Abstract
The effect of pulegone chiral center configuration on its antifeedant activity to Myzus persicae was examined. Biological consequences of structural modifications of (R)-(+)- and (S)-(−)-pulegone, the lactonization, iodolactonization, and incorporation of hydroxyl and carbonyl groups were studied, as well. The most active compounds were (R)-(+)-pulegone (1a) and δ-hydroxy-γ-spirolactones (5S,6R,8S)-(−)-6-hydroxy-4,4,8-trimethyl-1-oxaspiro[4.5]decan-2-one (5b) and (5R,6S,8S)-6-hydroxy-4,4,8-trimethyl-1-oxaspiro[4.5]decan-2-one (6b) derived from (S)-(−)-pulegone (1b). The compounds deterred aphid probing and feeding at preingestional, ingestional, and postingestional phases of feeding. The preingestional effect of (R)-(+)-pulegone (1a) was manifested as difficulty in finding and reaching the phloem (i.e., prolonged time preceding the first contact with phloem vessels), a high proportion of probes not reaching beyond the mesophyll layer before first phloem phase, and/or failure to find sieve elements by 20% of aphids during the 8-hr experiment. The ingestional activity of (R)-(+)-pulegone (1a) and hydroxylactones 5b and 6b resulted in a decrease in duration of phloem sap ingestion, a decrease in the proportion of aphids with sustained sap ingestion, and an increase in the proportion of aphid salivation in phloem. δ-Keto-γ-spirolactone (5R,8S)-(−)-4,4,8-trimethyl-1-oxaspiro[4.5]decan-2,6-dione (8b) produced a weak ingestional effect (shortened phloem phase). The postingestional deterrence of (R)-(+)-pulegone (1a) and δ-hydroxy-γ-spirolactones (5R,6S,8R)-(+)-6-hydroxy-4,4,8-trimethyl-1-oxaspiro[4.5]-decan-2-one (5a), 5b, (5S,6R,8R)-6-hydroxy-4,4,8-trimethyl-1-oxaspiro[4.5]decan-2-one (6a), 6b, and δ-keto-γ-spirolactone 8b prevented aphids from settling on treated leaves. The trans position of methyl group CH3–8 and the bond C5–O1 in lactone 6b appeared to weaken the deterrent activity in relation to the cis diastereoisomer (5b).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Substances that alter insect behavior have attracted much attention as potential crop protection compounds that, at least in part, might replace traditional pesticides (Unelius et al. 2006); they comprise repellents, oviposition inhibitors, antifeedants, etc. The most widely known antifeedants belong to different chemical groups and come from natural sources (Ley and Toogood 1990; Wawrzyniak 1996; Simmonds 1998). Plant terpenoids are one of the major classes of secondary metabolites synthesized by plants that are toxic, unpalatable, or at least repellent to herbivores. As such, they function as defensive agents (Pickett 1991; Harrewijn et al. 2001; Wittstock and Gershenzon 2002). Discovery of insect control substances include, among others, ajugarin, azadirachtin, and polygodial. The sesquiterpenoid polygodial was successfully applied in the field against bird cherry-oat aphid Rhopalosiphum padi. It gave results similar to those obtained with the broad-spectrum pyrethroid cypermethrin (Pickett et al. 1994). Monoterpenoids are components of many plant essential oils and often show insecticidal, feeding deterrent, and repellent activities (Simmonds 1998). Citral appeared toxic to Mediterranean fruit fly Ceratitis capitata, American cockroach Periplaneta americana, and housefly Musca domestica (Salvatore et al. 2004; Choi et al. 2006; Price and Berry 2006) and repellent to termites Coptotermes formosanus (Zhu et al. 2001) and mosquitoes Aedes aegypti (Oyedele et al. 2002).
Annual world crop loss due to aphids is estimated at 2% of all losses attributed to insect feeding (Wellings et al. 1989). Apart from removing vital fluids from plant sieve elements, aphids are effective vectors of virus diseases: approximately 60% of all plant viruses are spread by aphids. According to Blackman and Eastop (1985), the peach aphid Myzus persicae (Sulz.) can infest plants belonging to over 40 different families that include many economically important ones, and it has the ability to transmit more than 100 plant viruses. Elimination or at least reduction of penetration of plant tissues by aphids may save plants from infection by pathogenic agents (Martin et al. 1997).
Aphids respond to many monoterpenoids. The repellent properties of linalool and α-terpineol to Myzus persicae were reported by Hori (1998, 1999). Gutierrez et al. (1997) found that geraniol inhibited Myzus persicae settling on host plants. (S)-Limonene restrained phloem sap ingestion and had other negative effects on the behavior of the peach potato aphid (Halarewicz-Pacan et al. 2003). Citral and linalool had repellent effects that were manifested in a significant decrease in time spent on leaves, decreased total and mean time of penetration, and lower numbers of probes compared to controls. Citral, linalool, (S)-limonene, α-ionone, and camphene reduced the total and mean probing time of aphids and settling on the leaves (Gabrys et al. 2005).
