Abstract
The labial gland secretions from males of the North American bumblebees Bombus morrisoni Cresson and B. rufocinctus Cresson were analyzed by gas chromatography/mass spectrometry. In both species, 3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraenyl acetate was found as the major compound of a complex mixture of alkenols, acetates, hydrocarbons, and wax-type esters. In addition to a mixture of saturated and mono-unsaturated straight chain hydrocarbons, the labial gland of both species contained the isoprenoid hydrocarbons (6E, 10E)-3,7,15-trimethyl-3-methylene-hexadeca-1,6,10,14-tetraene [β-springene], three isomers of 3,7,11,15-tetramethyl-hexadeca-1,3,6,10,14-pentaene [α-springene], and two further unidentified cyclic diterpenes. In B. morrisoni, 3,7,11-trimethyl-2,6,10-dodecatrien-1-ol and 3,7,11,15-tetramethyl-6,10,14-hexadecatrien-1-ol were detected as characteristic alcohols, as well as small amounts of 9-hexadecenol and hexadecanol. Furthermore, a large peak of hexadecyl dodecanoate and minor amounts of 9-hexadecenyl, 9-octadecenyl, and eicosenyl 9-tetradecenoate were found as typical esters in this species. In B. rufocinctus, 9-hexadecenol, hexadecanol, and 9-octadecenol were present in considerable amounts, with their acetates and 9-tetradecenoic, tetradecanoic, hexadecanoic, and 11-octadecenoic acids. The chemical composition of cephalic labial glands in male bumblebees with perching behavior is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bumblebee species can be classified into three main groups according to their male premating behavior (Schremmer 1972; Lloyd 1981; Bergman 1997; Hovorka et al. 1998): (1) species exhibiting patrolling behavior, in which males establish flight paths with scent marks; (2) species showing perching behavior, in which males wait individually at prominent places and dart passing queens or other moving objects; and (3) species showing nest entrance-waiting behavior, in which males, often in groups, wait for emerging queens directly at the nest entrance. Whereas the patrolling behavior, the most common type of premating behavior among bumblebee and cuckoo-bumblebee species has been investigated in many species, the perching behavior and the nest entrance-waiting behavior are less common, less investigated, and consequently not well understood.
Males with perching behavior are easily recognized by their large bulging eyes. Bumblebees with this type of premating strategy occur worldwide, e.g., Bombus mendax, B. confusus, and B. vorticosus in Europe; B. asiaticus, B. rufofaciatus, and B. kashmirensis in Asia; and B. nevadensis, B. griseocollis, B. morrisoni, and B. rufocinctus in North America. In many of these species, scent marking of the perching site has been observed (Haas 1949; Alcock and Alcock 1983; O’Neill et al. 1991; Williams 1991; Kindl et al. 1999). Male labial gland secretions of only a few species with perching behavior have been investigated chemically (Hovorka et al. 1998; Bertsch et al. 2004; Rasmont et al. 2005). In this paper, we investigated the cephalic labial glands of males of the North American bumblebees B. morrisoni and B. rufocinctus.
Methods and Materials
Materials
Males of B. morrisoni Cresson and B. rufocinctus Cresson were collected at 40°22′41″ N, 120°19′42″ W near Litchfield (about 30 km east of Susanville, CA, USA) where these species are abundant. Males of both species are low in numbers, shy, and fast in flight. Ten individuals of each species were caught when they were feeding at Chrysothamnus in late afternoon. Individual gland contents may be variable and change quantitatively and qualitatively (a) during a lifetime (see Sobotnik et al. 2008 for B. terrestris) and (b) during daytime (when secretions are used for scent marking in the morning, glands may be depleted later in the day; see Bergman 1997 for B. lapidarius). In our field collections, old males were recognized by their frayed wings and were discarded. The borders of the wings of all males used for gland preparation were smooth, indicating active males. Males were transported alive to the laboratory and fed with a honey solution for 2 days in flight cages so that they could refill their glands. Then, they were frozen after a short flight activity very early in the morning. The cephalic part of the labial glands was dissected from the head of frozen males and placed in vials (glands from five males per vial) containing 0.2-ml pentane. As the aim of this investigation was not to study the variability of gland content but to elucidate the complexity of a composition of bumblebee male labial glands, we pooled the glands of five males into a single vial. This method allowed us to detect even minor compounds present in the glands.
