Abstract
In phytophagous insects, experience can increase positive responses towards non-host plant extracts or induce oviposition on non-host plants, but the underlying chemical and behavioral mechanisms are poorly understood. By using the diamondback moth, Plutella xylostella, its host plant Chinese cabbage, and a non-host plant Chrysanthemum morifolium, as a model system, we observed the experience-altered olfactory responses of ovipositing females towards volatiles of the non-host plant, volatiles of pure chemicals (p-cymene and α-terpinene) found in the non-host plant, and volatiles of host plants treated with these chemicals. We assessed the experience-altered oviposition preference towards host plants treated with p-cymene. Naive females showed aversion to the odors of the non-host plant, the pure chemicals, and the pure chemical-treated host plants. In contrast, experienced females either became attracted by these non-host odors or were no longer repelled by these odors. Similarly, naive females laid a significantly lower proportion of eggs on pure chemical-treated host plants than on untreated host plants, but experienced females laid a similar or higher proportion of eggs on pure chemical-treated host plants compared to untreated host plants. Chemical analysis indicated that application of the non-host pure chemicals on Chinese cabbage induced emissions of volatiles by this host plant. We conclude that induced preference for previously repellent compounds is a major mechanism that leads to behavioral changes of this moth towards non-host plants or their extracts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Studies of phytophagous insects have demonstrated a range of learned behavior that includes habituation, associative learning, food aversion learning, and induced preference (Papaj and Prokopy 1989; Schoonhoven et al. 2005). Experience-altered behavior has been observed in feeding (e.g., Jermy 1987; Cunningham et al. 1998a, 2004; del Campo et al. 2001; Held et al. 2001) and oviposition (e.g., Cunningham et al. 1998b; Rojas and Wyatt 1999). Earlier investigations on experience-altered host selection and oviposition behavior in adults were usually done with a range of host plants and generally showed that experience could enhance foraging efficiency in a changing host environment (Stanton 1984; Stephens 1993; Landolt and Molina 1996; Cunningham et al. 1998b). More recent investigations done with non-host plants or their extracts have shown that early adult experience could induce oviposition on non-host plants or a preference for host plants treated with non-host extracts (Liu et al. 2005; Liu and Liu 2006; Zhang et al. 2007). These recent findings have implications in both insect–plant evolution (Cunningham et al. 2001) and behavioral control of insect pests (Cunningham et al. 1999; Jallow et al. 2004) because oviposition on non-host plants is an essential step for host range expansion, and an induced preference for non-host plant-derived repellents may render these chemicals ineffective. However, the chemical and behavioral mechanisms for this kind of experience-altered behavior towards non-host plants or their extracts are only superficially understood.
Odors emitted by a plant or its crude extract are a complex blend of individual volatile components, each with a potential effect on insect behavior (Schoonhoven et al. 2005). Host and non-host plants of a given insect may share common volatile compounds (Bengtsson et al. 2006). In some cases, it is possible that the odors of a non-host plant or its extract contain repellent as well as attractive compounds, and an experience-altered behavior may be due to a complex of chemical, neurophysiological, and behavioral causes. For example, preference for non-host plant odors by ovipositing females following experience may result from either an induced preference for the repellent compounds or a habituation to the repellents combined with an unchanged response to the attractive compounds. It has been suggested that these alternative explanations can be investigated by experiments that use individual pure chemicals found in non-host plants (Liu et al. 2005).
By using the diamondback moth Plutella xylostella, its host plant Chinese cabbage, and a non-host plant (Chrysanthemum morifolium) as a model system, we have shown that experience of the non-host plant extract results in the moths being more attracted to host plants treated with the non-host plant extracts and depositing a higher proportion of eggs on host plants treated with the extract than on untreated host plants (Liu et al. 2005). The same model system is used in this study. Here, we first identified two chemicals, α-terpinene and p-cymene, from the volatiles of Chrysanthemum. We next observed the olfactory responses of naive and experienced P. xylostella females to odors of the non-host plant, odors of the two chemicals, and odors of host plants treated with the two chemicals. Finally, we observed the oviposition preference of naive and experienced females towards chemical-treated host plants. These results demonstrate that induced preference for repellent chemicals is a major mechanism leading to behavioral changes towards non-host plants or their extracts.
