Abstract
A growing body of evidence indicates that odors are used in individual, sexual, and species recognition in vertebrates, and may be reliable signals of quality and compatibility. Petrels are seabirds that exhibit an acute sense of smell. During the breeding period, many species of petrel live in dense colonies on small oceanic islands and form pairs that use individual underground burrows. Mates alternate between parental duties and foraging trips at sea. Returning from the ocean at night (to avoid bird predators), petrels must find their nest burrow. Antarctic prions, Pachyptila desolata, are thought to identify their nest by recognizing their partner’s odor, suggesting the existence of an individual odor signature. We used gas chromatography and mass spectrometry to analyze extracts obtained from the feathers of 13 birds. The chemical profile of a single bird was more similar to itself, from year to year, than to that of any other bird. The profile contained up to a hundred volatile lipids, but the odor signature may be based on the presence or absence of a few specific compounds. Our results show that the odor signature in Antarctic prions is probably endogenous, suggesting that in some species of petrels it may broadcast compatibility and quality of potential mates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Optimal mate choice relies on signals that broadcast compatibility and quality of potential mates. The interaction of such signals with the evolution of mate choice is largely acknowledged on the grounds of theoretical considerations and empirical demonstrations involving mainly visual- or acoustic-based communication systems (Maynard Smith and Harper 2003). Nevertheless, a growing body of evidence indicates that odors are broadly used in individual, sexual, and species recognition in vertebrates, and may be reliable signals of quality and compatibility (Wyatt 2003).
Birds were once considered to have a poor sense of smell, but more recent findings have modified the knowledge of this subject. Many birds species are sensitive to odors, and actually use olfaction in some behavioral tasks (for a review, see Roper 1999). However, compared to other organisms, information on the production or social use of self-produced odor compounds in birds has been largely neglected. Recent studies suggest that odor may play an important role in mate choice of birds (Zelano and Edwards 2002; Bonadonna and Nevitt 2004; Soini et al. 2007).
Procellariiform seabirds (petrels and albatrosses) have the most highly developed olfactory anatomy and neuroanatomy known in birds (Bang 1966). The first suggestion of the function of this anatomy was for finding food (Grubb 1972), and this was subsequently shown for many petrel species (reviewed by Roper 1999 and Nevitt and Bonadonna 2005a). Odors may also help petrels and albatrosses to navigate when flying across the oceans (Nevitt and Bonadonna 2005b).
Within species, petrels are faithful for life to their breeding colony and to their partner. In many species, pairs breed in underground burrows. Monogamous pairs lay a single egg, and partners alternate parental duties and foraging trips to sea (Warham 1996). Returning from the sea, many burrowing petrel species approach the colony and nest at night (to avoid avian predators, Mougeot and Bretagnolle 2000). Odors play a fundamental role in finding the burrow, and an odor signature of the nest facilitates burrow recognition in the dark (Bonadonna et al. 2003, 2004).
We recently showed that Antarctic prions, Pachyptila desolata, were able to recognize their own as well as their partner’s odor, suggesting that personal odors may play a role in the nest’s olfactory signature. This was the first evidence of individual recognition through odor in a bird species. Moreover, quite unexpectedly, we discovered a pattern of scent discrimination that is well-documented in mammals: Antarctic prions avoided self-odor when presented with the scent of another conspecific (Bonadonna and Nevitt 2004). Such behavior was first reported in mice, and was related to major histocompatibility complex genes (MHC), kin recognition, and inbreeding avoidance (Manning et al. 1992). This could also be the case in petrels, as high phylopatry may expose them to the risk of inbreeding and should select for mechanisms that allow for kin discrimination (Zelano and Edwards 2002).
Even after years of preservation, collected petrel feathers smell distinctive to the human nose (Bonadonna, personal observation). Birds protect and waterproof their feathers by preening them with the products of the uropygial gland (Stettenheim 1972). These products may carry odors that play a role in the behavior of birds (Soini et al. 2007). For example, odors produced by this gland play a role in sexual behavior of domestic ducks, Anas platyrhynchos (Balthazart and Schoffeniels 1979; Jacob et al. 1979). In petrels, the products of this gland may be species-specific (Jacob and Hoerschelmann 1982) and may constitute an olfactory signature on petrel feathers.