Gas Chromatography/Mass Spectrometry
A Finnigan MAT TSQ700 gas chromatograph (GC)/tandem mass spectrometer (MS) was employed. GC was carried out on a Hewlett-Packard Ultra 1 column (50 m, 0.2 mm i.d., 0.11 μm film thickness) in a splitless mode with helium as carrier gas at an inlet pressure of 300 kPa. The split valve was opened for 1 min. Initial temperature of 120°C was held for 1 min, then increased at 8°C/min to 280°C, at 3°C/min to 310°C, and at 1°C/min to 320°C. This temperature was held for 10 min. Mass spectrometer conditions were interface temperature 300°C, source temperature 130°C, electron energy 70 eV, emission current 0.2 mA, and electron multiplier 1,400 V. When using the positive ion chemical ionization (CI) mode, ammonia CI gas pressure was 70 Pa. Compounds were identified by comparing their mass spectra with those of the NIST’02 Library (National Institute of Standards and Technology, USA) and by retention times and molecular ions from CI spectra. To determine the position of double bonds, derivatization with dimethyl disulfide was used as described by Buser et al. (1983).
Results
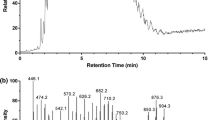
The labial glands of both B. morrisoni and B. rufocinctus males contained a mixture of acyclic diterpenes (alcohols, acetates, and hydrocarbons), two cyclic terpenes, and various straight-chain fatty acid derivates (alcohols, esters, and both saturated and unsaturated hydrocarbons with C21–C31). Typical chromatograms of the male cephalic labial gland secretions of B. rufocinctus and B. morrisoni are shown in Fig. 1. The compounds are summarized in Table 1.
In labial glands of male B. morrisoni, the major compound was 3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraenyl acetate (Fig. 1, 82% of total peak area, peak 20). In addition, considerable amounts of (6E, 10E)-3,7,15-trimethyl-3-methylene-hexadeca-1,6,10,14-tetraene (peak 6), 3,7,11,15-tetramethyl-hexadeca-1,3,6,10,14-pentaene (peak 9), 3,7,11,15-tetramethyl-6,10,14-hexadecatrien-1-ol (peak 18), and hexadecyl dodecanoate (peak 30) were detected. Traces of 3,7,11-trimethyl-2,6,10-dodecatrien-1-ol (peak 3), two more isomers of 3,7,11,15-tetramethyl-hexadeca-1,3,6,10,14-pentaene (peak 7 and 11), two unidentified cyclic diterpenes (peak 13a and 14), 9-hexadecenyl 9-tetradecenoate (peak 31), 9-octadecenyl 9-tetradecenoate (peak 33), and eicosenyl 9-tetradecenoate (peak 34) complete the pattern of substances found. Small amounts of 9-hexadecenol (peak 4) and hexadecanol (peak 5) were identified. The peak area of hexadecyl dodecanoate (30) with about 4.5% of the total peak area shows that considerable amounts of hexadecanol may be used by this species to form esters.
In labial glands of male B. rufocinctus, the major compound was 3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraenyl acetate (Fig. 1, 79% of total peak area, peak 20). Furthermore, considerable amounts of 9-hexadecenol (peak 4), (6E, 10E)-3,7,15-trimethyl-3-methylene-hexadeca-1,6,10,14-tetraene (peak 6), and 3,7,11,15-tetramethyl-hexadeca-1,3,6,10,14-pentaene (peak 9) were detected. Minor amounts of two more isomers of 3,7,11,15-tetramethyl-hexadeca-1,3,6,10,14-pentaene (peak 7 and 11) and 3,7,11,15-tetramethyl-6,10,14-hexadecatrien-1-ol (peak 18) were found. 9-Octadecenol (peak 13b) was also present in considerable amounts co-eluting with the unidentified cyclic diterpene detected in the GC of B. morrisoni (peak 13a). Small amounts of an isomer of this unidentified cyclic diterpene (peak 14), 9-tetradecenoic acid (peak 1), tetradecanoic acid (peak 2), hexadecanoic acid (peak 8), 9-octadecenoic acid (peak 17), 9-hexadecenyl acetate (peak 10), hexadecyl acetate (peak 12), and 9-octadecenyl acetate (peak 17) were detected.