Methods and Materials
Insects and Plants
The P. xylostella culture was established from a field collection on a cabbage farm in a suburb of Hangzhou, China, in October 2004 and maintained on common cabbage, Brassica oleracea L. var. capitata, cultivar Jing-feng No. 1 in a temperature-controlled room at 26 ± 1°C, 60–70% RH and 14L/10D photoperiod. The culture was replenished once a year with field-collected material.
A non-host plant of P. xylostella, C. morifolium cultivar Xiaoyangju, was used in this study. Plants of C. morifolium were first collected from Tongxiang, Zhejiang, China, and their shoots were transplanted in a potting mix (a mixture of peat moss, vermiculite, organic fertilizer, and perlite in a 10:10:10:1 ratio) in 1.5 l pots and grown in a greenhouse under ambient temperature and humidity and natural lighting. Plants of C. morifolium were used at the vegetative stage bearing about 20 leaves. The host plant, Chinese cabbage Brassica campestris L. ssp. pekinensis, cultivar Zao-shu No. 5, was used in the olfactory and oviposition tests. Chinese cabbage plants were cultivated in the above-mentioned potting mix in 1.5 l pots in greenhouses under ambient temperature and humidity and natural lighting to the five to six fully extended true-leaf stage when used in tests.
Collection and Analysis of Volatile Compounds of C. morifolium
The volatile compounds of C. morifolium were collected by using solid-phase microextraction (SPME, Supelco, Shanghai, China) and analyzed with a gas chromatograph and mass spectrometer (GC-MS, Agilent HP6890N-HP5973, Agilent, Palo Alto, CA, USA).
The SPME holder had fiber covered with 100 μm polydimethylsiloxane. Before each session of extraction, the fiber was conditioned at 250°C for 30 min. For volatile extraction by SPME, the plant sample was enclosed in a glass container (16 cm diameter and 35 cm height). The SPME needle was injected through the septum of the sample container as well as the extended plunger to expose the fiber. Following equilibration between the fiber and the volatile sample for 30 min, the fiber was retracted into the needle. The SPME device was removed from the container and inserted directly into the GC for desorption and analysis. The port was operated in splitless mode. After 3 min, the split valve was opened, and the fiber was removed at the same time. This treatment and analysis were replicated twice.
Chemical constituents of the volatiles were identified following GC-MS analysis on an HP 6890N Series GC System connected to an HP 5973 Network Mass Selective Detector with an HP-5 column (30 m × 0.32 mm i.d., 0.25 μm film thickness). The temperature program for GC was as follows: the initial temperature was maintained at 40°C, held for 2 min, heating up at 8°C/min to 120°C, and then at 1.5°C/min to 160°C. The carrier gas was helium (1 ml/min). The mass spectrum was repetitively scanned from 35 to 450 a.m.v. every 2 s. The MS was used in election impact ionization mode at 70 eV.
Chemical constituents of the volatile were identified by mass spectrum matching in the NIST98 library as well as by comparison of retention time and the fragmentation patterns of the mass spectra with those of authentic commercial samples.
Individual Pure Chemicals
The pure chemicals, α-terpinene and p-cymene, were purchased from Sigma-Aldrich Chemicals, Poole, UK. Chemicals were dissolved in distilled water (containing 2% acetone) to make the test solutions. Solutions of α-terpinene or p-cymene were always applied at a concentration of 500 mg/l.
Analysis of Cabbage Plant Volatiles
Headspace analysis was conducted to investigate volatile emission by cabbage plants that were untreated (control) or treated with a solution of pure chemicals. Cabbage plants were removed from the pots, and care was taken to ensure that roots were not damaged; then, the entire soil and root system was covered in aluminum foil. Preliminary tests showed that this treatment had little effect on plant growth and volatile collection. A volume of 10 ml solution of either α-terpinene or p-cymene was applied to each of the clean, undamaged cabbage plants. Plants that served as controls were sprayed with 10 ml of distilled water (containing 2% acetone). The plants were left to dry for 1 h before being placed into the collection vessels.
