Abstract
Many species of freshwater fish with relatively simple mating strategies release hormonally derived sex pheromones in urine. However, it is not known whether species with more complex reproductive strategies use specialized urinary chemical signals. We addressed this by using the Mozambique tilapia (Oreochromis mossambicus Peters 1852), a lek-breeding species in which males establish dominance hierarchies and visiting females mate preferentially with territorial/dominant males. We measured urination frequency of territorial males in social isolation and in the presence of females that were either ready to spawn or had finished spawning. In groups of fish, we monitored the volume of urine stored in subordinate and dominant males to determine if urine volume and olfactory potency (by recording electro-olfactograms, EOG, in females) are related to the male’s social rank. Dominant, territorial males stored more urine than subordinates and released it in short pulses, the frequency of which increased in the presence of females ready to spawn but not in the presence of post-spawn females. Urine from subordinate and dominant males was fractionated by liquid chromatography and fractions tested for olfactory potency by using the EOG, with the most potent fraction analyzed by mass spectrometry (MS). The olfactory system of females was sensitive to a urinary compound that was more abundant in the urine of dominant males than in that of subordinates. MS analysis suggested the compound is a sulfated aminosterol-like compound with a formula of C29H40N2O10S. Therefore, we suggest that dominant/territorial tilapia males dramatically increase urination frequency in the presence of females ready to spawn and that the urinary odorant acts as a pheromonal signal of dominance, thereby influencing female spawning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although sex pheromones are known to mediate reproduction in fish, detailed information on chemical identities, physiological origin, control of release, and function is available only from a small number of species with relatively simple reproductive strategies (Stacey and Sorensen 2006). Among these, the goldfish (Carassius auratus) is the best studied. It has a scramble-competition polygynous mating system, representative of many other cyprinids; females release steroids and prostaglandins via the gills and/or the urine, which act as hormonal pheromones that affect the reproductive physiology and behavior of males. Sexually receptive females control the release of urinary prostaglandin pheromones while exhibiting simple behaviors. This is thought to represent a specialization of pheromone release related to marking spawning habitat, so as to lure males (Appelt and Sorensen 2007). Cichlids, in contrast, employ a range of relatively complex mating systems (Barlow 1991). Typical among the mouth-brooding cichlids is polygynandry, wherein males defend a spawning territory that is visited by females and where males display conspicuous courtship behaviors (Barlow 1991; Nelissen 1991). The possibility of chemical communication in these reproductive interactions, however, has been little studied in this group.
The Mozambique tilapia (Oreochromis mossambicus Peters 1852) is a maternal mouth-brooding cichlid that spawns repeatedly throughout the year, with the females having a regular ovulatory cycle of 15–20 days (Coward and Bromage 2000). Males aggregate in breeding arenas or leks, where territorial males adopt a characteristic black coloration and defend small territories centered on the nests (pits) that they dig in the sand (Bruton and Boltt 1975). Females visit the leks when ready to spawn but then collect and brood the fertilized eggs and subsequent fry away from the leks (Fryer and Iles 1972). In captivity, male tilapia form a stable linear hierarchy (Oliveira and Almada 1996, 1998b), with the largest alpha males receiving the majority of spawning females (Oliveira and Almada 1996). Previous studies have found that the olfactory system of females is highly sensitive to substances released to the water by territorial males and to male body fluids, with urine being of special importance (Frade et al. 2002). This suggests the possibility of male urinary pheromones being involved in tilapia reproduction and, if males control their release, for chemical communication in this species.
In the present study, we tested whether male tilapia send chemical signals to females and whether this ability depends on male social status. Three experiments were conducted. First, we assessed urination frequency of males, with and without pre-ovulatory (ready to spawn) and post-ovulatory (post-spawn) females, to determine whether they may use urinary pheromone(s) to communicate. Second, we formed groups of fish and monitored the volume of urine stored in subordinate and dominant males to determine if urine volume and urine olfactory potency on females are related to male social rank. Finally, to characterize the urinary odorants, samples of urine collected from subordinate and dominant males were fractionated by liquid chromatography, the resulting fractions were tested for olfactory potency on females, and the most potent fraction was analyzed by mass spectrometry.
Methods and Materials
Experimental Animals
Before the experiments, groups of fish (one male and three or four females) were housed for several weeks in re-circulating aquaria. The aquaria (93 × 60 × 55 cm) contained 200 l dechlorinated tap water at 27°C and sand substrate. The photoperiod was 12 L:12 D, and the fish were fed twice a day with commercial cichlid food (Nutrafin basix®; Rolf C. Hagen, Inc., Montreal, Canada). Spawning occurred in all fish groups, but the eggs were removed to stimulate the initiation of a new ovulatory cycle. Each female was observed over at least two ovulatory cycles before use in behavioral experiments to ensure the predictability of ovulation. “Pre-ovulatory” females were used in experiments on the day before or on the day predicted for their next ovulation; their ovulation cycle ranged from 9 to 16 days (mean ± SD; 15.0 ± 3.1 days; N = 7). “Post-ovulatory” females were used 1–3 days after their last ovulation.