Compared to the chemistry of labial glands of other male bumblebee species, the proportion of hydrocarbons and esters was low in the species studied here. Only few wax-type esters were found (Table 1). Typical GCs of the species studied here also contained four diterpenes, i.e., isomers of springene. Their molecular ion was m/z = 272. Their mass spectra were identical with those of (6E, 10E)-7,11,15-trimethyl-3-methylene-hexadeca-1,6,10,14-tetraene [β-springene] (peak 6) and α-springene (peaks 7, 9, 10). The relative retention times of (6E, 10E)-7,11,15-trimethyl-3-methylene-hexadeca-1,6,10,14-tetraene [β-springene], (3Z,6E,10E)-3,7,11,15-tetramethyl-hexadeca-1,3,6,10,14-pentaene, and (3E,6E,10E)-3,7,11,15-tetramethyl-hexadeca-1,3,6,10,14-pentaene [the α-springenes] were as reported by Burger et al. (1981). As expected, the mass spectra of the three isomers of 3,7,11,15-tetramethyl-hexadeca-1,3,6,10,14-pentaene [α-springene] were nearly identical.
Peaks 13a and 14 in the secretions could not be identified. The mass spectra of both compounds exhibited molecular ions at m/z = 272, indicative of a hydrocarbon of the molecular formula C20H32. The molecular mass was confirmed by CI (NH3) and the pseudo molecular ion m/z 273 ([M+H]+.), whereas the pseudo molecular ion m/z 290 ([M+NH4]+) could not be detected. The mass spectrum of peak 13a was characterized by the following fragment ion m/z (%) values: 272 (19, M+), 135 (100), 120 (11), 119 (10), 107 (90), 105 (36), 93 (43), 91 (33), 79 (11), 77 (13), 69 (46), 53 (13), 41 (33). The mass spectrum of peak 14 was nearly identical. The most intense fragment ions m/z 135 ([C10H15]+) and m/z 107 ([C8H11]+) were observed in cyclic and polycyclic hydrocarbons indicating peak 13a and peak 14 to be isomers of a cyclic terpene.
Discussion
The labial glands of most bumblebee males with the patrolling premating behavior contain a pattern of straight chain primary alcohols [C12–C26] (Bergström et al. 1981, Valterová and Urbanová 1997). The corresponding acetates are often found only in minor amounts or as traces. Indeed, sometimes they are completely absent and occur only because of an aging process of the prepared glands. Our study and other previous studies show that the secretions of male bumblebees with the perching premating behavior differ from bumblebees with patrolling premating behavior in the occurrence of acetates as the leading components (Table 2).
In B. (Cullumanobombus) rufocinctus, 3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraenyl acetate [geranylgeranyl acetate] and 9-octadecenol contributed 84% to the total peak area. In B. (Separatobombus) morrisoni, 3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraenyl acetate [geranylgeranyl acetate] and 3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraen-1-ol [geranylgeraniol] contributed 84% to the total peak area. In B. (Separatobombus) griseocollis, tetradecyl acetate and 3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraenyl acetate [geranylgeranyl acetate] contributed 97% to the total peak area (Bertsch et al. 2004). In B. (Confusibombus) confusus, (Z)-9-octadecenyl acetate and 3,7,11,15-tetramethyl-6,10,14-hexadecatrien-1-ol [geranylcitronellol] contributed 77% to the total peak area (Hovorka et al. 1998), and in B. (Sibiricobombus) vorticosus, 3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraenyl acetate [geranylgeranyl acetate] and 3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraen-1-ol [geranylgeraniol] contributed 87% to the total peak area (Rasmont et al. 2005). In all labial gland secretions of males with perching behavior, two compounds dominate the marking secretions, with an acetate being the dominant compound.