Volatiles were collected in a system modified from Turlings et al. (1998). Air was first pushed through a glass bottle with water to be humidified, a flow meter to measure and regulate the air flow, and a charcoal filter to purify the air. The moist and purified air then entered a glass cylinder (16 cm diameter and 35 cm height) at 400 ml·min−1. To create a laminar flow, the air was forced through a glass frit at the top of the cylinder. Approximately 4 cm above the bottom, there was a 25 mm vertical female ground–glass connector for a collection trap. The trap was a glass tube (10 cm long, 5 mm diameter) that contained 30 mg of 80/100 mesh Super-Q (Altech, USA). The air passing over the plant was pulled through Super-Q adsorbent and vented out. The air-inlet, air-outlet, filter, and sampling vessel were connected with Teflon tubing. Each collection session lasted for 24 h. After each collection session, each trap was rinsed with 1 ml methylene dichloride, and the solution was concentrated to 100 μl by a stream of pure nitrogen. Volatile samples of control and chemical-treated cabbage plants were refrigerated at −4°C until they were analyzed. Each treatment was replicated twice.
Compounds were analyzed by using HP6890N GC equipped with an HP-5 column (30 m × 0.32 mm i.d., 0.25 μm film thickness) and a flame ionization detector. A volume of 1 μl of sample was injected in splitless mode with nitrogen (1.6 ml/min) as the carrier gas. The column temperature was programmed to maintain at 40°C for 4 min and then increase at 8°C/min to 220°C. The temperature of the injector slit was 250°C.
Experience Treatment
Test cohorts of P. xylostella were reared on common cabbage from egg to pupation in a temperature-controlled room at 26 ± 1°C, 60–70% RH and 14L/10D photoperiod. Pupae were collected 2–3 days after pupation, taken out of their cocoons, and thoroughly washed in distilled water using the procedure described by Liu and Liu (2006). The pupae were placed in a clean, ventilated cage (45 × 45 × 45 cm) for adult emergence and mating. Adults were provided with 15% honey-water as food upon emergence but were not exposed to any plant odors (away from the rearing room) until either being used as untreated controls or being exposed to various experience treatments at 2–3 days post-emergence.
To investigate the effects of experience on the olfactory and oviposition preference of P. xylostella females, we treated the test insects by using the following procedures:
-
(1)
Experience with non-host plant: For this treatment, a group of individuals from the test cohorts were moved manually (with a fine soft brush) at the late fourth instar from common cabbage to C. morifolium to force them to pupate on the non-host plants. The insects were left to emerge naturally on the plants. Adults were provided with 15% honey-water and enclosed with the non-host plants in a cage until being used in the olfactometer bioassay at 2–3 days post-emergence. A group of untreated adults reared from common cabbage were bioassayed as the “No experience” (naive) control for this experience treatment.
-
(2)
Experience with pure chemicals: We used a clear, cylindrical, and ventilated plastic container (14.5 cm in height, with a top of 9 cm diameter and a base of 13 cm diameter) as the arena for experience treatments. The inner surface of the container was sprayed evenly with 1 ml of a chemical solution. A piece of filter paper (9 cm diameter) was placed at the bottom of the container, and 2 ml of a chemical solution was applied evenly onto the filter paper with a small pipette. The container with the treated filter paper was dried for 1 h, and then four females were introduced to experience the chemical for 10 min before they were used immediately in olfactory or oviposition preference tests. “No experience” control females were handled in the same manner except that the arena for experience was treated with distilled water (containing 2% acetone) only.
-
(3)
Experience with chemical-treated host plants: Prior to each test, a cabbage plant was sprayed with 10 ml of a chemical solution and placed in a ventilated cage (45 × 45 × 45 cm) to air dry for 1 h. A group of untreated females was then transferred from the emergence cage onto the treated plant in the cage to experience the treated plant for 30 min before they were used immediately in olfactory or oviposition preference tests. A group of untreated females was taken from the emergence cage to be bioassayed as “No experience” control adults.
Basic Procedure for Testing Olfactory Preference
We used a Y-tube olfactometer to observe the olfactory responses of P. xylostella females (for details of the olfactometer, see Zhang et al. 2007). The two arms were connected to two separate glass containers holding the odor sources. The size of the glass containers varied from 6.5 cm diameter × 8.5 cm height for holding a piece of filter paper to 16 × 35 cm height for holding a plant. A flow meter was connected to each arm of the olfactometer. A pump was used to draw air over a charcoal filter and then through the olfactometer at a rate of 400 ml·min−1. As moths were most active at night, we conducted all observations in a dark room (lit with only a 15-W red light) at 26 ± 1°C between 1900 and 2300 hours.