We hypothesized that feather scents of Antarctic prions constitute an individual olfactory signature, and we predicted that the scents produced by the feathers of the same bird in two different years should be more similar to each other than the scents produced by the feathers of two different birds in the same year. We report the results of our study herein.
Methods and Materials
Feather samples
Feather samples were collected on Ile Verte (49°51′S, 70°05′E) in the Kerguelen Archipelago (southern Indian Ocean). The first sample was collected in the 2002–2003 austral summer from 10 pairs of ringed Antarctic prions, P. desolata, (20 birds), and again in the 2003–2004 and 2004–2005 austral summers (hereafter called years). We thus obtained feather samples for three consecutive years on seven females and six males (individuals may not survive and pairs may not nest every year, Warham 1996). Fifty to 200 mg of feathers were cut from the duvet around the uropygial gland with steel scissors cleaned in methanol between samples. Feathers were stored in aluminum paper, in a closed plastic bag, and refrigerated. Plastic bags were opened just before chemical analysis.
Chemical analysis
Feathers of each sample were weighed and immersed in 4 ml dichloromethane for 24 hr. After this, the feathers were removed, and the solvent allowed to evaporate to 250 μl in a fume hood. Twenty microliters of n-dodecane (purity 99.8%, Sigma Aldrich Switzerland) in dichloromethane (200 μg/ml) were added to each extract as an internal standard. The samples were analyzed on a Varian CP3800 gas chromatograph (Varian, Palo Alto, CA, USA), equipped with a flame ionization detector and a Varian CP-Sil 5 CB Low Bleed MS (25 m × 0.25 mm, ID, film thickness 0.25 μm) column. The oven was programmed as follows: 50°C for 2 min, 3°C min−1 to 100°C, 2.7°C min−1 to 140°C, 2.4°C min−1 to 170°C, and 10°C min−1 to 290°C. An on-column injector at 250°C was used with helium (1 ml min−1) as carrier gas. To determine the identity of compounds, gas chromatography–mass spectrometry was employed using a Varian CP3800 coupled to a Varian Saturn 2000 ion trap mass spectrometer equipped with splitless injection and the same capillary column as above. The scan range of the ion trap was 38 to 350 m/z. The oven temperature program was the same as that above except the initial temperature of 50°C was held for 3 min. Total ion chromatograms and mass spectra were recorded in the electron impact ionization mode at 70 eV. Identification of compounds was based on mass spectral fragmentation pattern, using various libraries [Wiley 138 and NBS 75K libraries (McLafferty 1998), NIST98 (Stephen 1998), and the compilation by Adams (1995)], and chromatographic retention index (RI).
Selection of compounds and statistical analyses for comparison of chemical profiles
We concentrated our analysis on compounds with retention times between 3 and 40 min in the FID chromatogram, on the basis that compounds with RI of greater than 1,700 on an apolar column do not have sufficient vapor pressure to be perceived by birds. Birds do not have a “wet” nose such as some mammals (e.g., dogs, mice, cats, etc) or a vomeronasal organ (Bang and Wenzel 1985) and, therefore, are likely only to perceive molecules that are borne by an air flow. From the 39 samples, we distinguished 99 different peaks; some were found in most samples, others in only a few. We considered each compound as a distinct dependent variable (99 variables) and each bird in each year (hereafter called bird/year) as a sampling unit (39 units). The data set contained a large number of potential dependent variables compared to the low number of sampling units. Therefore, we reduced the number of compounds (dependent variables) by using two different working hypotheses. Firstly, we hypothesized that the olfactory signature of birds is a bouquet composed of compounds present in all birds in different relative concentrations (Alberts 1992). Compounds of high occurrence (HOC) were selected as follows. We considered the third year profile of a bird (i.e., the profile obtained from the most recent feather sample). We used a compound only if it was present in the third year profile (i.e., the most recent feather sample) of at least 10 individual birds; 28 compounds fulfilled this criterion. Then, for all 13 birds and for each year, we considered the amount of these compounds (amount = 0 if compound was absent) and ran a principal component analysis (PCA; proc PRINCOMP in SAS 8.2) on the correlation matrix. This generated synthetic dependent variables that captured systematic associations among compounds.