A mixture of substances with high and lower volatility is characteristic of the scent glands in male bumblebees. Bergman and Bergström (1997) detected the main component, farnesol, of the labial glands of B. (Pyrobombus) pratorum in headspace samples from marked leaves, but they could not detect 3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraenyl acetate [geranylgeranyl acetate], also produced by the glands, in these samples. Kindl et al. (1999) showed that 3,7,11,15-tetramethyl-6,10,14-hexadecatrienyl acetate [geranylcitronellyl acetate], though present only in minor amounts in the labial glands of B. (Confusibombus) confusus, could be detected in headspace samples of the male-marked perch (dry flower head of Centaurea stoebe). O’Neill et al. (1991) studied marking behavior and labial glands of male B. rufocinctus. Their results suggest that males of this species mark leaves of Symphoricarpus and Ribes with at least three components of labial gland secretion. Even though these compounds were not identified, the retention times given for them indicate that they might be hexadecenol (O’Neill et al., Fig. 4, peak 12.31), octadecenol (peak 14.29), and 3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraenyl acetate (peak 16.37). Less volatile compounds left on leaves may remain detectable until the next day, helping the bumblebees to find and reconstruct the activity, of the previous day. Evaporation of alcohols and acetates is a first-order process (release rate is proportional to the amount of pheromone present) with long half-lives (Butler and McDonough 1981; McDonough et al. 1989) strongly depending on size, weight, and polarity of the compound molecule.
In labial gland secretions of B. (Pyrobombus) pratorum, two isomers of 3,7,11-trimethyl-2,6,10-dodecatriene [farnesene] were identified, the only isoprenoid hydrocarbons detected in bumblebee labial glands so far (Valterová et al. 1997). (3E, 6E, 10E)-3,7,11,15-Tetramethyl-hexadeca-1,3,6,10,14-pentaene and 7,11,15-trimethyl-3-methylene-hexadeca-1,6,10,14-tetraene [α- and β-springene, respectively] detected in the labial glands of B. (Separatobombus) griseocollis were newly identified isoprenoid hydrocarbons in bumble bee secretions (Bertsch et al. 2004). Meanwhile, these substances also have been detected in B. (Sibiricobombus) vorticusus (Rasmont et al. 2005), a male bumblebee with perching habit. (6E, 10E)-β-Springene has been isolated from a diversity of organisms, including the paracloacal gland of reptiles [the American alligator (Ibrahim et al. 1998) and the smooth-fronted caiman (Avery et al. 1993)], the dorsal secretions of mammals [collared peccary (Waterhouse et al. 1996), white-lipped peccary (Waterhouse et al. 2001), and springbok (Burger et al. 1978, 1981)], the Dufour glands of insects [Australian ant Nothomyrmecia macrops (Billen et al. 1988), the Old World army ant Aenictus rotundatus (Oldham et al. 1994), the ectoparasitoid Habrocon hebetor (Fukushima et al. 1990; Howard et al. 2003), and as a trace component in the stingless bee Nannotrigona testaceicornis (Cruz-Lopez et al. 2001)]. Additional isomers of α-springene described so far are 3,7,11,15-tetramethyl-hexadeca-1,4,6,10,14-pentaene (Waterhouse et al. 1996; Tayassu tajacu, Mammalia) and 2,6,11,15-tetramethyl hexadeca-2,6,8,10,14-pentaene (Burger et al. 1981; see Table 1; Antidorcas marsupialis, Mammalia, and Zhou et al. 2006; Citrus grandis, Spermatophyta).