For each replicate of a given treatment, four females were released into the stem of the olfactometer and left in the olfactometer to respond for 15 min. When a moth walked up to the upwind end of an olfactometer arm, either within 4 cm to the blocking screen or into the corresponding moth-trapping bulb at the end of 15 min, it was recorded as making a choice for that odor. When a moth did not reach the end of either arm within 15 min, it was recorded as “no response”. Each moth was used only once. After running five replicates, the connections of the odor sources to the olfactometer arms were exchanged to remove any asymmetrical bias in the set-up. The olfactometer tube and glass container were thoroughly washed with alcohol at the end of the observations for a given treatment and dried overnight in an oven at 200°C.
Olfactory Response to the Non-host Plant C. morifolium
Females of P. xylostella were taken haphazardly from the “Experience with non-host plant” and the corresponding “No experience” control treatments and observed by using the Y-tube olfactometer procedure described above. One arm of the Y-tube olfactometer was connected to a glass container that held a fresh C. morifolium plant and the other arm to an empty glass container. For each of the two treatments, we conducted 20 replicates to bioassay a total of 80 females. The test plants were replaced by new ones of the corresponding categories after every five replicates.
Olfactory Response to Pure Chemicals
For bioassays of females with prior experience of either α-terpinene or p-cymene, 2 ml solution of a chemical were applied evenly onto a piece of filter paper (9 cm diameter) with a pipette. The treated filter paper was air-dried for 1 h. Another piece of filter paper was treated similarly with 2 ml of distilled water (containing 2% acetone) as the control. The treated and control pieces of paper were placed separately into the two odor source containers of the Y-tube olfactometer. Four females of a given treatment were then observed in the olfactometer by using the procedure described above. For each experience treatment or its corresponding “No experience” control, we conducted 10 or 15 replicates to bioassay a total of 40 or 60 females, and fresh odor sources (i.e., fresh treated and control pieces of papers) were used for each replicate.
Olfactory Response to Cabbage Plants Sprayed with a Pure Chemical
Prior to each bioassay, one cabbage plant was sprayed with 10 ml solution of either α-terpinene or p-cymene, and another cabbage plant was sprayed with 10 ml of distilled water (containing 2% acetone) as the control. The treated and control plants were air-dried for 2 h and then placed separately into the two odor-source containers of the Y-tube olfactometer. Four females of a given treatment were then observed in the olfactometer by using the procedure described above. For each experience treatment or its corresponding “No experience” control, we conducted 10 replicates to bioassay a total of 40 females. The test plants were replaced by new ones of the corresponding categories after every five replicates.
Oviposition Preference
A dual-choice bioassay was used to determine whether the responses of ovipositing females would change following prior experiences of p-cymene. Cabbage plants were first sprayed with a solution of p-cymene (see above), while the control plants were sprayed with distilled water (containing 2% acetone), 10 ml solution per plant. Sprayed plants were air-dried for 2 h. One treated plant and one control plant were placed in diagonally opposite corners of a cage (55 × 55 × 55 cm), and the position of the plants adjusted so that the foliage of the two plants was approximately 20 cm apart at the closest point. Three P. xylostella females were then released into the center of each cage between 1900 and 2000 hours. After 12 h in darkness at 26 ± 1°C, the moths were removed, and the eggs deposited on each of the two plants were counted.
We tested the oviposition preference of females of each of the following three experience treatments towards control and p-cymene-treated cabbage plants: no experience, experience of p-cymene-treated filter paper, and experience of p-cymene-treated cabbage. We conducted 11–13 replicates (each in a separate cage) for each of the treatments.
Statistical Analysis
For the olfactometer bioassays, the number of females choosing each of the two odor arms in each of the experience or “No experience” treatments was compared with a replicated G test of goodness-of-fit with the null hypothesis of no preference, and for the observations on each odor source, a G test of a 2 × 2 table of independence was used to compare the relative proportions of responding and non-responding females between naive and experienced treatments. For the oviposition preference tests, a replicated goodness-of-fit G test was used to compare the numbers of eggs deposited on each pair of plants in a given plant treatment with the null hypothesis of no preference, and a G test of a 2 × 2 table of independence was used to compare the overall relative proportions of eggs laid on the control and treated host plants between females from any two experience treatments (Sokal and Rohlf 1995).