Secondly, we hypothesized that the olfactory signature of birds is a bouquet composed of compounds present in a few birds, but with different association patterns, independent of compound concentration (Alberts 1992). Compounds of low occurrence (LOC) were selected as follows. We used a compound only if it was present in the third year profile of, at most, 5 individual birds; 21 compounds fulfilled this criterion. For all 13 birds and for each year, we ran a multiple correspondence analysis (MCA; proc CORRESP in SAS 8.2) on the contingency table to generate synthetic dependent variables that captured systematic presence/absence associations among compounds.
For each synthetic variable from PCA and MCA analyses, we estimated the variance among birds over years and the variance among years with respect to birds. These variances were accounted for in mixed effects linear models (proc MIXED in SAS 8.2) by the random factors “bird” and “year,” respectively. An estimate of the residual variance was also provided. The measure of repeatability of the olfactory signature of an individual bird was computed as the ratio of the variance among birds consistent over years (i.e., “bird random effect”) to total variance (intraclass correlation coefficient [ICC]). The blend of volatile compounds was considered as an individual olfactory signature if the variance component “bird” differed significantly from zero and if the ICC was higher than 50%. The fixed factor “sex” was also included in the models.
Results and Discussion
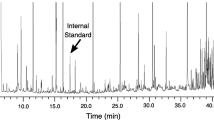
Representative chromatograms obtained from extracts of a single bird in each of the 3 yr are given (Fig. 1). The compounds identified in our analyses (Table 1) and used in our PCA and MCA were lipids. This is not surprising, considering that these compounds were probably derived from the uropygial gland, which principally produces waxes and greases to protect feathers (Jacob and Ziswiler 1982). Among the identified volatile compounds are fatty alcohols, fatty aldehydes, and hydrocarbons (Table 1).
Representative flame ionization detection chromatograms for Antarctic prion number KA26622. a, b, and c Chromatograms obtained with feathers of the first, second, and third year respectively. In the third year chromatogram, numbers on peaks refer to Table 1
Lipids are important as odors of vertebrates (Alberts 1992; Hagelin et al. 2003; Burger 2005), and some of the compounds we identified in Antarctic prions have already been identified as mammalian semiochemicals. For example, alkanes, such as decane and octane, are specific odor compounds from forehead hairs of white-tailed deer, Odocoileus virginianus (Gassett et al. 1997) and interdigital secretions of bontebok, Damaliscus dorcas (Burger et al. 1999). Aldehydes such as (E)-2-hexenal, dodecanal, and nonanal are present in urine of deer mouse, Peromyscus maniculatus (Ma et al. 1999) or in bontebok, (Burger et al. 1999), and alcohols such as dodecanol, heptanol, and tetradecanol are present in the preorbital secretion of grysbok, Raphicerus melanotis (Burger et al. 1996). Given the structure of these compounds and their function in other vertebrates, it seems likely that these compounds are produced endogenously through the oxidation or reduction of fatty acids. This is consistent with recent findings in other birds such as the dark-eyed junco, Junco hyemalis or green woodhoopoe, Phoeniculus purpureus (Burger et al. 2004; Soini et al. 2007).
Analysis of the variation in HOC
The first three PCA components each accounted for a noticeable percentage (>25%) of the variation in the concentration of each of the 28 compounds selected. These three components represented variation in the concentration of 24, 3, and 1 distinct compounds and accounted for 76%, 8%, 5% of the total variation in concentration of compounds, respectively. For these three synthetic variables, the variance estimates for the factors “bird” and “year” did not differ significantly from zero. The variation among birds over years accounted for a small fraction of the total variation with the possible exception of the second principal component (Table 2). It is possible that fatty acids present on feather samples collected in the first 2 yr continued to be modified during preservation, changing the relative quantity of derived “signature compounds”. No sex effect was detected for any of the three synthetic variables.
According to these results, variation in the concentration of HOC was not likely to constitute an individual olfactory signature. However, we cannot exclude the possibility that our sampling protocol was not subtle enough to detect differences and that a possible signature of same compounds at different relative concentration exists. More sophisticated individual odor sampling protocols are needed to test this.