Visualization of Urine and Quantifying Patterns of Urination Behavior
To determine whether males use urine to communicate during reproductive behavior, the urination frequency of a male was assessed in social isolation and while a female was present. In each replicate, a male was taken from its family tank and isolated in a glass tank (79 × 35 × 45 cm, 50 l, 27°C, aerated), with a sand substratum, for 24 h before the experiment. The male was lightly anesthetized by immersion in iced water for 2–3 min and injected in the dorsal musculature with 100 μl patent blue violet (100 mg ml−1 in 0.9% NaCl; Sigma-Aldrich), a dye that is released mainly through the urine and allows monitoring of the frequency of urine release (Appelt and Sorensen 1999, 2007; Barata et al. 2007). The fish was then placed back into its tank, and after the first urine pulse, urination was measured and fish behavior was recorded on video for 45 min (social isolation). Then, a pre- or post-ovulatory female (taken from a different family tank) was introduced to the tank and urine release and behavior recorded for another 45 min. Frequency and duration of each urine pulse was recorded by the observer with a hand-held computer (Psion Organizer LZ64) programmed for timing the start and end of each urine pulse. The procedure was replicated with seven males (mean ± SD, standard length = 156.3 ± 18.6 mm, and body weight = 119.5 ± 18.6 g) and seven pre-ovulatory females (length = 147.0 ± 20.9 mm; weight = 100.1 ± 41.8 g), and with another group of seven males (length = 155.0 ± 26.2 mm; weight = 120.7 ± 48.2 g) and seven post-ovulatory females (length = 146.1 ± 18.5 mm; weight = 96.6 ± 45.9 g). After each replicate, the males were returned to their original family tanks, but the females were deeply anesthetized (500 mg l−1 3-aminobenzoic acid ethyl ester, MS222, Sigma-Aldrich), and then a sharp blow to the head was applied to assure death before assessing their ovarian condition. The ovaries of each female were weighed and the gonadosomatic index (GSI = gonads mass/body mass × 100) calculated. A random sample of ten eggs was also weighed, and their diameters measured under a binocular microscope.
Male behavior was classified as not displaying (hovering or active swimming when the female was present), courting (including nest digging), or aggressive. Female behavior was classified as not displaying, receptive (following the male during its courtship displays), or submissive. These behaviors are described in detail by Baerends and Baerends van Roon (1950) and by Oliveira (1995). Total duration of all behaviors was quantified by using The Observer Video-Pro 4.0 software (Noldus Information Technology, Wageningen, The Netherlands).
Quantifying Urine Stored in Subordinate and Dominant Males
To determine if urine volume stored in the bladder is related to male social behavior and to obtain urine for testing olfactory potency and mass spectrometry analysis, males in social groups were monitored daily. Two groups (A and B) of 12 males and ten females (previously kept in family tanks as described above) were sequentially housed for 15 days in a plastic tank (128 × 110 × 50 cm) containing dechlorinated tap water (approximately 600 l) at 25–27°C; the photoperiod was 12 h, and feeding was once a day. The males were tagged with colored plastic labels (T-Bar extra small anchor—FF-94, Floy Tag Inc., Seattle, WA, USA) attached to the muscle near the dorsal fin and their behavior observed each morning, midday, and afternoon over 10 days (30 observations per male). Systematic behavioral observations started on the four day after formation of each fish group. The frequency of submissive (escape from an aggressive opponent, submission displays during agonistic interactions, or absence of dark coloration without social interaction) and dominant behaviors (aggressive displays, nest-digging, courtship displays towards females, or dark coloration without social interaction) was recorded over 5 min for each male. Although the tank had no sand, some males exhibited nest-digging behavior by nipping the bottom, as they normally do with a sand substratum. At the end of each afternoon’s observations, each male was slightly anesthetized (50 mg l−1 MS222), and urine was taken by gently squeezing the area immediately above and anterior to the genital papilla. Squeezing of this abdominal area caused erection of the genital papilla, and a jet of urine was collected. Urine volume was measured by weighing, and the urine was stored at −20°C until use (see below). The fish were allowed to recover and placed back in the tank.
The standard length and weight of each fish were measured at the start of each replicate (mean ± SD; group A: male length = 137.8 ± 5.6 mm, male weight = 81.6 ± 11.3 g, female length = 103.7 ± 9.6 mm, female weight = 39.9 ± 9.1 g; group B: male length = 144.4 ± 6.3 mm, male weight = 86.4 ± 10.0 g, female length = 111.2 ± 10.4 mm, female weight = 46.6 ± 10.2 g) and at the end to calculate their daily growth. The GSI of males of different social rank was compared between six dominant males (three from each group) and six subordinates (two from fish group A and four from group B) following the procedure described above for females. Samples of urine obtained from these males were assessed for olfactory potency on females, fractionated by liquid chromatography, and the resulting fractions assessed for olfactory potency and analyzed by mass spectrometry (see below). Samples of urine collected daily from all males in the two replicates were also assessed for olfactory potency to determine whether there is a relationship between males’ social rank and the olfactory potency of their urine (see below).
Liquid Chromatography Coupled with Mass Spectrometry
To determine which odorants are found in the urine of dominant and subordinate males, solid-phase extraction (SPE) of urine samples was carried out followed by fractionation and analysis by liquid chromatography–mass spectrometry (LC–MS). Urine samples from the same male were pooled for analysis. Urine (2 ml) was passed through a C18 cartridge (Sep-Pak®, Waters, Milford, MA, USA) and eluted with methanol (2 ml). Half of the eluent was kept at −20°C until used for assessment of olfactory potency on females by recording the electro-olfactogram (EOG; see below), while the other half was fractionated by LC–MS. Distilled water (2 ml) was subjected to the same procedure to generate a control eluent.