Studies of the perching behavior of bumblebees have revealed that no activity of virgin females (gynes) is detected near perches that have been marked by males with labial glandular secretion. This effect of male labial glandular secretion in male–female interactions has been shown in B. (Separatobombus) griseocollis (Alcock and Alcock 1983), in B. (Cullumanobombus) rufocinctus (O’Neill et al. 1991), and in B. (Confusibombus) confusus (Kindl et al. 1999). The question whether male labial glandular secretions mark small territories or individual perches and thus, mediate male–male interactions, need to be addressed in future studies.
References
Alcock, J., and Alcock, J. P. 1983. Behaviour in two bumblebees, Bombus nevadensis auricomus and B. griseocollis (Hymenoptera: Apidae). J. Zool. 200:561–570.
Avery, J. W., Shatagati, A., Turner, A., Wheeler, J. W., and Weldon, P. J. 1993. β-Springene in the paracloacal gland secretions of the smooth-fronted caiman (Paleosuchus trigonatus). Biochem. Syst. Ecol. 21:533–534.
Bergman, P. 1997. Chemical Communication in Bumblebee Premating Behaviour. PhD Thesis, Göteborg University, Sweden.
Bergman, P., and Bergström, G. 1997. Scent marking. Scent origin, and species specificity in male premating behaviour of two Scandinavian bumblebees. J. Chem. Ecol. 23:1235–1251.
Bergström, G., Svensson, B. G., Appelgren, M., and Groth, I. 1981. Complexity of bumble bee marking pheromones: Biochemical, ecological and systematical interpretations, pp. 175–183, in P. E., Howse, J. L., and Clément (eds.). Biosystematic of Social Insects. Systematic Association Special Volume No. 19, Academic Press, London, New York.
Bertsch, A., Schweer, H., and Titze, A. 2004. Analysis of the labial gland secretions of the male bumblebee Bombus griseocollis (Hymenoptera: Apidae). Z. Naturforsch. 59c:701–707.
Billen, J. P. J., Jackson, B. D., and Morgan, E. D. 1988. Secretion of the Dufour gland of the ant Nothomyrmecia macrops (Hymenoptera: Formicidae). Experientia 44:715–719.
Burger, B. V., LeRoux, M., Spies, H. S. C., Truter, V., and Bigalke, R.C. 1978. Mammalian pheromone studies III. (E, E)-7,11,15-trimethyl-3-methylene-hexadeca-1,6,10,14-tetraene, a new diterpene analogue of beta-farnesene from the dorsal gland of the springbok, Antidorcas marsupialis. Tetrahedon Lett. 5221–5224.
Burger, B. V., LeRoux, M., Spies, H. S. C., Truter, V., and Bigalke, R. C. 1981. Mammalian pheromone studies. 4. Terpenoid compounds and hydroxy esters from the dorsal glands of the springbok Antidorca marsupialis. Z. Naturforsch. 36c:340–343.
Buser, H. R., Arn, H., Guerin, P., and Rauscher, S. 1983. Determination of double bond positions in mono-unsaturated acetates by mass spectrometry of dimethyl disulfide adducts. Anal. Chem. 55:818–822.
Butler, L. I., and McDonough, L. M. 1981. Insect pheromones: Evaporation rates of alcohols and acetates from natural rubber septa. J. Chem. Ecol. 7:627–633.
Cruz-Lopez, I., Flavia, E., Patricio, L. R. A., and Morgan, E. D. 2001. Secretion of stingless bees: The Dufour gland of Nannotrigona testaceicornis. J. Chem. Ecol. 27:69–80.
Fukushima, J., Kuwamara, Y., Yamada, A., and Suzuki, T. 1990. New non-cyclic homo-diterpene from the sting glands of Bracon hebetor Say (Hymenoptera, Braconidae). Agric. Biol. Chem. 54:809–810.
Haas, A. 1949. Arttypische Flugbahnen von Hummelmännchen. Z. Vergl. Physiol. 31:281–307.
Hovorka, O., Urbanová, K., and Valterová, I. 1998. Premating behavior of Bombus confusus males and analysis of their labial gland secretion. J. Chem. Ecol. 24:183–193.
