Abstract
We tested whether changes in long-term nutrient availability would affect the xylem quality and characteristics of Scots pine trees as a food source for the larvae of the xylophagous wood borer Hylotrupes bajulus L. (Cerambycidae). We looked for an effect of host plant growth and xylem structural traits on H. bajulus larval performance, and looked for delayed effects of long-term forest fertilization on xylem chemical quality. In general, larval performance was dependent on larval developmental stage. However, the growth of larvae also varied with host plant quality (increases in the concentration of nitrogen and carbon-based secondary compounds of xylem were correlated with a decrease in the larval growth rate). The greater annual growth of trees reduced tracheid length and correlated positively with second-instar H. bajulus growth rate. This is consistent with the hypothesis that intrinsic growth patterns of host plants influence the development of the xylophagous wood borer H. bajulus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wood-boring beetles (Coleoptera: Cerambycidae) are predominantly xylophagous insects and are commonly referred to as long-horned beetles when they are adults and as round-headed borers when they are larvae (Linsley 1959). They attack the xylem of host plants in a variety of conditions ranging from living trees to dead decaying wood (Linsley 1959; Hanks 1999). Since they have adapted to such highly variable hosts, wood borer species have tremendous diversity, and many of these species are serious pests of forest trees. The larvae of the old house borer, Hylotrupes bajulus L. (Coleoptera: Cerambycidae) feed on the sapwood of dying or dead host species such as pine, spruce, or fir in both natural and managed forests as well as construction timber used in buildings. H. bajulus larvae may consume heartwood only if the sapwood has been destroyed by the larvae (Robinson and Cannon 1979). The larvae spend most of their lifetime (2–12 years) inside the trunk consuming the xylem tissue. Although the taxonomy, species distributions, and larval host ranges of long-horned beetles have been extensively studied, little is known about their chemical ecology in relation to growth and survival (Ljungkvist 1983; Allison et al. 2004).
Nitrogen is a vital component of the diet of insects, and the levels of nitrogenous compounds can influence the interactions between herbivorous insects and their host plants (Mattson 1980; Holopainen et al. 1995). In forestry, nitrogen fertilizers are used to enhance timber production, but much less attention has been paid to the effects of fertilizers on the characteristics of xylem and on the performance of the xylophagous insects that attack woody plants (Kytö et al. 1996). The modification of plant resource balances by fertilization treatments might change the structure of the wood, e.g., tracheid properties (Mäkinen et al. 2002), as well as have effects on mechanical properties of xylem (Lucas et al. 2000). This, in turn, might have an impact on the performance of herbivores. These are all topics that need clarification. It is known that the carbon-based secondary compounds (e.g., terpenes and resin acids) in conifers deter insect pests and fungal pathogens (Gershenzon and Croteau 1991; Nerg et al. 2004). Xylem resin reduces the feeding activity of H. bajulus larvae (Holm and Ekbom 1958). This study examined the effects of a long-term increase in nutrient availability on tree growth, tracheid properties, the nutritional value of xylem tissue, and the growth of wood-boring H. bajulus L. larvae.
Methods and Materials
Site Description and Treatments
The Scots pine (Pinus sylvestris L.) trees used in this study were part of an earlier experiment established by the Finnish Forest Research Institute in which several experimental sites in Finland were set up in the 1950s to investigate the effects of forest fertilization on tree growth. The experimental sites used were SITE1—Padasjoki (61°23′N, 25°3′E), SITE2—Vilppula (62°4′N, 24°29′E), and SITE3—Punkaharju (61°40′N, 29°18′E) (Turtola et al. 2002). Three fertilization treatments were applied to the plots at each site: 1) plots with no fertilization (control), 2) plots fertilized with nitrogen (N), and 3) plots fertilized simultaneously with calcium, nitrogen, and phosphorus (CaNP). There was one plot per treatment at each site, as individual trees were considered to act as replicates in these forestry experiments designed in the 1950s. The size of each plot was 40 × 40 m, with a 30 × 30 m area delimited for tree felling centered in each plot. Fertilization treatments (year of fertilization) were initiated in SITE1 when the trees were 12 yr old: N (1958, 1963, 1968, 1973, 1978, 1983, 1988, 1993, 1998); Ca (1959, 1978); P (1958, 1978, 1993); SITE2 when trees were 12 yr old: N (1959, 1964, 1969, 1974, 1979, 1984, 1989, 1994); Ca (1959, 1979); P (1959, 1979, 1994); SITE3 when trees were 14 yr old: N (1959, 1969, 1979, 1989); Ca (1959, 1979); P (1959, 1979). Fertilization treatments: N=82 kg ha−1 of nitrogen applied as ammonium sulfate in 1958–1959, 92 kg ha−1 as urea in 1963–1969, and 150–180 kg ha−1 as ammonium nitrate in 1973–1998; Ca=calcium applied as limestone, first at 2000 kg ha−1, and later at 4000 kg ha−1; P=phosphorus, initially applied at 29 kg ha−1 as finely ground rock phosphate, and subsequently at 40 kg ha−1 as superphosphate.