For LC–MS, the unconjugated bile acid chenodeoxycholic acid (CDCA; Steraloids Inc, Newport, RI, USA) was added (1 μg) to each of the sep-pack eluates (internal standard) to standardize retention times and injection volumes between samples of male urine. Eluates were dried under a stream of nitrogen, reconstituted in 110 μl methanol/water (60/40, v/v) and transferred to autosampler glass vials for LC–MS. The LC column (Nova-Pak reversed-phase C18, 4 μm, Waters Chromatography Division, Milford, MA, USA) was coupled to a mass spectrometer with electrospray ionization (ESI; LCQ Classic, Thermo Electron Corporation, Waltham, MA, USA). The mobile phase was isocratic at 15% methanol for 4 min, increased linearly from 15% to 100% methanol from 4 to 91 min, and allowed to run at 100% methanol for 5 min before ramping back to 15% methanol for the next sample. The flow was 1 ml min−1. After passing through the column, the sample was routed to a splitter—10% to the mass spectrometer and 90% collected into 30 3-min fractions for 90 min by using an autosampler (FC203, Gilson Inc., Madison, WI, USA). The 12 samples were run in succession, alternating between subordinate and dominant males. The fractions from each male were kept at −20°C until assessment of olfactory potency on females (see below).
Mass spectra were acquired with an ion trap mass spectrometer equipped with an ESI source and analyzed with Xcalibur software (Thermo Electron Corporation). The ion trap was operated in the negative ion mode with a spray voltage of 5 kV. A stream of 99% pure nitrogen at 60 psi was used as the sheath gas. Data were collected continuously in the range m/z 200–950. The relative peak areas (normalized to the area of the internal standard, CDCA) in the LC fractions that had significant olfactory potency were determined with Xcalibur software. Positive-ion MS was performed on olfactory-active fractions to determine whether any other compound could be present that was not detectable in the negative-ion mode. MS–MS at various energies was performed in the negative-ion mode in an attempt to ascertain more structural information about compounds in active fractions.
To deduce the chemical formula of compounds in active fraction(s), negative-ion high-resolution electrospray ionization was performed with a QSTAR1 MS (Applied Biosystems, Foster City, CA, USA) equipped with a hybrid quadrupole/time-of-flight detector. The most likely formulae were deduced by making several conservative assumptions about structure: (1) the presence of between 0 and 40 carbon atoms (high numbers were used so as to be conservative), (2) the presence of between 0 and 90 hydrogen atoms, (3) an even number of (or no) nitrogen atoms with a maximum of 12 nitrogens, (4) at least one sulfur and four oxygen atoms (consistent with the presence of at least one sulfate group as observed with MS/MS) with a maximum of three sulfur and 12 oxygen atoms, and (5) a mass accuracy within 15 ppm (a conservative measure, as a properly calibrated instrument should measure within 10 ppm). We identified all potential molecular formulae that fit this description and then systematically eliminated remaining formulae when the measured isotopic distribution pattern (i.e., the entire spectra, including the parent ion and all of its isotopes) did not match a structure’s theoretical isotopic distribution pattern (for details on this procedure, see Sorensen et al. 2005 and Hoye et al. 2007).
Olfactory Sensitivity of Females to Male Urine
Whole urine, urine extracts obtained by SPE, and LC fractions were tested for olfactory potency on females. The EOG was recorded as previously described (Frade et al. 2002). Briefly, male tilapia were anesthetized in water containing 100 mg l−1 MS222 and immobilized with gallamine triethiodide (3 mg kg−1 in 0.9% saline). The fish were placed in a padded Perspex® V-clamp, and their gills irrigated with aerated water containing MS222 (50 mg l−1). The olfactory rosette was exposed by removing the ring of cartilage surrounding the nostril and continually irrigated with dechlorinated, charcoal-filtered tap water (6 ml min−1). Stimulus-containing water was introduced into this flow via a three-way solenoid valve. At least 1 min was allowed to elapse between successive stimuli. The DC voltage was recorded by two glass micropipettes filled with 0.9% NaCl in 4% agar, one being placed close to the olfactory epithelium, and the other placed lightly on the skin of the head. The signal was amplified (×103) and recorded on a PC running Axoscope software (version 1.1, Axon Instruments, Inc., Foster City, CA, USA). The peak amplitude of the EOG to a given stimulus was blank-subtracted and normalized to the response to 10−5 M l-serine (standard).
Stimuli were made up on the day of recording by using the same dechlorinated, charcoal-filtered tap water used to irrigate the fish’s nostril. Urine collected each day from males in the social groups was tested at a dilution of 1:104 in water (v/v) on the same female, with six females (mean ± SD; length = 114.0 ± 4.7 mm; weight = 47.6 ± 6.1 g) used to assess the olfactory potency of all urine samples. Untreated urine from six subordinate and six dominant males and the corresponding urine SPE eluents were tested on each of the three females at a dilution of 1:104 (v/v). Using another group of females, the SPE urine eluent and corresponding LC fractions of one male of each social rank were tested on the same female; the stimuli from each male were tested on three females. Therefore, 18 females (mean ± SD; length = 123.1 ± 18.1 mm; weight = 62.0 ± 33.4 g) were used to assess the olfactory potency of these male-derived stimuli at a dilution of 1:104 (v/v; an equivalent dilution of the untreated sample urine). As a control, methanol was tested at 0.015% to 0.1% in water (the range of methanol concentration in urine extracts and LC fractions).