Howard, R. W., Baker, J. E., and Morgan, E. D. 2003. Novel diterpenoids and hydrocarbons in the Dufour gland of the ectoparasitoid Habrobracon hebetor (Say) (Hymenoptera: Braconidae). Arch. Insect Biochem. Physiol. 54:95–109.
Ibrahim, S. A., Avery, J. W., Weldon, P. J., and Wheeler, J. W. 1998. Age-class differences in lipids from the paracloacal glands of the American alligator (Alligator missippiensis). Z. Naturforsch. 53c:201–209.
Kindl, J., Hovorka, O., Urbanová, K., and Valterová, I. 1999. Scent marking in male premating behavior of Bombus confusus. J. Chem. Ecol. 25:1489–1500.
Lloyd, J. E. 1981. Sexual selection: Individuality, identification, and recognition in a bumblebee and other insects. Florida Entomol. 64:89–107.
McDonough, L. M., Brown, D. F., and Aller, W. C. 1989. Insect sex pheromones: Effect of temperature on evaporation rates of acetates from rubber septa. J. Chem Ecol. 15:779–790.
Oldham, N. J., Morgan, E. D., Gobin, B., Schoeters, E., and Billen, J. 1994. Volatile secretions of an Old World army ant, Aenictus rotundatus, and chemotaxonomic implications of army ant Dufour gland chemistry. J. Chem. Ecol. 20:3297–3305.
O’Neill, K. M., Evans, H. E., and BJOSTAD, L. B. 1991. Territorial behavior in males of three North American species of bumblebees (Hymenoptera, Apidae, Bombus). Can. J. Zool. 69:604–613.
Rasmont, P., Terzo, M., Aytekin, A. M., Hines, H., Urbanova, K., Cahlikova, K., and Valterova, I. 2005. Cephalic secretions of the bumblebee subgenus Sibiricobombus Vogt suggest Bombus niveatus Kriechbaumer and Bombus vorticusus Gerstaecker are conspecific (Hymenoptera, Apidae, Bombus). Apidologie 36:571–584.
Schremmer, F. 1972. Beobachtungen zum Paarungsverhalten der Männchen von Bombus confusus Schenk. Z Tierpsychol. 31:503–512.
Sobotnik, J., Kalinková, B., Cahliková, L., Weyda, F., Ptacek, V., and Valterová, I. 2008. Age-dependent changes in structure and function of the male labial glands in Bombus terrestris. J. Insect Physiol. 54:204–214.
Valterová, I., and Urbanová, K. 1997. Chemiké signály cmeláku [Chemical signals of bumble bees]. Chem. Listy 91:846–857(in Czech).
Waterhouse, J. S., Ke, I., Pickett, J. A., and Weldon, P. J. 1996. Volatile components in dorsal gland secretions of the collared peccary, Tayassu tajacu (Tayassuidae, Mammalia). J. Chem. Ecol. 22:1307–1314.
Waterhouse, J. S., Hudson, M., Pickett, J. A., and Weldon, P. J. 2001. Volatile compounds in dorsal secretions of the white-lipped peccary, Tayassu peccari, from Bolivia. J. Chem. Ecol. 27:2459–2469.
Williams, P. 1991. The bumble bees of the Kashmir Himalaya (Hymenoptera: Apidae, Bombini). Bull. Br. Mus. Nat. Hist. (Ent.) 60:1–204.
Zhou, J., Zhou, Ch., Jiang, X., and Xie, L. 2006. Extraction of essential oil from shaddock peel and analysis of its components by gas chromatography-mass spectrometry. J. Cent. South Univ. Technol. 13:44–48.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bertsch, A., Schweer, H. & Titze, A. Chemistry of the Cephalic Labial Gland Secretions of Male Bombus morrisoni and B. rufocinctus, Two North American Bumblebee Males with Perching Behavior. J Chem Ecol 34, 1268–1274 (2008). https://doi.org/10.1007/s10886-008-9538-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-008-9538-8