Abstract
Megaplatypus mutatus (=Platypus mutatus) (Coleoptera: Platypodidae) is an ambrosia beetle that is native to South America. It attacks only standing live trees and causes severe stem breakage and death in commercial poplar (Populus) plantations. Previous work showed that male M. mutatus emits a sex pheromone composed mainly of (+)-sulcatol and sulcatone. We collected male volatile emissions during the hours of maximum emergence by using a specific polar microextraction phase; analyzed the extract by GC-MS; and tested the biological activity of selected compounds in the extract with a walking behavioral assay. Female M. mutatus emerged primarily between 7 and 11 h. In the chemical analyses of volatiles, a third compound, 3-pentanol, was identified in a small percentage of samples. Walking behavioral bioassays with video image analysis showed that at the doses tested, 3-pentanol elicited an attractive response from females.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ambrosia beetles (Coleoptera: Platypodidae, Platypodinae, Platypodini) are a significant group of forest pests that affect weakened or felled trees. Their name derives from the fungus that they inoculate when they penetrate the xylem of their host and on which larvae feed. Megaplatypus mutatus (=Platypus mutatus) (Chapuis) is an ambrosia beetle native to South America (Wood 1993, 2007) that only attacks standing, live trees, mining deeply into the xylem through large tunnels that are later colonized by the fungus that they transport, Raffaelea santoroi (Guerrero). This weakens the structural integrity of the tree, causing severe stem breakage and mortality in commercial plantations of poplar Populus spp. (Santoro 1963; Achinelli et al. 2005; Alfaro et al. 2007). The attack is initiated when the male penetrates the bark and excavates a gallery that is several centimeters long. With the particles of boring dust (frass) that it produces, the male beetle builds a crown-like arrangement that surrounds the entrance to the gallery (Fig. 1); volatiles emitted from the center of the crown attract individuals of the opposite sex (Santoro 1963; Milligan and Ytsma 1988).
In a previous study in our laboratory, volatile emissions released from the male gallery were collected with a solid phase microextraction fiber (SPME) device coated with a non-polar, non-specific phase. These samples were analyzed by GC-MS and revealed two main components: (+)-6-Methyl-5-hepten-2-ol [(+)-sulcatol] and its related ketone, 6-methyl-5-hepten-2-one (sulcatone). Walking behavioral bioassays with an olfactometer confirmed an attractive response by females to (+)-sulcatol and sulcatone. These results suggested that M. mutatus emits a sex pheromone composed mainly of (+)-sulcatol and sulcatone (Gonzalez Audino et al. 2005).
Santoro (1963) reported that M. mutatus emerge primarily at sunrise. Observations (Gatti Liguori, unpublished results) suggested that newly emerged M. mutatus are susceptible to dehydration once they are outside their gallery system in the xylem. Therefore, it would be reasonable to expect that, immediately after emergence, female flight is orientated to locate males via the volatile plume emitted early in the morning. The response is necessary to initiate courtship and copulation.
Here, we report an analysis of the frequency profile of diurnal and nocturnal female emergence. Furthermore, volatile emission was analyzed during the morning hours when female emergence and courtship and mating occur at high frequency. Finally, the behavioral activities of candidate compounds from the volatile blend were evaluated by using the female response in a walking behavioral assay.
Methods and Materials
Biological Material
Insects were collected from our Institute’s plantation of Populus canadensis Moench and Quercus palustris Muenchh. located in Villa Martelli, Province of Buenos Aires, Argentina (34°22′ S, 58°30′W). Beetles were collected during emergence by using plastic traps that were specially designed to avoid antagonistic interactions (Gatti Liguori et al. 2007) and immediately sexed. Only females that emerged within a period of 3 h or less were used in laboratory experiments.
Temporal Pattern of Female Emergence
The number of emerged females was recorded every 2 h from 7–23 h, between 1 and 20 October, 2006. A relative frequency chart was made based on the data collected. The times of day with the highest frequency of emergence were used to select the time to collect and analyze male volatiles.