Statistical Analysis
Frequency and duration of urine pulses from males and behavioral data of both sexes were compared between social contexts or between female reproductive conditions (social isolation, pre- or post-ovulatory female) by either the Student’s t tests (paired samples) or one-way analysis of variance (ANOVA). The non-parametric Kendall’s correlation was used to evaluate (within each of the two social groups of fish) the relationship between the mean urine volume per day and male size or mean dominance index per day (calculated as the ratio of the summed frequency of all dominant behaviors and the summed frequency of all dominant and submissive behaviors). The daily growth rate (length and weight) within each fish group was tested for difference from zero with the Student’s t test; the relationship between growth rate and the mean dominance index per day was evaluated by the Kendall’s correlation. Univariate regression was used to evaluate the relationship between the mean EOG amplitudes evoked by daily urine samples of each male and fish group (fixed factor), mean urine volume per day, dominance index per day, and two-way interactions between the continuous covariates and the fixed factor. The Student’s t test for independent samples was used to compare the relative area of LC peaks in fractions that had significant olfactory potency between subordinate and dominant males. Normalized EOG amplitudes (mean from three females for each stimulus) evoked by crude urine, SPE eluents, and LC fractions from subordinate and dominant males were compared by one-way repeated measures ANOVA with male type as between-group variable and stimulus type as within-subject variable. Statistical analyses were carried out with SPSS® 14.0 for Windows software (SPSS Inc., 1989–2005), and in all cases, statistical significance was set at P < 0.05. All data are shown as mean ± SEM.
Ethical Statement
Our research has followed the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (European Treaty Series No. 123), the ASAB/ABS Guidelines for the Use of Animals in Research (published in Animal Behavior, 2003, 65:249–255), and the Guidelines for the Use of Fishes in Research by the American Fisheries Society (http://www.fisheries.org/afs/publicpolicy/guidelines2004.pdf).
Results
Male Urination Frequency and Behavior
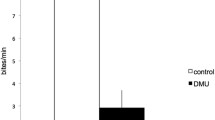
In isolation, male tilapia urinated at low frequency (about once every 10 min) and expelled urine in short-duration pulses (about 1 s). When together with a pre-ovulatory female, males significantly increased their urination frequency; this did not happen with a post-ovulatory female (Fig. 1). No obvious changes in the release of feces or intestinal fluids were seen. The duration of urinary pulses was not different among the three social contexts (isolation, 1.43 ± 0.27 s, N = 44; with pre-ovulatory female, 1.26 ± 0.14 s, N = 225; with post-ovulatory female, 1.31 ± 0.12 s, N = 78; one-way ANOVA, F 2,344 = 0.16, P = 0.856).
Urination of male tilapia in isolation and in the presence of a female. Urination frequency (mean ± SEM) with a pre-ovulatory female (black bar, N = 7) was significantly higher than in isolation (open bar, N = 7; asterisk Student’s t test for paired samples, t 6 = 5.46, P < 0.005). With a post-ovulatory female (gray bar, N = 7), the frequency of urination was not different from that when in social isolation (open bar, N = 7; t 6 = 1.77, P = 0.127)
Male behavior was not different in the presence of pre- or post-ovulatory females. Males were never indifferent to the presence of a female and spent most of the time swimming in her vicinity (no display), sometimes displaying obvious courtship behaviors and, more rarely, aggressive behaviors (Fig. 2a). Pre- and post-ovulatory females did not differ in their behavior either. The females spent most of the time swimming in the vicinity of the male and sometimes exhibiting receptive or submissive behaviors, depending on the male’s behavior (Fig. 2b). The pattern of urine release varied among males; higher urination frequency was not necessarily associated with specific behaviors of either the male or the female. For example, a male might urinate at high frequency during clear courtship displays or while he was swimming in the vicinity of a pre-ovulatory female without displaying obvious courtship (Fig. 3, example 1). Also, a male would sometimes urinate with high frequency in the presence of a pre-ovulatory female without displaying obvious courtship (Fig. 3, example 2). Finally, in the presence of a post-ovulatory female, the male would sometimes exhibit clear courtship behaviors without urinating at high frequency (Fig. 3, example 3).
Behavior of male and female tilapia. a Males in the presence of pre-ovulatory females (black bars, N = 7) or post-ovulatory females (gray bars, N = 7) did not differ (Student’s t tests for independent samples, df = 12, P > 0.05) in the time (mean ± SEM) spent in different behavioral classes. b Pre-ovulatory females (black bars, N = 7) and post-ovulatory females (gray bars, N = 7) did not differ in the time spent in different behavioral classes in the presence of a male (Student’s t tests for independent samples, df = 12, P > 0.05)
Examples of male behavior, release of urine, and female behavior. Each example (1 to 3) shows the male’s behavior (top horizontal bar: white no display, gray courtship displays, black aggressive displays), the male’s urination (urine pulses of about 1 s are indicated by vertical black bars below the behavior of each male) and the female’s behavior (low horizontal bar: white no display, gray receptive, black submissive). In examples 1 and 2, both males were in the presence of a pre-ovulatory female, and in three, the male was in the presence of a post-ovulatory female
Analysis of females’ ovarian states confirmed that they were in different reproductive condition. The GSI of pre-ovulatory females (4.4 ± 1.7%) was higher than that of post-ovulatory females (1.4 ± 0.4%; Mann–Whitney U, z = 3.1, N 1 = N 2 = , P < 0.005). The average egg weight was also higher in pre-ovulatory females (2.48 ± 1.40 mg) than in post-ovulatory females (0.17 ± 0.06 mg; z = 3.1, P < 0.005). This was due to larger egg sizes between 1.6 to 2.5 mm (54.3 ± 24.4% of the eggs) in pre-ovulatory females, whereas in the post-ovulatory females, the eggs did not exceed 1.0 mm in diameter.