Results
Chemical Analysis of Plant Volatiles
Identification of volatile compounds of C. morifolium (Fig. 1) was done by comparing GC-MS fragmentation patterns for the major peaks with authentic standards and NIST98 libraries. The mass spectra of peak 1 and peak 2 were identified as α-terpinene and p-cymene, respectively. The remaining peaks were not identified due to lack of authentic standards at the time.
GC-MS analysis of volatile compounds emitted from C. morifolium using authentic standards for comparison of retention times and mass spectra. Total ion current chromatogram of C. morifolium compared with that of two authentic standards. The mass spectra of peak 1 and peak 2, identified as α-terpinene and p-cymene, respectively, are shown together with that of the respective authentic standards
Analysis of the volatiles released from control cabbage plants and cabbage plants sprayed with a solution of α-terpinene or p-cymene indicated that qualitative differences occurred in the volatiles collected following treatment with each of the two chemicals (Fig. 2). α-Terpinene- or p-cymene-treated cabbage plants emitted more volatiles in addition to the pure chemical applied to the plants compared to the volatile compounds from untreated plants.
Olfactory Response
In the olfactometer choice tests with volatiles of “C. morifolium vs. air”, a significantly lower proportion of females in the “No experience” (naive) control made their first choice towards the nonhost plant than air; in contrast, the proportions of females making their first choice towards C. morifolium or air did not differ significantly when the females had a prior experience of the nonhost plant (Table 1).
In the olfactometer choice tests with “α-terpinene vs. air”, a significantly lower proportion of females in the naive control made their first choice towards α-terpinene; in contrast, a significantly higher proportion of females made their first choice towards α-terpinene when the females had a prior experience of the chemical (Table 2).
In the olfactometer choice tests that compared volatiles of “α-terpinene-treated cabbage vs. air”, a significantly lower proportion of females in the naive control made their first choice towards α-terpinene-treated cabbage than air; in contrast, the proportions of females making their first choice towards α-terpinene-treated cabbage or air were similar when the females had a prior experience with α-terpinene-treated cabbage plants (Table 3).
In the olfactometer choice tests with “p-cymene vs. air”, a significantly lower proportion of females in the naive control made their first choice towards p-cymene than air, whereas a significantly higher proportion of females made their first choice towards p-cymene than air when the females had a prior experience of the chemical (Table 4).
Similarly, for “p-cymene-treated cabbage vs. air”, a significantly lower proportion of females in the naive control made their first choice towards p-cymene-treated cabbage than air; in contrast, a significantly higher proportion of females made their first choice towards p-cymene-treated cabbage than air when the females had a prior experience of p-cymene-treated cabbage plants (Table 5).
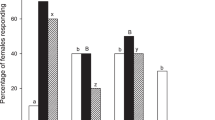
Prior experience reduced the relative proportion of non-responding females in all cases, although the reductions reached a significant level only in the treatments with two odor sources (Fig. 3).
Percentages of P. xylostella females without or with prior experience exhibiting “no response” in the Y-tube olfactometer in various treatments: 1 without or with prior experience of C. morifolium plant (see Table 1 for sample sizes); 2 without or with prior experience of α-terpinene-treated paper (see Table 2 for sample sizes); 3 without or with prior experience of α-terpinene-treated plant (see Table 3 for sample sizes); 4 without or with prior experience of p-cymene-treated paper (see Table 4 for sample sizes); 5 without or with prior experience of p-cymene-treated plant (see Table 5 for sample sizes). Bars in the same group with different letters differ significantly at P = 0.05 (G test of a 2 × 2 table of independence)
Oviposition Preference
In the choice tests that used p-cymene-treated and control cabbages, the numbers (mean ± SE) of eggs laid per replicate (i.e., for three females) were 114.4 ± 10.9, 85.0 ± 12.5, and 79.3 ± 14.2 for the naive females, females with prior experience of p-cymene, and females with prior experience of p-cymene-treated plants, respectively. These means did not differ significantly among the three groups of females (one-way analysis of variance, F 2,33 = 2.74, P = 0.079). The naive females laid a significantly higher proportion of eggs (55.6%) on the control plants than on the p-cymene-treated plants (44.4%; G p = 18.6, df = 1, P < 0.001; G H = 104.2, df = 12, P < 0.001). In contrast, females with a prior experience of p-cymene deposited a significantly higher proportion of eggs (57.6%) on the p-cymene-treated plants than on the control plants (42.4%; G p = 21.9, df = 1, P < 0.001; G H = 126.2, df = 10, P < 0.001). However, the proportions of eggs laid on the p-cymene-treated and control plants (50.7% vs. 49.3%) did not differ significantly for females with prior experience of p-cymene-treated plants (G p = 0.2, df = 1, P = 0.67; G H = 179.4, df = 11, P < 0.001; Fig. 4). As indicated by the G H statistic, the variations among replicates were large in all three treatments.