Sampling
All trees from the 30 × 30 m area were divided into three groups according to their basal area. From each of these groups, two trees were randomly selected for felling. In April 2000, 18 trees (six per treatment) from each site were harvested. For chemical and xylem property analysis, consecutive xylem disks (about 2 cm thick) with bark from breast height (H1; 130 cm from ground level) and canopy height (H2; 0.7 × tree height) were sawed. The xylem disks were frozen and stored at −20°C before analysis. All xylem used in the following analysis was taken from the sapwood. The analyses of nutrient and tracheid properties were performed on xylem disks from both H1 and H2, while for secondary compound analysis, only xylem disks from H1 were used. To examine the growth performance tests of H. bajulus L. larvae, an additional 20-cm-long xylem disk was sawed from each tree, just above the disks taken from H1 and H2 for chemical and xylem property analysis. The sapwood of these xylem disks was sawed into blocks 80 × 40 × 20 mm and air-dried at room temperature before use in the insect performance tests. The sapwood diameter from the latest 20 annual rings was measured.
Tracheid Properties
At H1, tracheid length was measured from annual ring numbers 12, 30, and 40 from the pith outwards. The annual ring number 12 was at the border of the sapwood and heartwood. At H2, tracheid length was measured from annual ring numbers 5, 10, and 12 from the pith outwards. Early- and latewood samples were separated from these annual rings and macerated with glacial acetic acid/hydrogen peroxide (1:1, v/v) at 60°C overnight. The suspension was washed with distilled water and stained with safranin (1%, 10 sec). Tracheids were placed on glass slides, and 50 unbroken tracheids were measured with a light microscope.
Chemical Analysis
A 0.125-g (dry weight) powdered xylem sample was weighed for the nitrogen (N) analysis. Nitrogen was analyzed with a LECO CHN 2000 analyzer (USA). The analyzer was calibrated with EDTA (containing 40.97 % carbon, 9.57 % nitrogen, and 5.48 % hydrogen) as a standard with every eighth sample being a pine control sample. The performance of the apparatus was monitored once a day with certified reference samples (BRC 101 and NIST 1547). The total nitrogen content was calculated as mg g−1 dry weight. Other xylem characteristics, such as, cellulose, hemicellulose, lignin, and starch were measured (Heijari et al. 2005), but these parameters did not have any significant effect on H. bajulus performance (data not shown). In the monoterpene analysis, sapwood samples (two replicate wood pieces) were extracted with n-hexane as described earlier by Manninen et al. (2002). Wood resin acids were extracted from freeze-dried and powdered xylem samples (two replicate wood pieces) with petroleum ether-diethyl ether. Monoterpene and resin acid extracts were analyzed by gas chromatography-mass spectrometry (Hewlett Packard GC type 6890, MSD 5973) using a 30-m-long HP-5MS (0.25 mm ID, 0.25-μm film thickness, Hewlett Packard) capillary column (Manninen et al. 2002). Helium was used as the carrier gas. The temperature program for monoterpenes rose from 50°C to 250°C, and for resin acids from 50°C to 270°C. The heating rate was 5°C min−1. The SCAN technique (mass numbers from m/z 30 to 350 were recorded; signal ions in monitoring; 93, 133, 136, 161, 204 m/z) was used for monoterpene samples, and the technique of selected ion monitoring (SIM) 299, 301, 314, 316 m/z for resin acid samples. For quantification of resin acids and terpenes, calibrations were made from known amounts of available pure compounds relative to known amounts of the internal standard (1-chloro-octane for monoterpenes and heptadecanoic acid for resin acids).