Social Hierarchies, Urine Volume, and Olfactory Potency of Urine
In two groups of fish, males maintained a stable social hierarchy over 10 days of observation. In each group, three males were dominant (mean dominance index per day higher than 0.5), occupying a fixed territorial position in the tank, showing dark coloration and frequently displaying courtship or aggression. Among the other males of lower social rank, two in each group adopted intermittently dominant and submissive behaviors (mean dominance index per day between 0.2 and 0.4), whereas the remaining seven males in each group were clearly subordinate (mean dominance index per day lower than 0.2) and rarely or never exhibited territorial/dominant behaviors (e.g., dark coloration, courtship, or aggressive displays). Although the urine volume collected daily from each male varied, males of higher social rank consistently produced more urine than males of a lower social rank; within each group, the mean urine volume collected per male was positively correlated to the male’s social rank (Fig. 4a) rather than its size either at the start (group A: length, T = 0.28, P = 0.206; weight, T = 0.25, P = 0.265; group B: length, T = 0.38, P = 0.084; weight, T = 0.12, P = 0.583) or at the end of observations (group A: length, T = 0.15, P = 0.486; weight, T = 0.20, P = 0.369; group B: length, T = 0.37, P = 0.107; weight, T = 0.24, P = 0.075). In both groups, males had a small daily growth that was not correlated with social rank (Table 1).
Urine volume, urine olfactory potency, and dominance index of donor male tilapia in groups. Male’s dominance index was calculated as the ratio of the summed frequency of dominant behaviors and the summed frequency of dominant and submissive behaviors (see “Materials and Methods”). a Scatter plot of mean dominance index per day (±SEM, N = 10 d) and mean urine volume per day (±SEM, N = 10 days) from each male in fish group A (N = 12; filled circles) or B (N = 12; open circles). In both groups, the urine volume was positively correlated (Kendall’s correlation) to the male’s dominance index (group A, T = 0.60, P < 0.001; group B, T = 0.51, P < 0.05). b Relationship between dominance index (mean per day ± SEM, N = 10 d) of males in fish group A (open circles, N = 12) and B (filled circles, N = 12), and normalized EOG amplitudes evoked by corresponding male urine (diluted 1:10,000) collected each day (mean ± SEM, number of urine samples varied between one and ten)
Univariate regression analysis used to evaluate the relationship between the mean olfactory potency of urine and fish group (fixed factor), mean urine volume per day, and mean dominance index per day provided a significant explanatory regression model (R 2 adj = 0.61, F 7,16 = 7.01, P = 0.001). The variation in the mean olfactory potency of male urine was explained by variation in the mean dominance index per day (F 1,18 = 4.67, P = 0.044) and not by differences between the two male groups (F 1,18 = 1.65, P = 0.215), variation in the mean urine volume collected per day (F 1,18 = 0.480, P = 0.497), or by the interaction between the fish group and male behavior or urine volume (F 1,18 = 0.502, P = 0.488). The mean EOG amplitudes elicited by male urine increased with the mean dominance index per day (Fig. 4b).
Odorants in the Urine of Dominant and Subordinate Males
Urine and C18 SPE eluents of urine from six dominant males evoked higher amplitude EOG responses than those of six subordinate males; matching samples of urine and SPE eluent from either group of males were not significantly different (Fig. 5). These dominant and subordinate males differed in the mean dominance index per day and mean urine volume per day; however, they did not differ in length, weight, daily growth, or GSI (Table 2).
Olfactory potency of male tilapia urine and extracts of urine on females. EOG amplitudes (mean ± SEM) evoked by urine and solid-phase extracts of urine (diluted 1:10,000) from subordinate (open bars; N = 6) and dominant (filled bars; N = 6) males. The urine and extracts of urine from dominant males elicited higher EOG amplitudes than those from subordinate males (asterisk between groups, F 1,10 = 6.12, P < 0.05); within each male type, no significant differences were found between the EOG responses elicited by the urine and the extract of urine (within subjects, F 1,10 = 0.58, P = 0.46)
EOG responses to liquid chromatography (LC) fractions of SPE eluent showed that fraction 15 (eluted from the LC column between 42 and 45 min) of both dominant and subordinate males evoked the largest EOG amplitudes in females, followed by fraction 16 (eluted between 45 and 48 min; Fig. 6a). The two fractions from dominant males evoked higher EOG amplitudes than the same two fractions from subordinate males (fraction 15: F 1,10 = 8.04, P < 0.05; fraction 16: F 1,10 = 12.98, P < 0.01; Fig. 6b). For both dominant and subordinate males, no significant difference was found between the olfactory potency of the original SPE eluent and LC fraction 15 (Student’s t tests for paired samples: dominant males, t 5 = 0.07, P = 0.946; subordinate males, t 5 = 2.30, P = 0.07), suggesting that fraction 15 contained most of the olfactory potency found in the total SPE eluent. Control SPE eluent and methanol alone at 0.015% to 0.1% in water did not evoke EOG amplitudes higher than the water blank (data not shown).