Our interest in pulegone and its likely antifeedant effect on Myzus persicae was inspired by reports on the compound’s broad spectrum biological activity. Pugelone is repellent to birds, including the common starling Sturnus vulgaris, red-winged blackbird Agelaius phoeniceus, and Northern bobwhite Colinus virginianus (Avery et al. 1996; Mason and Epple 1998). It is also toxic to the German cockroach Blatella germanica, Musca domestica, and storage pests [rice weevil Sitophilus oryzae, red flour beetle Tribolium castaneum, and the sawtoothed grain beetle Oryzaephilus surinamensis (Franzios et al. 1997; Lee et al. 2003)]. Pulegone downgrades reproduction and development of the pea aphid Acyrthosiphon pisum, probably by killing aphid symbionts Buchnera sp., which is attributed to its bactericidal activity (Harrewijn et al. 2001). The fungicidal effect of pulegone has also been reported (Gata-Gonçalves et al. 2003).
Pulegone is the major component of essential oils of several species of Mentha (e.g., M. pulegium, M. arvensis, M. longifolia, and M. spicata) and occurs in two isomeric forms: (R)- and (S)-pulegone (Phatak and Heble 2002; Vetere et al. 2002; Conover and Lyons 2005). The enantiomers of a chiral compound may differ significantly in respect to their interaction with biological receptors, thus resulting in a different biological activity (Juza et al. 2000). The biological activity depends not only on the stereochemistry but is related also to the presence of various functional groups. Often, natural antifeedants are lactones with one or more additional functional groups (Ley and Toogod 1990; Wawrzeńczyk et al. 1997). From the practical point of view, the use of plant-derived antifeedants on a large scale is not justified economically. Synthetic analogs of natural compounds are more accessible for application.
The aim of this work was to assess the effect of pulegone chiral center configuration on its antifeedant activity to Myzus persicae and to study the biological consequences of structural modifications of (R)-(+)- and (S)-(−)-pulegone: the lactonization, iodolactonization, and incorporation of hydroxy and carbonyl groups. The biological study included various aspects of aphid host plant selection and acceptance processes. The behavioral responses of Myzus persicae to pure and structurally modified pulegone enantiomers were investigated to reveal the biological basis of their deterrent activity.
Methods and Materials
Chemicals
(R)-(+)-Pulegone (>98%), (S)-(−)-pulegone (>99%), (1S,2R,5S)-(+)-isopulegol (>99%), and (1R,2S,5R)-(−)-isopulegol (>99%) were purchased from Aldrich and Fluka. Test lactones 3–8 (Fig. 1) were synthesized from optically pure isomers of pulegone and isopulegol. The synthetic methodology for compounds 3–6, based on the orthoacetate modification of the Claisen rearrangement and iodolactonization of γ,δ-unsaturated acids or acidic lactonization of epoxy esters, was described earlier (Dams et al. 2004a,b). δ-Hydroxy-γ-spirolactones 5–6 were oxidized with pyridinium dichromate to the corresponding δ-keto-γ-spirolactones 7–8 (Dams et al. 2004b). The structures of synthesized lactones were determined on the basis of spectroscopic and crystallographic methods. The enantiomeric purity of the final products was higher than 97% as determined by gas chromatography by using chiral columns: CP-Cyclodextrin-β-2,3,6-m-19, 25 m × 0.25 mm × 0.25 μm and Chirasil-Val-L, 25 m × 0.25 mm × 25 μm. It was not possible to obtain pure enantiomers of 6a and 6b. To evaluate their activity, it was necessary to use 6a/6b mixtures. The activity of 6a was studied as a mixture 5a/6a = 28%:72% and 6b–5b/6b = 28%:72%.
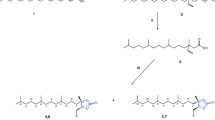
Structures of pulegone and pulegone-derived lactones. 1a (R)-(+)-pulegone, 1b (S)-(−)-pulegone; 2a (5R,6S,8R)-(+)-6-iodo-4,4,8-trimethyl-1-oxaspiro[4.5]decan-2-one, 2b (5S,6R,8S)-(−)-6-iodo-4,4,8-trimethyl-1-oxaspiro[4.5]decan-2-one; 3a (5R,8R)-(−)-4,4,8-trimethyl-1-oxaspiro[4.5]dec-6-en-2-one, 3b (5S,8S)-(+)-4,4,8-trimethyl-1-oxaspiro[4.5]dec-6-en-2-one; 4 4,4,8-trimethyl-1-oxaspiro[4.5]decan-2-one; 5a (5R,6S,8R)-(+)-6-hydroxy-4,4,8-trimethyl-1-oxaspiro[4.5]-decan-2-one, 5b – (5S,6R,8S)-(–)-6-hydroxy-4,4,8-trimethyl-1-oxaspiro-[4.5]decan-2-one; 6a (5S,6R,8R)-6-hydroxy-4,4,8-trimethyl-1-oxaspiro[4.5]decan-2-one, 6b (5R,6S,8S)-6-hydroxy-4,4,8-trimethyl-1-oxaspiro[4.5]decan-2-one; 7a (5R,8R)-(−)-4,4,8-trimethyl-1-oxaspiro[4.5]decane-2,6-dione, 7b (5S,8S)-(+)-4,4,8-trimethyl-1-oxaspiro[4.5]decane-2,6-dione; 8a (5S,8R)-(+)-4,4,8-trimethyl-1-oxaspiro[4.5]decane-2,6-dione, 8b (5R,8S)-(−)-4,4,8-trimethyl-1-oxaspiro[4.5]decane-2,6-dione
Aphids, Plants, and Compound Application
Aphids (Myzus persicae) and plants (Chinese cabbage Brassica pekinensis) were reared in laboratory at 20°C, 65% RH, and 16:8 L/D photoperiod. Compounds were applied to the adaxial surface of leaves as 0.1% ethanolic solutions, 0.01 ml/cm2 of the leaf according to a method described by Polonsky et al. (1989). All biological assays were performed 1 hr after compound application to allow evaporation of the carrier solvent. Iodolactones were not studied in electronic penetration graph (EPG) experiments due to their instability.