Larval Performance
Laboratory grown Hylotrupes bajulus L. larvae were pre-grown on protein-yeast enriched pine sapwood to accelerate growth. The growth conditions were 27°C and 70% relative humidity (RH) in darkness. Adults and larvae were reared as described by Berry (1972) and the European Standard EN 47 (1988).
Two sawed Scots pine xylem blocks, from both H1 and H2, were placed against each other and held together with an elastic band. A groove (45 × 15 × 10 mm) was gouged into the point of contact. The double xylem block system was weighed, and one randomly selected second- (ca. 200 mg) or third-instar (ca. 355 mg) H. bajulus was enclosed into each groove under a glass plate, which was tightened by an elastic band to prevent the larvae from escaping. The double xylem block system (four replicates per tree per sampling height) with larvae were enclosed inside plastic boxes (volume 750 ml). The larvae were allowed to feed for 127 d in a dark culture room at 21°C and 60% RH. The mortality of the whole larval population feeding on xylem taken from H1 and H2 was 8.6% and 19.3% for I2L and 3.8% and 4.5% for I3L, with no statistical differences between sampling heights or instars (data not shown). After feeding, the relative growth rate (RGR) for the living larvae was calculated (RGR = (lnW 2 − lnW 1) / (t 1 − t 2), where W 1 and W 2 were the fresh biomass at the beginning (t 1) and end (t 2) of the sampling period, and ln is the natural logarithm; Waldbauer 1964).
After the feeding tests, larvae were placed on the Scots pine wood to complete their development. Emerging adults were allowed to copulate. Females were allowed to lay eggs in Petri dishes with Scots pine wood disks, and the eggs hatched after about 9 d. The neonatal larvae (I1L), mean weight 0.24 mg) were transferred after hatching into a 5-mm deep hole. The hole was drilled into the top of the 50 × 20 × 20 mm size Scots pine xylem blocks taken from the trees of different fertilization treatments and sites described above, both at H1 and H2. Progenies of each female were randomized among the treatments. Larvae were allowed to feed for 194 d in a dark culture room at approximately 25°C and 85% RH. The relative growth rate for the I1L larvae was calculated as described.
Statistical Analyses
The effect of fertilization treatments at each site was analyzed by analysis of variance (ANOVA) (GLM Univariate) and followed by the Tukey multiple range test (significance level P < 0.05). T test (t) (or Chi-Square for larval mortality) was used when differences between sampling heights were tested. The relative growth rate (RGR) of H. bajulus larvae was calculated separately for the different larval stages. Correlation coefficients (r) between RGR and consumed xylem with different xylem properties were tested by Pearson correlations. Statistical analyses were carried out with SPSS 11.5.1 for Windows statistical software package.
Results and Discussion
It is known that nitrogen in plant tissue can positively affect host utilization by insects (Mattson 1980; Holopainen et al. 1995). In our study, H. bajulus larval performance was similar in wood taken from fertilized and non-fertilized trees (Table 1). Interestingly, we found that for 2nd- and 3rd-instar H. bajulus, RGR was lower for xylem from canopy height than that found in xylem collected from breast height SITE2 (t 10 = −2.343, P = 0.041 and t 8 = −2.893, P = 0.020) and SITE3 (t 10 = −2.884, P = 0.018 and t 9 = −4.482, P = 0.002). Conversely, the mean (± SD) final weight of neonatal larvae was marginally lower 0.58 ± 0.16 mg at H1 compared to 0.72 ± 0.31 mg at H2 (Table 1). Similarly, Körting (1972) observed that neonatal larvae of H. bajulus had a three-fold higher growth rate on Scots pine canopy height xylem than at breast height xylem. The differences in neonatal larval growth rates at H1 and H2 may be a consequence of the within-tree variation in nitrogen concentration. As a consequence of its higher nitrogen concentrations, canopy height xylem may be a superior food source for neonatal H. bajulus larvae. The nitrogen concentration was significantly lower at H1 than at H2 in SITE1 in control (t 10 = −3.693, P = 0.004) and CaNP-fertilized trees (t 10 = −4.330, P = 0.001) and in SITE2 (t 10 = −5.766, P < 0.001) and SITE3 (t 10 = −3.983, P = 0.003) in N-fertilized trees (Fig. 1). Addition of nitrogen to Scots pine (Pinus sylvestris L.) plots increased the xylem nitrogen concentration (Fig. 1). This observed increase in nitrogen concentration is consistent with earlier studies (Helmisaari and Siltala 1989; Finér and Kaunisto 2000). We propose that for late instars, the nitrogen content of food is not as essential in the maintenance of daily metabolism as it is for neonatal larvae that are increasing their body mass.