Olfactory responses of female tilapia to liquid chromatography (LC) fractions of C18 solid-phase extraction eluents of male urine. a Typical electro-olfactograms (EOGs) recorded in response to stimuli (black horizontal bars) of LC fractions 14 to 17 (elution from the LC column between 40 and 51 min; each fraction corresponds to 3 min elution) diluted 1:10,000 in water. b Normalized EOG amplitudes (mean ± SEM) elicited by LC fractions (1 to 30 collected every 3 min) of extracts of urine (diluted 1:10,000) from subordinate (open bars; N = 6) and dominant males (filled bars; N = 6). LC fractions 15 (elution between 42 and 45 min), and 16 (elution between 45 and 48 min) from dominant males elicited higher EOG amplitudes than those from subordinate males (*P < 0.05; ** P < 0.01)
Chromatograms of urine SPE eluent of subordinate and dominant males both had about 25 detectable peaks (Fig. 7a and b). The average retention time (RT) for CDCA (internal standard) was 68.37 ± 0.078 min (mean ± SD). Fraction 15 corresponded to an apparent single peak (RT = 43.96 ± 0.45 min; range from start to finish of peaks in all 12 samples = 42.5–46.5 min), which, for some samples, tailed into fraction 16. The relative area of this peak (normalized to the area of the internal standard) was higher in dominant males (11.69 ± 1.14) than in subordinate males (6.12 ± 0.74; Student’s t test, t 10 = 4.08, P < 0.005). The MS of fraction 15 was composed of two negative ions at 609.0 and 511.4 m/z for both dominant and subordinate males (Fig. 7c) with the same retention time (data not shown) strongly suggesting that they were from a single compound. The difference of m/z 98 between the two ions suggests the loss of H2SO4, seen for all sulfated steroids tested by Fine (2006). MS/MS on m/z 609.0 yielded a dominant negative ion of m/z 511.4, demonstrating that the two ions are from a single compound, and MS/MS on m/z 511.4 yielded a dominant ion of m/z 493.3 (loss of 18), suggesting at least one hydroxyl group in the molecule (Fig. 7d). Positive-ion MS of the peak in fraction 15 yielded three ions: \({m \mathord{\left/ {\vphantom {m z}} \right. \kern-\nulldelimiterspace} z}535.2\left[ {{\text{M}} - {\text{H}}_2 {\text{SO}}_4^ + {\text{Na}}} \right]\), m/z 1,047.3 [2M-2(H2SO4)+Na], and m/z 1,070.4 [2M-2(H2SO4)+2Na]), consistent with fraction 15, containing a single compound. High-resolution MS analysis of the compound in fraction 15 yielded ions at m/z 609.2303 and 511.2768. Although 34 potential formulae met the required criteria, only C29H40N2O10S could explain the observed isotopic ratios that were measured (see “Materials and Methods”).
Representative liquid chromatography–mass spectrometry chromatograms of male tilapia urine extracts. Subordinate male (a) and a dominant male (b). In both chromatograms, the olfactory active peak of fraction 15 (42–45 min) is indicated by the arrow and corresponding retention time; chenodeoxycholic acid (CDCA; 1 μg) was added as internal standard. c Mass spectrum of the peak centered at 43.4 min in (b). Both ions are from a single compound; the smaller shows the loss of a sulfate from the larger. The spectrum for the peak at 44.1 min in (a) is identical (data not shown). d Mass spectrometry/mass spectrometry of 511.4 m/z in c. The dominant ion (m/z 493.3) shows the loss of H2O
Discussion
Territorial/dominant tilapia males seem to signal females by using a urinary odorant that may allow females to discriminate dominant from subordinate males. This is supported by three lines of evidence. First, territorial males urinate at higher frequency in the presence of females ready to spawn (pre-ovulatory) than they do in the presence of sexually inactive females (post-ovulatory) or in social isolation. Given that the duration of urine pulses did not change among the different social contexts, then a greater volume of urine must also be released when the male is in the presence of a spawning female. Second, a putative sulfated aminosterol-like compound with high olfactory potency is found in larger amounts in the urine of territorial/dominant males than in that of non-territorial/subordinate males. This is consistent with previous work that showed that males release sulfated steroid-like odorants in the urine (Frade et al. 2002). Third, subordinate males store less urine in the bladder than do males of higher social rank, agreeing with a previous study (Barata et al. 2007). Taken together, these results suggest that territorial/dominant males advertise social status by increasing the release of a potent urinary odorant in the presence of a spawning female; subordinate males are less capable of stimulating a female because they produce lesser quantities of the (putative sulfated aminosterol-like) odorant and are also less capable of increasing urination frequency because they store less urine.
The mixed-sex groups of fish were formed with males that previously had been kept separated from each other, all being territorial in their original tanks with four or five females, and where reproduction occurred regularly. However, when these males were placed together, a clear dominance hierarchy emerged. The dominant males were effective in defending a fixed territorial position and displayed courtship to approaching females, whereas males of lower social rank seldom or never adopted the black coloration typical of a territorial male and did not court females. This is in agreement with previous behavioral studies that showed that mating success is highly skewed toward dominant males in established groups of captive Mozambique tilapia (Oliveira and Almada 1996, 1998a). Our study also showed that dominant males store more urine than males of lower social rank, and urine of dominant males was of higher olfactory potency and related to a higher concentration of a sulfated aminosterol odorant. Therefore, we suggest that both the urine volume stored in the bladder and concentration of the sulfated aminosterol odorant reflect social dominance rather than reproductive capability (i.e., presence of mature testes).
The olfactory potency of the C18 (methanol) eluent of male urine was not significantly different from that of crude urine (Fig. 5). This indicates that the most active/important odorant(s) found in male urine are relatively non-polar (compared to substances that would have passed through the C18 adsorbent in the aqueous urine). Furthermore, because the olfactory potency of the only EOG-active LC fraction (no. 15) was not significantly different from that of the urine extract and it contained only a single compound (as indicated by mass spectrometric analysis), it seems likely that the olfactory potency of urine extract is largely due to a single odorant (of m/z 609). Mass spectrometric analyses indicated that the odorant is a sulfated steroid derivative with a molecular weight of 610 Da. High-resolution negative-ion mass spectral data pointed to a molecular formula of C29H40N2O10S; the presence of two nitrogens in the molecule indicates that this is a sulfated aminosterol-like compound. However, further structural studies (e.g., nuclear magnetic resonance spectroscopy) are required for complete identification and structure assignment. Interestingly, novel sulfated aminosterols have been identified as important constituents of the migratory pheromone of the sea lamprey Petromyzon marinus (Sorensen et al. 2005; Hoye et al. 2007). If further studies show that the putative urinary pheromone of male tilapia is an aminosterol, it would be the second instance that such a class of compound has been shown to act as an odorant and, possibly, as pheromones in vertebrates.