Percentages of eggs laid on control and p-cymene-treated cabbage plants by P. xylostella females with various prior experiences in choice tests. Asterisks on the left indicate significant difference from no preference within a choice test (***P < 0.001, n.s. not significant), and the bars of different treatments followed by different letters on the right indicate significant difference (P < 0.01) between them
Discussion
Odor compositions of host plants are taxon-specific, and the olfactory system of an insect has the capacity to distinguish these odors from others and use the plant chemical cues in host foraging (Schoonhoven et al. 2005). C. morifolium is a non-host plant of P. xylostella and produces many kinds of volatile compounds (Fig. 1). These volatile compounds can be obtained by various methods that involve extraction of the plant material. However, sampling air from around intact plants gives a more accurate picture of the volatile profile (Tholl et al. 2006). Our previous study showed that the extract of dried leaves of C. morifolium had a repellent effect on naive P. xylostella females, but experienced females were not repelled, and they were attracted instead by host plants treated with the non-host plant extract (Liu et al. 2005). In the present study, naive females of this insect showed an aversion to volatiles emitted from intact plants of C. morifolium, but females with a prior experience with the plant were not repelled by the plant volatiles (Table 1). In another study, P. xylostella females with a prior experience of pea, another non-host plant of the insect, showed preference for the non-host plant volatiles (Zhang et al. 2007). Thus, depending on the plant species, P. xylostella females can exhibit habituation or induced preference for volatiles emitted from non-host plants.
GC-MS analysis of volatile compound emitted from C. morifolium plants (by comparison with authentic standards) showed the presence of α-terpinene and p-cymene in the blend (Fig. 1). Naive females of P. xylostella showed aversion to both these volatiles at the concentration tested, but experienced females became attracted by them (Tables 2 and 4). Experience-altered responses towards volatiles of Chinese cabbage treated with each of the two compounds were similar to those towards the pure compounds, although the females with a prior experience of α-terpinene-treated plants only lost their aversion to the plant volatiles and did not become attracted by them (Tables 3 and 5). The somewhat different responses towards α-terpinene- and α-terpinene-treated plants were not surprising, as volatiles from α-terpinene-treated plants contained many more compounds in addition to α-terpinene (Fig. 2). The oviposition preference tests showed similar experience-altered behaviors to those revealed by the olfactory bioassays. Naive females showed a preference for oviposition on untreated plants, whereas experienced females either showed a preference for oviposition on p-cymene-treated plants or were no longer repelled by the treated plants (Fig. 4). The combined results of olfactory and oviposition bioassays demonstrate that experience-induced preference for previously repellent compounds is a major mechanism that leads to behavioral changes of ovipositing females towards non-host plants or their extracts and that increased acceptance of non-host plants or their extracts following experience does not have to involve initially attractive compounds (Liu et al. 2005). It is likely that similar behavioral changes towards repellents were involved in experience-induced preference for oviposition on substrates treated with neem-based products (Liu and Liu 2006).
Although experience-induced preference for repellents in ovipositing females of phytophagous insects has been demonstrated in only a few cases, it is likely a widespread phenomenon in view of the commonly observed evidence for habituation and induced preference to feeding deterrents in phytophagous larvae (Jermy 1987; Papaj and Prokopy 1989; Schoonhoven et al. 2005). In addition, experience-induced habituation and preference for repellents have been recorded in several saprophagous and blood-sucking dipterans (Jaenike 1982; McCall and Eaton 2001; Kaur et al. 2003). Experience-induced preference for repellents allows the insects to retain a degree of flexibility to more successfully deal with uncertainty in the environment, including ovipositing on poor host plants when highly suitable plants are not available (Stephens 1993). It may also play an important role in allowing insects to explore new host plants, a necessary precursor for the evolution of host range expansion (Janz et al. 2006; Zhang and Liu 2006; Zhang et al. 2007). For deployments of non-host plant-derived chemicals in behavioral manipulation of insect pests, effective strategies and techniques have to be developed to mitigate the decrease in responses to the repellents (Gould 1991; Akhtar and Isman 2003).
