J Chem Ecol 35, 488–494 (2009). https://doi.org/10.1007/s10886-009-9615-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-009-9615-7