The finding of a single odorant in the urine extract, the chemical identity of which does not resemble any known reproductive steroids, is intriguing. In male tilapia, the concentration of urinary steroids is modulated by social context; testosterone (T), 11-ketotestosterone (11KT), 17,20β-dihydroxy-4-pregnen-3-one (17,20β-P), and 17,20α-dihydroxy-4-pregnen-3-one (17,20α-P) are positively correlated to dominance (Oliveira et al. 1996). However, none of these steroids evokes significant EOG responses even at concentrations as high as 10−6 M (Frade et al. 2002), and the current study confirmed that the female olfactory system is insensitive to male urinary sex-steroids. This contrasts with the idea that pheromones are generally mixtures of compounds (Wyatt 2003) and that, in teleosts, reproductive pheromones are mixtures of sex hormones (and their metabolites) in which the ratio of components varies between species and gender and, within a sex, varies with reproductive status (Stacey and Sorensen 2006). However, we have not investigated thoroughly all possible urinary odorants. The methanol eluent from our C18 purification procedure should contain relatively low polarity compounds; more polar compounds should have passed through the column with the water of the urine. Therefore, it is possible that we missed a fraction of urinary odorants for females because we did not test the olfactory potency of the aqueous eluent.
Tilapia females have high olfactory sensitivity to male-conditioned water and to bile and intestinal fluids of males (Frade et al. 2002), suggesting that the urinary odorant found in this study may be detected as part of a mixture of odorants and that females may use the ratio, rather than the absolute amount, to discriminate between dominant and subordinate males. Possibly, dominant males but not subordinates change this ratio by increasing their urination rate in the presence of a female ready to spawn; subordinate males not only produce less of the urinary odorant but are apparently unable to store as much urine in the bladder. Subordinate males would thus be less capable of changing their profile of odorants when in the presence of a female ready to spawn. Although longer urine retention by dominant males would, at least partly, explain the higher concentration of the aminosterol odorant in their urine than in that of subordinate males, our results suggest that production of urinary odorants is higher in dominant than in subordinate males. Further research is required to determine the site of production of urinary odorants, especially the aminosterol-like compound.
Female reproductive condition affects the urination frequency of territorial males; males showed the same intensity of courtship behaviors with pre- or post-ovulatory females but urinated much more in the presence of pre-ovulatory females. The behavior of the two groups of females was not different (perhaps due to stress because of handling before their introduction in the male’s tank), suggesting that the increase in urine release by males was triggered by odors from pre-ovulatory females as previously suggested (Miranda et al. 2005). In contrast to males, females release urine in shorter pulses and at higher frequency, and this is, apparently, unaffected by the presence of a territorial male (Almeida et al. 2005). Our study appears to be the first to link the release of a urinary aminosterol-like odorant directly with both the social status of a male fish and its reproductive opportunity, which may be perceived through female odors (including urinary odorants).
Chemical signaling through urinary pheromones has been previously shown in female goldfish, which increase their release of urine containing the post-ovulatory hormonal pheromone, prostaglandin \({\text{F}}_{2\alpha } \) and, thereby, mark spawning sites and attract males (Appelt and Sorensen 2007). The goldfish scramble-competition mating system, floating spawning substrate, and prostaglandin pheromones in female urine are representative of many species of cyprinids (Stacey and Sorensen 2006). In other fish groups, different suites of hormonal products in the urine are used as pheromones (Colombo et al. 1980; Liley 1982; Katsel et al. 1992; Vermeirssen and Scott 2001; Stacey and Sorensen 2006) in what appears to be insipient specialization for chemical communication between the sexes. This varies taxonomically but may also be related to different life histories or reproductive strategies. In contrast, in tilapia, chemical signaling seems to be more complex occurring both between males (Barata et al. 2007) and reciprocally between males and females.
In summary, dominant/territorial tilapia males release urine in pulses of short duration, the frequency of which increases dramatically in the presence of females ready to spawn, but which does not increase in the presence of post-spawn females. Male urine is a vehicle of a putative sulfated aminosterol that is an important urinary odorant detected by the female’s olfactory system. Both the concentration of this odorant and the urine volume in the bladder are higher in dominant than in subordinate males, implying that males of low social rank are less capable of stimulating females ready to spawn. We suggest that the putative aminosterol in male urine may act as a pheromonal signal of dominance and influence female spawning.
References
Almeida, O. G., Miranda, A., Hubbard, P. C., Frade, P., Barata, E. N., and Canário, A. V. M. 2005. Urine as a social signal in the Mozambique tilapia (Oreochromis mossambicus). Chem. Senses 30:i309–i310.
Appelt, C. W., and Sorensen, P. W. 1999. Freshwater fish release urinary pheromones in a pulsatile manner, pp. 247–256, in R. E. Johnston, D. Müller-Schwarze, and P. W. Sorensen (eds.). Advances in Chemical Signals in Vertebrates. Kluwer, New York.
Appelt, C. W., and Sorensen, P. W. 2007. Female goldfish signal spawning readiness by altering when and where they release a urinary pheromone. Anim. Behav. 74:1329–1338.
Baerends, G. P., and Baerends van roon, J. M. 1950. An introduction to the study of the ethology of cichlid fishes. Behaviour 1(Suppl.):1–243.