Collection and Analysis of Male Volatiles
Live Populus alba L., Q. palustris Munchh, and Casuarina stricta L. trees that were at least 10 years old and had a diameter at breast height (dbh) of 20 cm were artificially infested with 59, 15, and 94 virgin males, respectively. Each insect was confined in a transparent plastic jar (60 × 30 mm) to ensure its interaction with the tree bark surface. The date and time at which tunneling activity began was recorded.
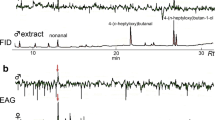
At times during maximal female emergence, males were collected from the frass crown arrangement with entomological forceps and immediately placed in a 20-ml glass vial (Scientific Specialties Service, Inc., Baltimore, MD, and Reno, NV, USA) with a teflon-coated cap (teflon septum with glass-reinforced polypropylene resin open cap). The physical integrity of each male was checked, and the volatiles from the headspace were collected at 30°C for 30 min by using a Solid Phase Microextraction (SPME) holder and fiber covered with a Carbowax®/divinylbenzene polar phase (Supelco Inc., Bellefonte, PA, USA). This coating is specific for low-molecular weight alcohols and ketones. Samples were analyzed immediately by GC-MS (see below), yielding a volatile emission profile for each insect.
Synthetic Chemicals
Sulcatone (6-methyl-5-hepten-2-one) and 3-pentanol were analytical grade (Sigma-Aldrich Co., St. Louis, MO, USA); (+)-sulcatol [(+)-6-methyl-5-hepten-2-ol] 99% chemical purity, was purchased from Pherotech Inc., Delta, B.C., Canada.
Linked Gas Chromatography–Mass Spectrometry (GC-MS) Analyses
SPME fibers were analyzed by GC-MS with a Shimadzu QP 5050A instrument equipped with a non-polar, fused silica HP-1 capillary column with crossed linked methyl silicone (50 m length, 0.32 mm diameter, and 0.52 µm thickness) (Hewlett Packard, Santa Clara, CA, USA). Samples were injected in the splitless mode. Volatiles from the SPME fibers were desorbed in the injection port at 190°C for 1.5 min. The GC column was held at 60°C for 1 min, after which the temperature was programmed to increase 3°C/min up to 105°C, and then 40°C/min to 250°C where it was maintained for 5 min. The carrier gas was helium with a head pressure of 30 kPa. The MS detector was set on electron impact mode at 70 eV.
Behavioral Bioassays
Walking behavior of female M. mutatus was evaluated in an experimental arena with a video-tracking technique (Alzogaray et al. 2000). The floor of the test arena was covered with a round piece of Whatman No. 1 filter paper (125 mm diameter; Whatman Ltd., Maidstone, UK), and a glass cover (20 × 20 mm) was placed in the center of the paper. Next, the filter paper and glass cover were both covered with a rectangular piece of wire mesh (100 × 100 mm; 1 mm mesh size). A colorless glass ring (100 mm diameter; 50 mm high) was used to confine the insects. The glass ring and wire mesh were washed previously with 96% ethanol and kept at 250-300°C for at least 1 week. A new glass cover and filter paper were used in each replicate.
A closed-circuit video camera that provided black and white images (VC 1910; Sanyo Electrical Co., Tokyo, Japan) was suspended 22 cm over the center of the test arena. A circular fluorescent tube (22 W, OSRAM, Buenos Aires, AR) was placed 64 cm above the video camera.
An image analyzer (Videomex V, Columbus, OH, USA) received input from the video camera, converting the analog signal into digital data. The resolution was 256 × 192 pixels, and the acquisition and processing speed was 30 fps. The video signal colors were inverted in the monitor, therefore white objects appeared black and vice versa. The presence of insects in the arena was determined by visual contrast between the individuals (white) and the arena background (dark), and scored as the number of “ON” pixels. The area occupied by the insects was recorded by using the Multiple Zone Motion Monitor for Videomex software.