The concentration of nitrogen (mean + SE) in Scots pine sapwood at breast- (H1; a) and canopy height (H2; b) in three experimental sites and fertilization treatments (control=no fertilization, N-fertilized=fertilization with nitrogen, CaNP-fertilized=fertilized with calcium, nitrogen and phosphorous). Means indicated with different letters within sites are significantly different according to ANOVA, P < 0.05, N = 6
Interestingly, we found that the performance of wood-boring Hylotrupes bajulus larvae was explained by the tree radial diameter; tree diameter correlated positively with the RGR of I2L in SITE2 (r = 0.609, P < 0.001, N = 34) and in SITE3 (r = 0.430, P = 0.010, N = 35). These results agree with Hanks et al. (2005) who reported that larvae of P. semipunctata (Cerambycidae) increased their body size more in large circumference eucalyptus host trees than in small trees. Furthermore, Ruel and Whitham (2002) found that pinyon pines (Pinus edulis) that had grown vigorously during the juvenile stage suffered greater herbivory when these trees matured and produced smaller growth rings. On the whole, these results provide evidence that some long-living wood-boring larvae may perform better in large host trees than in slow-growing trees. Future studies should examine larval performance in host trees undergoing different intrinsic growth patterns (e.g., provenance experiments).
Although, the differences in the trunk diameter and volume of trees from fertilized and non-fertilized plots were significant (see Turtola et al. 2002; Heijari et al. 2005), fertilization treatments did not cause any changes in carbon allocation to secondary compounds in the xylem. Total resin acid concentration per volume of xylem (mean ± SE) in SITE3 trees (3.8 ± 0.5 mg cm−3 dry weight) was significantly (F 2, 15 = 7.568, P = 0.005) higher than in SITE2 trees (2.5 ± 0.2 mg cm−3 dry weight) and also marginally (P = 0.061) higher than in SITE1 trees (2.7 ± 0.4 mg cm-3 dry weight). We observed that the total monoterpene concentration of sapwood had a negative effect on the growth rate of neonatal larvae (Fig. 2), whereas the concentration of resin acids did not affect larval growth. This result is consistent with earlier studies, which indicate that specific constitutive defense chemicals have effects on the performance of H. bajulus neonatal larvae and that early instars are more sensitive to host plant quality than late instars (Montgomery 1982), with high mortality occurring generally among early instars (Preszler and Price 1988). Furthermore, in SITE3, the generally lower growth and smaller diameter of the trees might be the reason for the high content of resin acids per volume of xylem. The inconsistent results among sites may also be caused by the fact that all of the sites were Myrtillus type forests, and thus might have been more nutrient-rich than the pines generally grown in Finland (Saarsalmi and Mälkönen 2001). Our study showed that the effect of fertilization on xylem constitutive defense was not significant. The genetic structure of the investigated stands might have differed substantially, and thus it is unlikely that the results would have differed in other tree-growing areas in Finland. Concomitantly, our study provides support for the conclusion of Herms (2002), who proposed that in general, fertilization treatments neither enhance nor reduce pest resistance in woody plants.