Behavioral Responses of Aphids
The antifeedant effect of pulegone and its structural analogs was assessed by watching the settling behavior of freely moving aphids and by electronically monitoring aphid stylet activities in plant tissues (probing behavior).
Settling of Myzus persicae was studied by using the “half-leaf test” (Polonsky et al. 1989). Compounds were applied to one half of a leaf; the other side of the midrib was coated with 0.1% ethanol and considered the “control.” Aphids were offered a choice between equal areas of treated and control surfaces. Aphids that settled on each side of the midrib (i.e., aphids that did not move and had their antennae directed backwards, indicating probing) were counted at 15-, 30-min, 1-, 2-, and 24-hr intervals after access to the leaf (8 replicates, 20 viviparous apterous females/replicate). The relative index of deterrence was calculated after Powell et al. (1997): R = (C − T)/(C + T). C represents the number of aphids settled on the control half of the leaf and T = the number of aphids settled on half of the leaf coated with the tested compound. R values may range from “−1” (indicating a good attractant) to “1” (indicating a good deterrent). Statistical differences in settling/no settling between treatment and control were tested for each substance by Student’s t-test.
Myzus persicae probing behavior was monitored by using electronic registration of aphid stylet penetration in plant tissues referred to as EPG. This technique is commonly used in insect–plant studies. Aphids and plants become incorporated into an electric circuit; the circuit is closed when aphids insert their stylets into plant tissues. A weak voltage is applied through the circuit, and all changes in electrical properties are recorded as EPG waveforms that can be correlated with aphid activities and stylet position in plant tissues (Tjallingii 1994). The values of parameters derived from EPG recordings, e.g., the duration of probing, duration of phloem sap ingestion, number of probes, etc., reflect the suitability of a food source to the aphids (Mayoral et al. 1996). After attachment of the wire electrode, aphids were starved for 1 hr before the start of an experiment. The probing behavior of 16 apterous females was tested per substance and monitored continuously for 8 hr with a four-channel DC EPG recording device. Signals were analyzed with Probe 2.1 software provided by W.F. Tjallingii. The following EPG patterns were distinguished: np (non penetration—aphid stylets remained outside of the plant), A, B, C, F (pathway phase—penetration of non-phloem tissues), E1 (salivation into sieve elements), and E2 (ingestion of phloem sap). The E1/E2 transition patterns were included in E1. A number of sequential (i.e., describing the sequence of events during the recording) and non-sequential (i.e., referring to frequency, total, and average duration of patterns) parameters were calculated (Van Helden and Tjallingii 1993). Results were analyzed statistically with analysis of variance (Kruskal–Wallis test) by using Statistica 6.1 (StatSoft axxp505b946905ar28).
Results
(R)-(+)-Pulegone and its Analogs
Significantly fewer aphids settled on the leaf halves coated with (R)-(+)-pulegone (1a), (+)-δ-iodo-γ-spirolactone (2a), δ-hydroxy-γ-spirolactones cis(5R,6S,8R)-(+)-5a and trans(5S,6R,8R)-6a than on control leaf halves. The relative indices of deterrence after 24-hr observation were 0.43, 0.72, 0.25, and 0.31, respectively. The unsaturated γ-spirolactone (5S,8R)-(−)-γ-spirolactone (3a) showed strong deterrent properties during the first 2 hr of an experiment (R = 0.82). However, this activity had ceased by 24 hr (Fig. 2).
The most distinctive modification of aphid behavior during probing (EPG experiments) was observed on plants treated with (R)-(+)-pulegone (1a) (Table 1, Fig. 2). Total time of probing was significantly shorter on treated leaf halves—72% of 8-hr experimental time—compared to 90% for control plants. Aphids penetrated mainly peripheral tissues (78% of penetration time compared to 47% on control tissue). The time before reaching phloem vessels was nearly twice as long with non-penetration accounting for one third of that time. On (R)-(+)-pulegone-treated plants, aphid probes that preceded the first phloem phase were numerous (2.6 times as many as on the control half of leaves), but the probing was short, usually not longer than 2–10 min (83% of all probes). Moreover, some aphids failed to reach phloem vessels. Among those that got through to the phloem, most did not ingest sap for longer than 10 min (i.e., there was no sustained sap ingestion in more than 30% of aphids). The high proportion of salivation during penetration of phloem vessels (18% of all activities in sieve elements) was noteworthy. In 44% of aphids, recurrent alterations between E1 and E2 during every period of phloem phase were observed (the E1/E2 transition patterns were included in E1; data not shown). In general, aphid behavior generally did not change on leaf halves treated with δ-hydroxy-γ-spirolactones 5a and 6a compared to control leaf halves. However, on these plants, as on plants treated with (R)-(+)-pulegone (1a), aphids usually did not show sustained sap ingestion during the first contact with phloem vessels (Table 1).