Barata, E. N., Hubbard, P. C., Almeida, O. G., Miranda, A., and Canário, A. V. M. 2007. Male urine signals social rank in the Mozambique tilapia (Oreochromis mossambicus, Peters 1852). BMC Biology 5:54 DOI 10.1186/1741–7007–5–54.
Barlow, G. W. 1991. Mating systems among cichlid fishes, pp. 173–190, in M. H. A. Keenleyside (ed.). Cichlid Fishes: Behaviour, Ecology and EvolutionChapman & Hall, London.
Bruton, M. N., and Boltt, R. E. 1975. Aspects of the biology of Tilapia mossambica Peters (Pisces: Cichlidae) in a natural freshwater lake (Lake Sibaya, South Africa). J. Fish Biol. 7:423–445.
Colombo, L., Marconato, A., Belvedere, P. C., and Frisco, C. 1980. Endocrinology of teleost reproduction. A testicular steroid pheromone in the black goby, Gobius jozo L. Boll. Zool. 47:355–364.
Coward, K., and Bromage, N. R. 2000. Reproductive physiology of female tilapia broodstock. Rev. Fish Biol. Fish. 10:1–25.
Fine, J. M. 2006. Isolation, identification, and biological characterization of the Sea Lamprey (Petromyzon marinus) migratory pheromone. PhD dissertation. University of Minnesota, Twin Cities.
Frade, P., Hubbard, P. C., Barata, E. N., and Canário, A. V. M. 2002. Olfactory sensitivity of the Mozambique tilapia to conspecific odours. J. Fish Biol. 61:1239–1254.
Fryer, G., and Iles, T. D. 1972. The Cichlid Fishes of the Great Lakes of Africa: Their Biology and Evolution. Oliver & Boyd, Edinburgh.
Hoye, T. E., Dvornikovs, V., Fine, J. M., Anderson, K. R., Jeffrey, C. S., Muddiman, D. C., Shao, F., Sorensen, P. W., and Wang, J. 2007. Details of the structure determination of the sulfated steroids PSDS and PADS—new components of the sea lamprey (Petromyzon marinus) migratory pheromone. J. Org. Chem. 72:7544–7550.
Katsel, P. L., Dmitrieva, T. M., Valeyev, R. B., and Kozlov, Y. P. 1992. Sex pheromones of male yellowfin baikal sculpin (Cottocomphorus grewingki): isolation and chemical studies. J. Chem. Ecol. 18:2003–2010.
Liley, N. R. 1982. Chemical communication in fish. Can. J. Fish. Aquat. Sci. 39:22–35.
Miranda, A., Almeida, O. G., Hubbard, P. C., Barata, E. N., and Canário, A. V. M. 2005. Olfactory discrimination of female reproductive status by male tilapia (Oreochromis mossambicus). J. Exp. Biol. 208:2037–2043.
Nelissen, M. H. J. 1991. Communication, pp. 225–240, in M. H. A. Keenleyside (ed.). Cichlid Fishes. Behaviour, Ecology and EvolutionChapman & Hall, London.
Oliveira, R. F. 1995. Etologia social e endocrinologia comportamental da tilápia Oreochromis mossambicus (Teleostei, Cichlidae). PhD dissertation, Universidade de Lisboa.
Oliveira, R. F., and Almada, V. C. 1996. Dominance hierarchies and social structure in captive groups of the Mozambique tilapia Oreochromis mossambicus (Teleostei, Cichlidae). Ethol., Ecol. Evol. 8:39–55.
Oliveira, R. F., and Almada, V. C. 1998a. Mating tactics and male-male courtship in the lek-breeding cichlid Oreochromis mossambicus. J. Fish Biol. 52:1115–1129.
Oliveira, R. F., and Almada, V. C. 1998b. Mating tactics and male-male courtship in the lek-breeding cichlid Oreochromis mossambicus. J. Fish Biol. 52:1115–1129.
Oliveira, R. F., Almada, V. C., and Canario, A. V. M. 1996. Social modulation of sex steroid concentrations in the urine of male cichlid fish Oreochromis mossambicus. Horm. Behav. 30:2–12.
Sorensen, P. W., Fine, J. M., Dvornikovs, V., Jeffrey, C. S., Shao, F., Wang, J., Vrieze, L. A., Anderson, K., and Hoye, T. R. 2005. Mixture of new sulphated steroids functions as a migratory pheromone in the sea lamprey. Nature Chem. Biol. 1:324–328.
Stacey, N., and Sorensen, P. 2006. Reproductive pheromones, pp. 359–412, in K. A. Sloman, R. W. Wilson, and S. Balshine (eds.). Behaviour and Physiology of Fish. Academic, London.
Vermeirssen, E. L. M., and Scott, A. P. 2001. Male priming pheromone is present in bile, as well as in urine, of female rainbow trout. J. Fish Biol. 58:1039–1045.
Wyatt, T. D. 2003. Pheromones and Animal Behaviour. Communication by Smell and Taste. Cambridge University Press, Cambridge.
Acknowledgment
This work was funded by the Ministry of Science and Higher Education and European Social Funds through the Portuguese National Science Foundation (FCT), project POCTI/BSE/38815/2001, and Ph.D. fellowship SFRH/BD/6750/2001 to P. Frade. We thank João Reis (Universidade do Algarve, Faro, Portugal) for technical support and Prof. Luís Dias (Universidade de Évora) for useful discussion on the statistical methods. We also thank the reviewers for their comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barata, E.N., Fine, J.M., Hubbard, P.C. et al. A Sterol-Like Odorant in the Urine of Mozambique Tilapia Males Likely Signals Social Dominance to Females. J Chem Ecol 34, 438–449 (2008). https://doi.org/10.1007/s10886-008-9458-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-008-9458-7