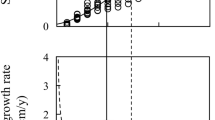
At the breast (H1) and canopy height (H2), the fertilization treatments had no significant effects on tracheid length (data not shown). In SITE3, tracheids in xylem collected from H1 and H2 were significantly (F 2, 15 > 5.141, P < 0.05 and F 2, 15 > 4.915, P < 0.023) longer than in trees growing at the other sites. In the pooled data, there was a negative correlation between the growth of annual rings and tracheid length (Fig. 3), but this was not associated with the fertilization treatments. Similarly, a fertilization experiment with Norway spruce (Picea abies) observed decreased tracheid length (Mäkinen et al. 2002). Lucas et al. (2000) detected differences between the mechanical properties of xylem, and this in turn had an impact on the performance of herbivores. We found that the RGR of I2L exhibited a significant positive correlation (r = 0.359, P = 0.009, N = 53) with the latewood tracheid length, and xylem consumption of I3L showed a positive correlation (r > 0.542, P < 0.030, N = 16) with both early- and latewood breast height tracheid lengths. These results indicate that cell properties could influence the growth of late instar larvae, but not the growth of the neonatal larvae. Furthermore, xylem cell properties may explain some of the differences occurring in growth performance of xylophagous H. bajulus larvae.
Linear correlation between tracheid length (mm) and annual diameter growth (mm) at breast height in early (closed circles) and latewood (open circles) of Scots pine. Data (pooled from all sites) are from 12th, 30th, and 40th annual rings of xylem. Equations for relationships and R 2 are shown (y EW=earlywood and y LW=latewood), P < 0.05
In summary, the nitrogen concentration of xylem had a positive effect on wood borer performance, but the impact was stronger on early instar than on late instar H. bajulus larvae. It is likely that fertilization treatments induce changes in the growth and nutrient concentrations of xylem, but that these do not lead to any major changes in tracheid properties or in constitutive defense level of xylem. The effect of long-term fertilization on the performance of wood-boring xylophagous H. bajulus larvae was notable, and our results suggest that variation in the growth of Scots pine forests can have effects on the utilization of xylem by H. bajulus larvae.
References
Allison, J. D., Borden, J. H., and Seybold, S. J. 2004. A review of the chemical ecology of the Cerambycidae (Coleoptera). Chemoecology 14:123–150.
Berry, R. W. 1972. A rearing procedure for the house longhorn beetle Hylotrupes bajulus L. Int. Biodeterior. Biodegrad. 8:141–144.
European Standard EN 47. 1988. Wood preservatives; determination of the toxic values against larvae of Hylotrupes bajulus (Linnaeus) (Laboratory method). European Committee for Standardization, Brussels.
Finér, L., and Kaunisto, S. 2000. Variation in stemwood nutrient concentrations in Scots pine growing on peatland. Scand. J. Forest Res. 15:424–432.
Gershenzon, J., and Croteau, R. 1991. Terpenoids, pp. 165–219, in G. A. Rosenthal and M. R. Berenbaum (eds.). Herbivores, their interactions with secondary plant metabolites. Academic Press, New York.
Hanks, L. M. 1999. Influence of the larval host plant on reproductive strategies of Cerambycid beetles. Annu. Rev. Entomol. 44:483–505.
Hanks, L. M., Paine, T. D., and Millar, J. G. 2005. Influence of the larval environment on performance and adult body size of the wood-boring beetle Phoracantha semipunctata. Entomol. Exp. Appl. 114:25–34.
Heijari, J., Nerg, A.-M., Kaakinen, S., Vapaavuori, E., Raitio, H., Levula, T., Viitanen, H., Holopainen, J. K., and Kainulainen, P. 2005. Resistance of Scots pine wood to Brown-rot fungi after long-term forest fertilization. Trees 19:729–735.
Helmisaari, H.-S., and Siltala, T. 1989. Variation in nutrient concentration of Pinus sylvestris stems. Scand. J. Forest Res. 4:443–451.
Herms, D. A. 2002. Effects of fertilization on insect resistance of woody ornamental plants: Reassessing an entrenched paradigm. Environ. Entomol. 31:923–933.
Holm, C., and Ekbom, P. 1958. The significance of house longhorn beetle as a destroyer of buildings and its control. State Institute of Technical Research, Helsinki.