(S)-(−)-Pulegone and its Analogs
Significantly fewer aphids settled on leaf halves treated with (5S,6R,8S)-(−)-δ-iodo-γ-spirolactone (2b), δ-hydroxy-γ-spirolactones: cis (5S,6R,8S)-(−)-5b, trans (5R,6S,8S)-6b, and trans (5R,8S)-(−)-δ-keto-γ-spirolactone (8b) than on control leaf halves. The relative indices of deterrence after 24-hr were 0.34, 0.49, 0.48, and 0.67, respectively (Fig. 3).
Aphid probing on plants treated with (S)-(−)-pulegone (1b) was similar to that on control plants. The most noticeable changes occurred after application of hydroxylactone 5b (Table 2). Although aphid stylet penetration was not inhibited on 5b-treated leaf halves, the total duration of phloem activities was reduced by a half, and fewer aphids showed sustained sap ingestion compared to controls [62 vs. 100% on control leaf halves or 94% on (S)-(−)-pulegone-treated plants]. The time to reach phloem elements was doubled, the duration of first phloem sap ingestion period was half, and the proportion of salivation in all activities in sieve elements was five times greater than on the control half of the leaves. On 8b-treated plants, 81% of aphids reached phloem vessels, but the duration of first phloem phase was half as long as on control plants. On 6b-treated plants, the first phloem phase was delayed by more than 2 hr on average, 32% fewer aphids reached sieve elements, and the proportion of salivation in the phloem phase was six times greater compared to controls. None or few aphids showed sustained sap ingestion during first phloem phase on plants treated with (S)-(−)-pulegone and lactones 5b, 8b, and 6b. Thirty-two percent (6b) and 38% (5b) of aphids failed to ingest phloem sap for longer than 10 min at a time (Table 2).
Discussion
Behavioral Aspects of Feeding Deterrent Activity of Pulegone and its Analogs
Frazier and Chyb (1995) suggested that insect feeding can be inhibited at three levels: preingestional, immediate effect associated with host finding and host selection processes involving gustatory receptors; ingestional, related to food transport and production, release, and digestion by salivary enzymes; and postingestional, long-term effects involving various aspects of digestion and absorption of food. Aphids differ from chewing herbivores with respect to the location of their main gustatory receptors. Mouthparts lack external chemoreceptors, and the taste organ is located in the hypopharynx, i.e., the hypopharyngeal gustatory organ; hence the ingestion of sap is crucial for recognition and acceptance of a host plant (Wensler and Filshie 1969; Ponsen 1987; Harrewijn 1990). Before reaching the phloem vessels of host food plants, aphids ingest small samples of parenchyma cell contents for gustatory purposes (Martin et al. 1997). Gabrys and Tjallingii (2002) showed that aphids can distinguish a host from a non-host plant before reaching sieve elements. Because aphid-probing behavior cannot be observed directly, the parameters derived from EPG recordings are used as reliable indicators of preingestional and ingestional factors that affect feeding. Long penetration times of non-phloem tissues compared to total penetration time, a large number of short vs. long probes before the first phloem phase, a relatively long time to first phloem phase within a probe, and a failure to find sieve elements may be interpreted as preingestional effects of antifeedants on aphids that restrain probing at the level of non-phloem tissues. Short (<10 min) probes are limited either to the epidermis (<2 min; Van Hoof 1958) or do not reach beyond the mesophyll layer (2−10 min; Gabrys et al. 1997). Similarly, the short total and mean duration of phloem sap ingestion and the high proportion of salivation during penetration of phloem vessels may point to feeding deterrence at the ingestional level (Mayoral et al. 1996; Gabrys and Pawluk 1999). Particularly, prolonged salivation is characteristic of aphid behavior on resistant plant cultivars or non-host plants (Van Helden and Tjallingii 1993; Wilkinson and Douglas 1998; Gabrys and Pawluk 1999).
In this study, pulegone and some of its structural analogs were found to deter aphid probing and feeding at the preingestional [(R)-(+)-pulegone], ingestional [(R)-(+)-pulegone, δ-hydroxy-γ-spirolactones 5b and 6b, δ-keto-γ-spirolactone 8b], and postingestional [(R)-(+)-pulegone (1a), δ-hydroxy-γ-spirolactones 5a, 5b, 6a, 6b, and δ-keto-γ-spirolactone 8b] levels.
The preingestional effect of (R)-(+)-pulegone (1a) was characterized by aphid difficulties in finding and reaching phloem tissues. These difficulties were manifested in: prolonged time preceding the first contact with phloem vessels (2.5 times as long as on controls), greater number of probes not reaching beyond mesophyll layers before first phloem phase (3.6 times as many as on controls), and a failure to find sieve elements by 20% of aphids during 8-hr experiments. Azadirachtin- and S-limonene-derived lactone have similar effects on Myzus persicae during the pre-phloem phase (Nisbet et al. 1993; Halarewicz-Pacan et al. 2003). On non-host plants or resistant varieties of crop plants, aphid probing may also be impeded at the level of peripheral tissues. Caillaud (1999) showed that Acyrthosiphon pisum did not reach or ingest phloem sap from non-host plants. There were only short probes to the mesophyll. Likewise, Aphis gosypii on resistant lines of Cucumis melo showed a high number of short probes that never reached phloem vessels (Garzo et al. 2004). Pathway activities of cabbage aphid Brevicoryne brassicae were suppressed on the non-host plant Vicia faba and accidental host plants Thlaspi arvense and Lunaria annua (Gabrys and Pawluk 1999).