Charleston et al. (2006) found that cabbage plants treated with extract of the syringa tree Melia azedarach emitted large quantities of volatiles as well as some additional compounds that were not detected in either the syringa extract or the untreated plants, suggesting that the syringa extract induced the emission of cabbage volatiles. Such enhanced emission of volatiles caused by interactions between a plant and the extract of another plant was observed also in this study (Fig. 2). As the enhanced emission of volatiles by the treated plant may influence the behavior of the herbivores as well as their natural enemies (Charleston et al. 2005, 2006), this kind of response has obvious implications in the deployment of non-host plant chemicals for behavioral manipulation of insect pests (Gould 1991; Isman 2006; Liu et al. 2006).
References
Akhtar, Y., and Isman, M. B. 2003. Binary mixtures of feeding deterrents mitigate the decrease in feeding deterrent response to antifeedants following prolonged exposure in the cabbage looper, Trichoplusia ni (Lepidoptera: Noctuidae). Chemoecology 13:177–182.
Bengtsson, M., Jaastad, G., Knudsen, G., Kobro, S., Backman, A. C., Pettersson, E., and Witzgall, P. 2006. Plant volatiles mediate attraction to host and non-host plant in apple fruit moth, Argyresthia conjugella. Entomol. Exp. Appl. 118:77–85.
Charleston, D. S., Kfir, R., Vet, L. E. M., and Dicke, M. 2005. Behavioural responses of diamondback moth Plutella xylostella (Lepidoptera: Plutellidae) to extracts derived from Melia azedarach and Azadirachta indica. Bull. Entomol. Res. 95:457–465.
Charleston, D. S., Gols, R., Hordijk, K. A., Kfir, R., Vet, L. E. M., and Dicke, M. 2006. Impact of botanical pesticides derived from Melia azedarach and Azadirachta indica plants on the emission of volatiles that attract parasitoids of the diamondback moth to cabbage plants. J. Chem. Ecol. 32:325–349.
Cunningham, J. P., West, S. A., and Wright, D. J. 1998a. Learning in the nectar foraging behaviour of Helicoverpa armigera. Ecol. Entomol. 23:363–369.
Cunningham, J. P., Jallow, M. F. A., Wright, D. J., and Zalucki, M. P. 1998b. Learning in host selection in Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Anim. Behav. 55:227–234.
Cunningham, J. P., Zalucki, M. P., and West, S. A. 1999. Learning in Helicoverpa armigera (Lepidoptera: Noctuidae): a new look at the behavior and control of a polyphagous pest. Bull. Entomol, Res. 89:201–207.
Cunningham, J. P., West, S. A., and Zalucki, M. P. 2001. Host selection in phytophagous insects: a new explanation for learning in adults. Oikos 95:537–543.
Cunningham, J. P., Moore, C. J., Zalucki, M. P., and West, S. A. 2004. Learning, odor preference and flower foraging in moths. J. Exp. Biol. 207:87–94.
Del Campo, M. L., Miles, C. I., Schroeder, F. C., Mueller, C., Booker, R., and Renwick, J. A. 2001. Host recognition by the tobacco hornworm is mediated by a host plant compound. Nature 411:186–189.
Gould, F. 1991. Arthropod behavior and the efficacy of plant protectants. Annu. Rev. Entomol. 36:305–330.
Held, D. W., Eaton, T., and Potter, D. A. 2001. Potential for habituation to a neem-based feeding deterrent in Japanese beetles, Popillia japonica. Entomol. Exp. Appl. 101:25–32.
Isman, M. B. 2006. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 51:45–66.
Jaenike, J. 1982. Environmental modification of oviposition behavior in Drosophila. Am. Nat. 119:784–802.
Jallow, M. F. A., Cunningham, J. P., and Zalucki, M. P. 2004. Intra-specific variation for host plant use in Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae): implications for management. Crop Prot. 23:955–964.