The arena image was divided into a central square (4 cm2, 5% of the total area) and a circular outer area. The center of the glass cover was located in the center of the virtual central square. A female M. mutatus was placed on the wire mesh and allowed to acclimatize for 5 min before starting the bioassay. During this time, the insect moved all around the arena. Insect movement was recorded for 60 min. During the first 30 min, 20 μl of carbon dioxide-free distilled water were placed on the glass cover. Then, 20 μl of the test solution were placed on the cover by using carbon dioxide-free distilled water as carrier. Temperature varied between 25 and 30°C. Water took about 30 min to evaporate within this temperature range. The first 30 min of each test was the control, and the remaining 30 min was the experimental treatment. Thus, the occupation level of the central circle during the first 30 min (control) was compared to the occupation level during second 30 min (following the introduction of the test substance).
For preparation of test solutions, sulcatol, sulcatone, and 3-pentanol were weighed and added to carbon dioxide free distilled water (100 ml total volume). Concentrations of stock solutions were chosen in order to obtain the required amounts of test substance in the 20 or 40 µl test aliquots. Solutions were sonicated and stored at 5°C until 1 h before they were used in the behavioral assays. At least four different concentrations of each compound were assayed. Carbon dioxide-free distilled water alone was used to evaluate the behavior of the control group. Each experiment was repeated at least eight times.
To assay the comparative responses to (+)-sulcatol and sulcatone (binary mixture) versus (+)-sulcatol, sulcatone, and 3-pentanol (ternary mixture), 40 µl of solution containing 22 µg of each component were tested in the same conditions. Each experiment was replicated al least 16 times.
We used the Central Area Occupation (CAO) parameter, defined as the total number of “ON” pixels in the central circle (where the test compound is placed) during a replicate (Fontán et al. 2002), to quantify insect behavior. A mean CAO value was obtained for each treatment and compared to its respective control.
Statistical Analysis
Data from the behavioral assay were analyzed by non-parametric Kruskal–Wallis ANOVA (STATISTICA’99 1999). Edition software by StatSoft, Inc (Kernel Release 5.5 A ©1984–1999).
Results
Temporal Pattern of Female Emergence
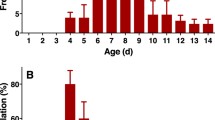
Analyses of female emergence with time of day showed that 75% of the females left the gallery between 7 and 11 h (Fig. 2). Based on these results, we performed our analysis of male volatiles by using males collected during the first hours of this interval, hypothesizing that pheromone emission would be in progress at this time.
Collection and Analysis of Male Volatiles
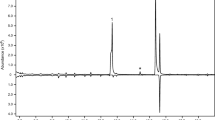
GC-traces showed that (+)-sulcatol and sulcatone were found in 91.7% of the male samples collected on P. alba, Q. palustris, and C. stricta trees between 7 and 11 AM. However, an additional peak was detected in 8.3% of the samples (Fig. 3). This peak was identified as 3-pentanol by comparison of its retention time and mass spectrum with an authentic standard. Although there was considerable variability among insects, we estimated a mean relative amount of 3-pentanol as 13.9 ± 6.4%, whereas sulcatone and sulcatol were 34.9 ± 9.3% and 51.2 ± 10.7%, respectively. 3-Pentanol was not found in any of the system blanks.
Behavioral Bioassays
Results were analyzed based on the Central Area Occupation (CAO) parameter. Significant occupation of the central area can be interpreted as an effective attraction to the source followed by an arrestment in the area (Fontán et al. 2002).
CAO values of female M. mutatus exposed to different concentrations of 3-pentanol revealed that doses between 1 and 8 µg elicited a significant behavioral response (P ≤ 0.05) when compared with their respective controls (Fig. 4). Thus, females were attracted to the stimulus source. Only the highest concentration (10 µg) failed to elicit any significant response from females. In this case, the value of CAO was not significant differently from its control (P = 0.07). This can be interpreted as a repellence or saturation phenomenon that attracts the insects to the plume, but then repels them when they get closer.