The ingestional effect of (R)-(+)-pulegone (1a) was characterized by a decreased duration of phloem sap ingestion (by as much as 50% compared to controls), decreased proportion of aphids with sustained sap ingestion (by 30%), and increased proportion of salivation in aphid activities in phloem (about 12 times). Furthermore, the ingestional effect of feeding deterrence was observed after application of δ-hydroxy-γ-spirolactones 5b and 6b. There was a decrease in the total duration of phloem phase, the duration of first phloem phase, and the proportion of phloem phase in activities related to stylet penetration (5b). Between 30–40% of aphids failed to ingest sap for longer than 10 min on 6b- and 5b-coated leaves, respectively, and the duration of salivation into sieve elements was five times longer than on control leaves. Weak ingestional effects occurred with δ-keto-γ-spirolactone 8b-treated leaves and were manifested in shortened phloem phase. Ingestion of the phloem sap for longer than 10 min reflects the acceptance of a plant by an aphid (Tjallingii and Mayoral 1992). The mechanism of resistance of plant varieties or cultivars to aphids is manifested in the reduction of time in sap ingestion (Cole et al. 1993; Sauge et al. 1998; Powell 2004). The presence of azadirachtin in the phloem sap results in the termination of feeding (Nisbet et al. 1993). A decrease in the duration of sap ingestion is a good indicator of the deterrent properties of specific chemical compounds—shown via the use of artificial diets by Givovich and Niemeyer (1995). Frequent interruptions of E2 in phloem phases and the relatively high proportion of salivation in aphid activities in phloem are possible responses of aphids to resistance mechanism in plants (Tjallingii 2001). Increased salivation duration is often reported in aphids that feed on resistant varieties of crop plants (Van Helden and Tjallingii 1993; Mayoral et al. 1996; Wilkinson and Douglas 1998). Sauge et al. (1998) found frequent alterations between E1 and E2 patterns during the phloem phase of Myzus persicae on resistant varieties of peach, and Van Helden and Tjallingii (1993) in Nasonovia ribis-nigri on lettuce. In the present study, such interesting phenomenon occurred after the application of (R)-(+)-pulegone (1a). Miles (1990) and Miles and Oertli (1993) suggest that aphid saliva may neutralize plant resistance factors in the phloem. Tjallingii and Cherqui (1999) showed that the watery saliva secreted into phloem elements contained various proteins, including detoxification enzymes. Leszczynski (2001) is of the opinion that the enzymes present in the saliva may help to metabolize toxic allelochemicals encountered during sap ingestion.
The postingestional deterrence by pulegone and some of its analogs may be assumed because of their influence on aphid settling. The effect of (R)-(+)-pulegone (1a) was long-term and discouraged aphids from settling on plants 24 hr after application, suggesting a role in postingestional activity. The δ-hydroxy-γ-spirolactones 5a and 6a derived from (R)-(+)-pulegone (1a) were probably also postingestional antifeedants. Application of these compounds did not affect aphid behavior during the pre-pholem or phloem phase of stylet penetration; however, aphids refused to settle on leaf halves treated with these compounds or with 5b, 6b, or 8b within 24 hr after treatment. The postingestional effect of these terpenoids will require a separate study into their influence on digestive physiology and/or metabolism in aphids.
Structure–Activity Relationships
The starting point for our experiments were two optical isomers of natural monoterpenoid pulegone: (R)-(+)- and (S)-(−)-pulegone. We found that only (R)-(+)-pulegone (1a) was deterrent to Myzus persicae; (S)-(−)-pulegone (1b) had no effect on aphid probing or feeding behavior. (R)-(+)-pulegone (1a) appears to be a feeding deterrent with the broadest spectrum of activity of the compounds studied. It affected aphid behavior during the initial, intermediate, and final (sap ingestion) phases of probing. (R)-(+)- and (S)-(−)-pulegone were the initial substrates for the synthesis of the bicyclic lactones with a p-mentane system, various functional groups, and different configuration of chiral centers. The incorporation of iodine into the bicyclic spirolactones (i.e., 2a and 2b) had the most dramatic impact on activity of all of the structural modifications. The deterrence indices of 2a and 2b were twice and four times as high as those of (R)-(+)-pulegone (1a) and (S)-(−)-pulegone (1b), respectively. Lactones with hydroxy and carbonyl groups were also active (5a, 5b, 6a, 6b, and 8b); however, there were differences in the activity levels among the enantiomers. Generally, lactones synthesized from (S)-(−)-pulegone (1b) were more active (e.g., hydroxylactones 5b and 6b) and showed broad spectrum deterrence while 5a and 6a deterred aphid settling but had no effect during the preingestive and ingestive stages of aphid probing. There was also a difference in activity between two diastereoisomeric hydroxylactones 5b and 6b, with the 5b having broader spectrum of activity. The trans position of methyl group CH3–8 and the bond C5–O1 in the lactone ring of 6b appeared to weaken the deterrent activity in relation to diastereoisomer 5b with cis configuration.