Holopainen, J. K., Rikala, R., Kainulainen, P., and Oksanen, J. 1995. Resource partitioning to growth, storage and defence in nitrogen fertilized Scots pine and susceptibility of the seedlings to the tarnished plant bug Lygus rugulipennis. New Phytol. 131:521–532.
Körting, A. 1972. Über Wachstumsunterschiede von Eilarven des Hausbockes (Hylotrupes bajulus L.) bei Aufzucht in den rindennahen Holzzonen von Kiefernstämmen. Z. Angew. Entomol. 72:149–156.
Kytö, M., Niemelä, P., and Larsson, S. 1996. Insects on trees: population and individual response to fertilization. Oikos 75:148–159.
Linsley, E. G. 1959. Ecology of Cerambycidae. Annu. Rev. Entomol. 4:99–138.
Ljungkvist, H. 1983. The discovery of the house longhorn beetle in a natural biotope. Entomol Tidskr 104:35.
Lucas, P. W., Turner, I. M., Dominy, N. J., and Yamashita, N. 2000. Mechanical Defence to Herbivory. Annu. Bot. (Lond.) 86:913–920.
Mäkinen, H., Saranpää, P., and Linder, S. 2002. Effect of growth rate on fibre characteristics in Norway spruce (Picea abies (L.) Karst.). Holzforschung 56:449–460.
Manninen, A.-M., Tarhanen, S., Vuorinen, M., and Kainulainen, P. 2002. Comparing the variation of needle and wood terpenoids in Scots pine provenances. J. Chem. Ecol. 28:211–228.
Mattson, W. J. 1980. Herbivory in relation to plant nitrogen content. Annu. Rev. Ecol. Syst. 11:119–161.
Montgomery, M. E. 1982. Life-cycle nitrogen budget for the gypsy moth, Lymantria dispar, reared on artificial diet. J. Insect Physiol. 28:437–442.
Nerg, A.-M., Heijari, J., Noldt, U., Viitanen, H., Vuorinen, M., Kainulainen, P., and Holopainen, J. K. 2004. Significance of wood terpenoids in the resistance of Scots pine provenances against the old house borer, Hylotrupes bajulus, and brown-rot fungus, Coniophora puteana. J. Chem. Ecol. 30:125–142.
Preszler, R. W., and Price, P. W. 1988. Host quality and sawfly populations: a new approach to life table analysis. Ecology 69:2012–2020.
Robinson, W. H., and Cannon, K. F. 1979. The life history and habits of the old house borer, Hylotrupes bajulus (L.), and its distribution in Pennsylvania. Melsheimer Entomol. Ser. 27:30–34.
Ruel, J., and Whitham, T. G. 2002. Fast-growing juvenile pinyons suffer greater herbivory when mature. Ecology 83:2691–2699.
Saarsalmi, A., and Mälkönen, E. 2001. Forest fertilization research in Finland: A literature review. Scand. J. Forest Res. 16:514–535.
Turtola, S., Manninen, A.-M., Holopainen, J. K., Levula, T., Raitio, H., and Kainulainen, P. 2002. Secondary metabolite concentrations and terpene emissions of Scots pine xylem after long-term forest fertilization. J. Environ. Qual. 31:1694–1701.
Waldbauer, G. P. 1964. The consumption, digestion and utilization of solanaceous and non-solanaceous plants by larvae of the tobacco hornworm, Protoparce sexta (Johan). (Lepidoptera: Sphingidae). Entomol. Exp. Appl. 7:253–269.
Acknowledgments
The Graduate School in Forest Sciences and The Academy of Finland (Research Council for Biosciences and Environment, projects 45066 and 43159) have financially supported this research. We thank the Finnish Forest Research Institute and Raino Lievonen for organizing the felling of trees and transportation of the xylem disks. We thank also Terhi Vuorinen, Jaana Rissanen, and Juhani Tarhanen for helping in the chemical analysis and Ewen MacDonald for revising the language of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heijari, J., Nerg, AM., Kainulainen, P. et al. Effect of Long-Term Forest Fertilization on Scots Pine Xylem Quality and Wood Borer Performance. J Chem Ecol 34, 26–31 (2008). https://doi.org/10.1007/s10886-007-9395-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-007-9395-x