The values of CAO for (+)-sulcatol were different from their respective controls for all doses tested (P ≤ 0.05). Therefore, a significant attraction to the source was observed whenever the insects were exposed to (+)-sulcatol (Fig. 5). There were no differences among the different concentrations (P > 0.05).
The values of CAO for sulcatone were different from their respective controls (P ≤ 0.05) at all doses tested. Thus, the insects were significantly attracted to the source in all cases of exposure to sulcatone (Fig. 6). No differences were observed among the different concentrations (P > 0.05).
In the assay to evaluate the role of 3-pentanol in the pheromone blend, the values for CAO for the binary mixture [(+)-sulcatol + sulcatone] were not significantly different from those of the ternary mixture [(+)-sulcatol + sulcatone + 3-pentanol] (Fig. 7).
Response of female M. mutatus (mean ± SE) measured as the Central Area Occupation (=number of on pixels) for the binary mixture and the ternary mixture compared to their respective controls. The binary mixture contained 22 µg each of (+)-sulcatol and sulcatone. The ternary mixture contained 22 µg each of (+)-sulcatol, sulcatone, and 3-pentanol. *Indicates significant difference compared to control values (P ≤ 0.05). **Indicates significant difference compared to control values (P ≤ 0.001). Controls were not significantly different
Discussion
Our results showed that during the assay period (1 to 20 October), female M. mutatus emerge preferably between 7 and 11 AM. This preference could be due to this insect’s particular susceptibility to dehydration (Gatti Liguori, unpublished results).
Chemical analyses of volatiles emitted by male M. mutatus between 7 and 11 AM showed that the extracts were composed primarily of sulcatone and (+)-sulcatol. However, an additional compound, 3-pentanol, was identified in a small percentage of samples. Walking behavioral bioassays with video image analysis showed that at the doses tested, 3-pentanol, (+)-sulcatol, and sulcatone each elicited an attractive response from female M. mutatus. As no significant differences in response were observed among the different concentrations, we cannot conclude whether one of the concentrations was more attractive than the others under the current the experimental conditions. The assay to compare the attractiveness of the two vs. three component mixtures did not show significant differences in their behavioral activity. So, although we have demonstrated that 3-pentanol is attractive to M. mutatus females, we were not able to address its role in the sex pheromone blend.
3-Pentanol has been described as a minor pheromonal component in West Indian sugarcane weevils, Metamasius hemipterus sericeus (Oliv.) (Perez et al. 1994), an insect pest of bananas, pineapples, palms, and sugarcane in Central and South America, the Caribbean, and Africa (Vaurie 1966). It has also been described in volatile emissions from the metasternal glands of Triatoma infestans (Klug), the etiological agent of Chagas disease (Manrique et al. 2006). For M. mutatus, we hypothesize based on the superior response to the ternary mixture with respect to its control (P < 0.001 vs. P = 0.01 for the binary mixture with the same test), that 3-pentanol could diminish the uncertainty in the process of location of the pheromone source (Witzgall and Arn 1990) and/or arrest the M. mutatus female at the point of origin of the plume (i.e., the male in the natural system).
Our efforts are now aimed at testing the role of 3-pentanol combined with (+)-sulcatol and sulcatone in the attraction of female M. mutatus in the field. We are also investigating whether the male proctodeum is the site of pheromone storage and/or production. We are evaluating the attractant activity of this male tissue compared to a blend of synthetic pheromone components, and are conducting an exhaustive chemical analysis of this tissue for minor pheromonal components.
References
Achinelli, F. G., Liljersthröm, G., Aparicio, A., Delgado, M., Jouanny, M., and Mastrandrea, C. 2005. Daños por taladrillo (Megaplatypus mutatus (= Platypus sulcatus)) en plantaciones de álamo (Populus spp.) de Alberti, Buenos Aires: análisis preliminar de la magnitud y distribución de fustes quebrados. Rev. Asoc. Ftal. Arg. 59:8–11. (In Spanish).