Our comparative study of the biological activity of enantiomeric pairs of pulegone and pulegone-derived lactones shows a correlation between chiral center configuration and expression of biological activity. Chirality is a unique character of most known biological processes, and enantiomers of bioactive molecules show different activities toward living organisms (Avalos et al. 2000). These studies confirm other reports that suggest that (R)-(+)-pulegone is a more bioactive compound than the (S)-isomer. For example, the hepatotoxicity and pneumotoxicity of pulegone is attributed to (R)-configuration of pulegone (Chan 2001). Addition of a lactone moiety and a hydroxyl group to (S)-(−)-pulegone introduced antifeedant properties not possessed by the inactive substrate, thus confirming the importance of the lactone moiety to expression of biological activity. Similar effects were obtained in feeding deterrence studies of pulegone and pulegone-derived spirolactones toward the Colorado potato beetle Leptinotarsa dacemlineata Say, lesser mealworm Alphitobius diaperinus Panzer, confused flour beetle Tribolium confusum Duv., grain weevil Sitophilus granarius L., and khapra beetle Trogoderma granarium Ev. (Szczepanik et al. 2005; Wawrzenczyk et al. 2005).
References
Avalos, M., Babiano, R., Cintas, P., Jimenez, J. L., and Palacios, J. C. 2000. From parity to chirality: chemical implications revisited. Tetrahedron: Asymmetry. 11:2845–2874.
Avery, M. L., Decker, D. G., Humphrey, J. S., and Laukert, C. C. 1996. Mint plant derivatives as blackbird feeding deterrents. Crop. Prot. 15:461–464.
Blackman, R. L., and Eastop, V. F. 1985. Aphids on the World’s Crops: An Identification Guide. Wiley, New York.
Caillaud, M. C. 1999. Behavioural correlates of genetic divergence due to host specialization in the pea aphid Acyrthosiphon pisum. Entomol. Exp. Appl. 91:227–232.
Chan, K. K. 2001. Quantitation of monoterpenoid compounds with potential medicinal use in biological fluids. J. Chromatogr. A. 936:47–57.
Choi, W., Park, B., Lee, Y., Jang, D. Y., Yoon, H. Y., and Lee, S. 2006. Fumigant toxicities of essential oils and monoterpenes against Lycoriella mali adults. Crop. Prot. 25:398–401.
Cole, R. A., Rigall, W., and Morgan, A. 1993. Electronically monitored feeding behaviour of the lettuce root aphid (Pemphigis bursarius) on resistant and susceptible lettuce varieties. Entomol. Exp. Appl. 68:179–185.
Conover, M. R., and Lyons, K. S. 2005. Will free-ranging predators stop depredating untreated eggs in pulegone-scented gull nests after exposure to pulegone-injected eggs? Appl. Anim. Behav. Sci. 93:135–145.
Dams, I., Białońska, A., Ciunik, Z., and Wawrzeńczyk, C. 2004a. Lactones.21. Synthesis and odoriferous properties of lactones with the p-menthane system. J. Agric. Food Chem. 52:1630–1634.
Dams, I., Białońska, A., Ciunik, Z., and Wawrzeńczyk, C. 2004b. Lactones. 22. Synthesis of terpenoid lactones with the p-menthane system. Eur. J. Org. Chem. 2004:2662–2668.
Frazier, J. L., and Chyb, S. 1995. Use of feeding inhibitors in insect control, pp. 364–381, in R. F. Chapman, and G. de Boer (eds.). Regulatory Mechanisms in Insect FeedingChapman & Hall, New York.
Franzios, G., Mirotsou, M., Hatziapostolou, E., Kral, J., Scouras, Z. G., and Mavragani-tsipidou, P. 1997. Insecticidal and genotoxic activities of mint essential oils. J. Agric. Food Chem. 45:2690–2694.
Gabrys, B., Dancewicz, K., Halarewicz-pacan, A., and Janusz, E. 2005. Effect of natural monoterpenes on behaviour of the peach potato aphid Myzus persicae (Sulz.). IOBC/WPRS Bull. 28:29–34.
Gabrys, B., and Pawluk, M. 1999. Acceptability of different species of Brassicaceae as hosts for the cabbage aphid. Entomol. Exp. Appl. 91:105–109.
Gabrys, B., and Tjallingii, W. F. 2002. The role of sinigrin in host plant recognition by aphids during initial plant penetration. Entomol. Exp. Appl. 104:89–93.
Gabrys, B., Tjallingii, W. F., and Van beek, T. 1997. Analysis of EPG recorded probing by cabbage aphid on host plant parts with different glucosinolate contents. J. Chem. Ecol. 23:1661–1673.
Garzo, E., Palacios, I., and Fereres, A. 2004. Characterization of melon germplasm resistant to Aphis gossypii Glover, pp. 441–447, in J. J. Simon, C. A. Dedryver, C. Rispe, and M. Hulle (eds.). Aphids in a New MillenniumINRA, Paris.
Gata-Gonçalves, L., Nogueira, J. M. F., Matos, O., and De sousa, R. B. 2003. Photoactive extracts from Thevetia peruviana with antifungal properties against Cladosporium cucumerinum. J. Photochem. Photobiol. B. 70:51–54.
Givovich, A., and Niemeyer, H. 1995. Comparison of the effect of hydroxamic acids from wheat on five species of cereal aphids. Entomol. Exp. Appl. 74:115–119.