Janz, N., Nylin, S., and Wahlberg, N. 2006. Diversity begets diversity: host expansions and the diversification of plant-feeding insects. BMC Evol. Biol. 6:4 DOI 10.1186/1471-2148-6-4.
Jermy, T. 1987. The role of experience in the host selection of phytophagous insect, pp. 143–157, in R. F. Chapman, E. A. Bernays, and J. G. Stoffolano (eds.). Perspectives in Chemoreception and BehaviorSpringer-Verlag, New York.
Kaur, J. S., Lai, Y. L., and Giger, A. D. 2003. Learning and memory in the mosquito Aedes aegypti shown by conditioning against oviposition deterrence. Med. Vet. Entomol. 17:457–460.
Landolt, P. J., and Molina, O. 1996. Host-finding by cabbage looper moth (Lepidoptera: Noctuidae): learning of host odor upon contact with host foliage. J. Insect Behav. 9:899–908.
Liu, T. X., and Liu, S. S. 2006. Experience-altered oviposition responses to a neem-based product, Neemix®, by the diamondback moth, Plutella xylostella. Pest Manag. Sci. 62:38–45.
Liu, S. S., Li, Y. H., Liu, Y. Q., and Zalucki, M. P. 2005. Experience-induced preference for oviposition repellents derived from a non-host plant by a specialist herbivore. Ecol. Lett. 8:722–729.
Liu, S. S., Li, Y. H., and Lou, Y. G. 2006. Non-host plant extracts reduce oviposition of Plutella xylostella (Lepidoptera: Plutellidae) and enhance parasitism by its parasitoid Cotesia plutellae (Hymenoptera: Braconidae). Bull. Entomol. Res. 96:373–378.
McCall, P. J., and Eaton, G. 2001. Olfactory memory in the mosquito Culex quinquefasciatus. Med Vet. Entomol. 15:197–203.
Papaj, D. R., and Prokopy, R. J. 1989. Ecological and evolutionary aspects of learning in phytophagous insects. Annu. Rev. Entomol. 34:315–359.
Rojas, J. C., and Wyatt, T. D. 1999. The role of pre- and post-imaginal experience in the host-finding and oviposition behavior of the cabbage moth. Physiol. Entomol. 24:83–89.
Schoonhoven, L. M., Van Loon, J. J. A., and Dicke, M. 2005. Insect–Pant Biology. 2nd edn.Oxford University Press, New York.
Sokal, R. R., and Rohlf, F. J. 1995. Biometry: The Principle and Practice of Statistics, in Biological Research. 3rd edn.W. H. Freeman and Company, New York.
Stanton, M. L. 1984. Short-term learning and the searching accuracy of egg-laying butterflies. Anim. Behav. 32:33–40.
Stephens, D. W. 1993. Learning and behavioral ecology: incomplete information and environmental predictability, pp. 195–218, in D. R. Papaj, and A. C. Lewis (eds.). Insect Learning: Ecological and Evolutionary Aspects. Chapman & Hall, New York.
Tholl, D., Boland, W., Hansel, A., Loreto, F., Röse, U. S. R., and Schnitzler, J.-P. 2006. Practical approaches to plant volatile analysis. Plant J. 45:540–560.
Turlings, T. C. J., Lengwiler, U. B., Bernasconi, M. L., and Wechsler, D. 1998. Timing of induced volatile emissions in maize seedlings. Planta 207:146–152.
Zhang, P. J., and Liu, S. S. 2006. Experience induces a phytophagous insect to lay eggs on a nonhost plant. J. Chem. Ecol. 32:745–753.
Zhang, P. J., Liu, S. S., Wang, H., and Zalucki, M. P. 2007. The influence of early adult experience and larval food restriction on responses towards non-host plants in moths. J. Chem. Ecol. 33:1528–1541.
Acknowledgments
We thank Marcel Dicke, Wageningen University, The Netherlands, and Myron Zalucki, The University of Queensland, Australia, for thoughtful comments on the manuscript. This study was funded by the National Natural Science Foundation of China (project no. 30571225) and the National Basic Research and Development Program of China (2002CB111403).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, H., Guo, WF., Zhang, PJ. et al. Experience-Induced Habituation and Preference Towards Non-Host Plant Odors in Ovipositing Females of a Moth. J Chem Ecol 34, 330–338 (2008). https://doi.org/10.1007/s10886-008-9433-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-008-9433-3