Alfaro, R., Humble, L. M., Gonzalez Audino, P., Villaverde, R., and Allegro, G. 2007. The threat of the ambrosia beetle Megaplatypus mutatus (Chapuis) [=Platypus mutatus Chapuis] to world poplar resources. Forestry 80:471–479.
Alzogaray, R. A., Fontán, A., and Zerba, E. 2000. Repellency of deet to nymphs of Triatoma infestans. Med. Vet. Entomol. 14:6–10.
Fontán, A., González Audino, P., Martínez, A., Alzogaray, R. A., Zerba, E. N., Camps, F., and Cork, A. 2002. Attractant volatiles released by female and male Triatoma infestans (Hemiptera: Reduviidae), a vector of Chagas disease: chemical analysis and behavioral bioassays. J. Med. Entomol. 39:191–197.
Gatti Liguori, P., Zerba, E., and Gonzalez Audino, P. 2007. New trap for emergent Megaplatypus mutatus. Can. Entomol. 139:894–896.
Gonzalez Audino, P., Villaverde, R., Alfaro, R., and Zerba, E. 2005. Identification of volatile emissions from Platypus mutatus (=sulcatus) (Coleoptera: Platypodidae) and their behavioral activity. J. Econ. Entomol. 98:1506–1509.
Manrique, G., Vitta, A., Ferreira, R., Zani, C., Rikard, Unelius, C., Lazzari, C., Diotaiuti, L., and Lorenzo, M. 2006. Chemical communication in Chagas disease vectors. Source, identity, and potential function of volatiles released by the metasternal and Brindley’s glands of Triatoma infestans adults. J. Chem. Ecol. 32:2035–2052.
Milligan, R. H., and Ytsma, G. 1988. Pheromone dissemination by male Platypus apicalis White and P. gracilis Broun (Col., Platypodidae). J. Appl. Ent. 106:113–118.
Perez, A. L., Campos-Piedra, Y., Chinchilla, C. M., Oehlschlager, A. C., Gries, G., Gries, R., Castrillo, G., Giblin-Davis, R. M., Peña, J. E., Duncan, R. E., Gonzalez, L. M., Pierce, Jr, H. D., Mcdonald, R., and Andrade, R. 1995. Aggregation pheromones and host kairomones of the West Indian sugarcane weevil, Metamasius hemipterus sericeus (Oliv.) (Coleoptera: Curculionidae). J. Chem. Ecol. 23:869–888.
Santoro, H. F 1963. Bioecología de Platypus sulcatus Chapuis (Coleoptera: Platypodidae). Revista de Investigaciones Forestales 4:47–79. (In Spanish).
Statistica’99, 1999. StatSoft, Inc., Kernel Release 5.5 A ©1984–1999. Tulsa, OK (USA).
Vaurie, P. 1966. A revision of the Neotropical genus Metamasius (Coleoptera: Curculionidae, Rhynchophorinae). Species groups I and II. Bull. Am. Mus. Nat. Hist. 131:213–337.
Witzgall, P., and Arn, H. 1990. Direct measurement of the flight behavior of male moths to calling females and synthetic pheromones. Zeitschrift für Naturforschung 45c:1067–1069.
Wood, S. L. 1993. Revision of the genera of Platypodidae (Coleoptera). Great Basin Nat. 53:259–281.
Wood, S. L. 2007. Bark and Ambrosia Beetles of South America (Coleoptera, Scolytidae). Brigham Young University, M.L. Bean Life Science Museum, Provo, Utah, 900 pp.
Acknowledgments
This work was supported by the Agencia Nacional de Promoción Científica y Técnica (PICT 2005) and the University of San Martín. PGL received a doctoral grant from the University of San Martin. PGA, EZ, and RA are members of the Researcher Career of the CONICET of Argentina.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Gatti Liguori, P., Zerba, E., Alzogaray, R.A. et al. 3-Pentanol: A New Attractant Present in Volatile Emissions from the Ambrosia Beetle, Megaplatypus mutatus . J Chem Ecol 34, 1446–1451 (2008). https://doi.org/10.1007/s10886-008-9547-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-008-9547-7