Gutierrez, C., Fereres, A., eina, M., Cabrera, R., and Gonzaes-coloma, A. 1997. Behavioral and sublethal effects of structurally related lower terpenes on Myzus persicae. J. Chem. Ecol. 23:1641–1650.
Halarewicz-Pacan, A., Gabryś, B., Dancewicz, K., and Wawrzeńczyk, C. 2003. Enantiospecific effect of limonene and limonene-derived bicyclic lactones on settling and probing behaviour of the peach potato aphid Myzus persicae (Sulz.). J. Plant Prot. Res. 43:133–142.
Harrewijn, P. 1990. Resistance mechanisms of plant genotypes to various aphid species, pp. 117–130, in R. K. Campbell, and R. D. Eikenbary (eds.). Aphid–Plant Genotype InteractionsAmsterdam, Elsevier.
Harrewijn, P., Van oosten, A. M., and Piron, P. G. M. 2001. Natural Terpenoids as Messengers. Kluwer, Dordrecht, The Netherlands.
Hori, M. 1998. Repellency of rosemary oil against Myzus persicae in a laboratory and in a screenhouse. J. Chem. Ecol. 24:1425–1432.
Hori, M. 1999. Antifeeding, settling inhibitory and toxic activities of labiate essential oils against the green peach aphid, Myzus persicae (Sulzer) (Homoptera: Aphididae). Appl. Entomol. Zool. 34:113–118.
Juza, M., Mazzonti, M., and Morbidelli, M. 2000. Simulated moving-bed chromatography and its application to chirotechnology. Trends Biotechnol. 18:108–118.
Lee, S., Peterson, C. J., and Coats, J. R. 2003. Fumigation toxicity of monoterpenoids to several stored product insects. J. Stored. Prod. Res. 39:77–85.
Leszczynski, B. 2001. Rola allelozwiązków w oddziaływaniach owady–rośliny, pp. 61–86, in W. Oleszek, K. Głowniak, and B. Leszczyński (eds.). Biochemiczne oddziaływania środowiskoweAkademia Medyczna, Lublin.
Ley, S. V., and Toogood, P. L. 1990. Insect antifeedants. Chem. Br. 1:31–35.
Martin, B., Collar, J. L., Tjallingii, W. F., and Fereres, A. 1997. Intracellular ingestion and salivation by aphids may cause the acquisition and inoculation of non-persistently transmitted plant viruses. J. Gen. Virol. 78:2701–2705.
Mason, J. R., and Epple, G. 1998. Evaluation of bird repellent additives to a simulated pesticide carrier formation. Crop Prot. 17:657–659.
Mayoral, A. M., Tjallingii, W. F., and Castanera, P. 1996. Probing behaviour of Diuraphis noxia on five cereal species with different hydroxyamic acid levels. Entomol. Exp. Appl. 78:341–348.
Miles, P. W. 1990. Aphid salivary secretions and their involvement in plant toxicoses, pp. 131–147, in R. K. Campbell, and R. D. Eikenbary (eds.). Aphid-Plant Genotype interactionsElsevier Science, Amsterdam.
Miles, P. W., and Oertli, J. J. 1993. The significance of antioxidants in the aphid-plant interaction: the redox hypothesis. Entomol. Exp. Appl. 67:275–283.
Nisbet, A. J., Woodford, J. A. T., and Connolly, J. D. 1993. Systemic antifeedant effects of azadirachtin on the peach-potato aphid Myzus persicae. Entomol. Exp. Appl. 68:87–98.
Oyedele, A. O., Gbolade, A. A., Sosan, M. B., Adewoyin, F. B., Soyelu, O. L., and Orafidiya, O. O. 2002. Formulation of an effective mosquito-repellent topical product from Lemongrass oil. Phytomedicine. 9:259–262.
Phatak, S. V., and Heble, M. R. 2002. Organogenesis and terpenoid synthesis in Mentha arvensis. Fitoterapia. 73:32–39.
Pickett, J. A. 1991. Lower terpenoids as natural insect control agents, pp. 297–313, in J. B. Harborne, and F. A. Tomas-Barberan (eds.). Ecological Chemistry and Biochemistry of Plant TerpenoidsClarendon, Oxford.
Pickett, J. A., Wadhams, L. J., and Woodcock, C. M. 1994. Attempts to control aphid pests by integrated use of semiochemicals, pp 1239−1246. Brighton Crop Protection Conference—Pests and Diseases. British Crop Protection Council, Thornton Heath, UK.
Polonsky, J., Bhatnagar, S. C., Griffiths, D. C., Pickett, J. A., and Woodcock, C. M. 1989. Activity of qassinoids as antifeedants against aphids. J. Chem. Ecol. 15:933–998.
Ponsen, M. B. 1987. Alimentary tract, pp. 79–97, in A. K. Minks, and P. Harrewijn (eds.). Aphids, Their Biology, Natural Enemies and Control. Vol. AElsevier, Amsterdam.
Powell, G. 2004. Sieve element salivation and the transmission to ingestion, pp. 479–483, in J. J. Simon, C. A. Dedryver, C. Rispe, and M. Hulle (eds.). Aphids in a New MillenniumINRA, Paris.
Powell, G., Hardie, J., and Pickett, J. A. 1997. Laboratory evaluation of antifeedant compounds for inhibiting settling by cereal aphids. Entomol. Exp. Appl. 84:189–193.
Price, D. N., and Berry, M. S. 2006. Comparison of effects of octopamine and insecticidal essential oils on activity in the nerve cord, foregut, and dorsal unpaired median neurons of cockroaches. J. Insect Physiol. 52:309–319.
Salvatore, A., Borkosky, S., Willink, E., and Bardon, A. 2004. Toxic effects of lemon peel constituents on Ceratitis capitata. J. Chem. Ecol. 30:323–333.
Sauge, M. H., Kervella, J., and ahbe, Y. 1998. Probing behaviour of the green peach aphid Myzus persicae on resistant Prunus genotypes. Entomol. Exp. Appl. 89:223–232.
Simmonds, M. S. J. 1998. Chemoecology. The legacy left by Tony Swain. Phytochemistry. 49:1183–1190.
Szczepanik, M., Dams, I., and Wawrzenczyk, C. 2005. Feeding deterrent activity of terpenoid lactones with the p-menthane system against the Colorado potato beetle (Coleoptera: Chrysomelidae). Environ. Entomol. 34:1433–1440.
Tjallingii, W. F. 1994. Sieve element acceptance by aphids. Eur. J. Entomol. 91:47–52.
Tjallingii, W. F. 2001. Plant penetration by aphids as revealed by electrical penetration graphs. Aphids and other Homopterous Insects. 8:105–120.
Tjallingii, W. F., and Cherqui, A. 1999. Aphid saliva and aphid-plant interactions. Exp. Appl. Entomol. 10:169–174.
Tjallingii, W. F., and Mayoral, A. M. 1992. Criteria for host plant acceptance by aphids, pp. 280–282, in S. B. J. Menken, J. H. Visser, and P. Harrewijn (eds.). Proc. 8th Int. Symp. Insect-Plant RelationshipsKluwer, Dordrecht.
Unelius, C. R., Nordlander, G., Nordenhem, H., Hellqvist, C., Legrand, S., and Borg-karlson, A-K. 2006. Structure–activity relationships of benzoic acid derivatives as antifeedants for the pine weevil, Hylobius abietis. J. Chem. Ecol. 32:2191–2203.
Van helden, M., and Tjallingii, W. F. 1993. Tissue localisation of lettuce resistance to the aphid Nasonovia ribisnigri using electrical penetration graphs. Entomol. Exp. Appl. 68:269–278.
Van hoof, H. A. 1958. An Investigation of the Biological Transmission of a Non-Persistent Virus, p. 112, in A. Van Putten and B. Oortmeier (eds.). Meded. Inst. Planteziektenkundig. Wageningen Agricultural University, Alkmaar.
Vetere, V., Santori, G. F., Moglioni, A., Moltrasio iglesias, G. Y., Casella, M. L., and Ferretti, O. A. 2002. Hydrogenation of (−)-menthone, (+)-isomenthone, and (+)-pulegone with platinum/tin catalysts. Catal. Letters 84:251–257.
Wawrzenczyk, C., Dams, I., Szumny, A., Szczepanik, M., Nawrot, J., Pradzynska, A., Gabtys, B., Dancewicz, K., Magnucka, E., Gawdzik, B., Obara, R., and Wzorek, A. 2005. Synthesis and evaluation of antifeedant, antifungal and atibacterial activity of isoprenoid lactones. Pol. J. Environ. Stud. 14:Suppl69–84.
Wawrzeńczyk, C., Paruch, E., Olejniczak, T., Saletra, A., Nawrot, J., Prądzyńska, A., Halarewicz-pacan, A., and Gabryś, B. 1997. Lactones 4. The effect of the compound configuration on the feeding deterrent activity of some terpenoid lactones. Proc. 2nd International Conference on Insects: Chemical, Physiological and Environmental Aspects, September 14–19, Lądek Zdrój, Poland: 222–227.
Wawrzyniak, M. 1996. The effect of selected plant extracts on the cabbage butterfly, Pieris brassicae L. (Lepidoptera). Pol. J. Ent. 65:93.
Wellings, P. W., Ward, S. A., Dixon, A. F. G., and abbinge, R. 1989. Crop loss assessment, pp. 49–64, in A. K. Minks, and P. Harrewijn (eds.). Aphids, Their Biology, Natural Enemies and Control. Vol. CElsevier, Amsterdam.
Wensler, R. J. D., and Filshie, B. K. 1969. Gustatory sense organs in the food canal of aphids. J. Morphol. 129:473–492.
Wilkinson, T. L., and Douglas, A. E. 1998. Plant penetration by pea aphids (Acyrthosiphon pisum) of different plant range. Ent. Exp. Appl. 87:43–50.
Wittstock, U., and Gershenzon, J. 2002. Constitutive plant toxins and theirs role in defense against herbivores and pathogens. Curr. Opin. Plant. Biol. 5:1–8.
Zhu, B. C. R., Henderson, G., Chen, F., Fei, H., and Laine, R. A. 2001. Evaluation of vetiver oil and seven insect-active essential oils against the Formosan subterranean termite. J. Chem. Ecol. 27:1617–1625.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dancewicz, K., Gabrys, B., Dams, I. et al. Enantiospecific Effect of Pulegone and Pulegone-Derived Lactones on Myzus persicae (Sulz.) Settling and Feeding. J Chem Ecol 34, 530–538 (2008). https://doi.org/10.1007/s10886-008-9448-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-008-9448-9