Analysis of the variation in LOC
The first four MCA dimensions accounted for a substantial percentage (>20%) of the variation in the presence/absence of each of the 21 compounds selected. These four dimensions represented the variation in the presence/absence of 9, 4, 2, and 5 distinct compounds and accounted for 35%, 19%, 10%, and 8% of the total variation in the presence/absence of compounds, respectively. For the first two of these synthetic variables, variation among birds over years (“bird” factor) was statistically significant and accounted for a large fraction of the total variation (Table 3). Identified compounds responsible for the statistical difference were isododecane, 3-methylundecanoic acid, (E)-2-octenal, and isotridecane for the first synthetic variable, and octane, isopentadecane, (E)-2-dodecenol, and tetradecanal for the second synthetic variable. Therefore, we believe that these LOC compounds may constitute an individual olfactory signature repeatable over years (ICC = 0.66 and 0.51, respectively) that distinguishes individuals (“bird” effect P = 0.024 and 0.04, respectively; Fig. 2).
a Volatile compound profile of 13 birds (letter) over 3 yr (number) as described by 2 synthetic variables derived through MCA analysis from the variation in the presence/absence of 21 volatile LOCs. Open symbols represent females; filled symbols represent males. b Same representation as in a but only selected birds are presented to better show clustering
The effect of the sex of the bird was close to being significant for the third synthetic variable (P = 0.051; Table 3). However, in recent experiments in which odors of male and female birds were presented simultaneously in a Y-maze, neither male nor female Antarctic prions showed any preferences (Bonadonna et al. unpublished data). More work is needed to resolve this discrepancy.
In summary, our results, although preliminary and lacking direct behavioral confirmation for the compounds, suggest the existence of an endogenous individual olfactory signature in Antarctic prions, originating from the uropygial gland: an individual is more similar to itself with regard to volatile compounds, from year to year, than it is to a conspecific. This is consistent with behavioral studies on partner-odor recognition (Bonadonna and Nevitt 2004). As this odor is likely endogenous, it might also broadcast the compatibility and quality of a potential mate as previously proposed (Zelano and Edwards 2002). To our knowledge, this is the first demonstration of a putative odor signature in birds.
References
Adams, R. P. 1995. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy. Allured Publishing Corporation, Carol Stream, IL.
Alberts, A. C. 1992. Constraints on the design of chemical communication-systems in terrestrial vertebrates. Am. Nat. 139:S62–S89.
Balthazart, J., and Schoffeniels, E. 1979. Pheromones are involved in the control of sexual behavior in birds. Naturwissenschaften 66:55–56.
Bang, B. G. 1966. The olfactory apparatus of tubenosed birds (Procellariiformes). Acta Anat. 65:391–415.
Bang, B. G., and Wenzel, B. M. 1985. Nasal cavity and olfactory system, pp. 195–225, in A. S. King and J. McLelland (eds.). Form and Function in Birds, vol. 5. Academic, London.
Bonadonna, F., and Nevitt, G. A. 2004. Partner-specific odor recognition in an Antarctic seabird. Science 306:835.
Bonadonna, F., Hesters, F., and Jouventin, P. 2003. Scent of a nest: discrimination of own-nest odours in Antarctic prions, Pachyptila desolata. Behav. Ecol. Sociobiol. 54:174–178.
Bonadonna, F., Villafane, M., Bajzak, C., and Jouventin, P. 2004. Recognition of burrow’s “olfactory signature” in blue petrels, Halobaena caerulea: an efficient discrimination mechanism in the dark. Anim. Behav. 67:893–898.
Burger, B. V. 2005. Mammalian semiochemicals. Chemistry of Pheromones and Other Semiochemicals II 240:231–278.
Burger, B. V., Tien, F. C., Le Roux, M., and Mo, W. P. 1996. Mammalian exocrine secretions.10. Constituents of preorbital secretion of grysbok, Raphicencs melanotis. J. Chem. Ecol. 22:739–764.
Burger, B. V., Nell, A. E, Spies, H. S. C., Le Roux, M., Bigalke, R. C., and Brand, P. A. J. 1999. Mammalian exocrine secretions. XII: constituents of interdigital secretions of bontebok, Damaliscus dorcas dorcas, and blesbok, D-d. phillipsi. J. Chem. Ecol. 25:2057–2084.
Burger, B. V., Reiter, B., Borzyk, O., and Du Plessis, M. A. 2004. Avian exocrine secretions. I. Chemical characterization of the volatile fraction of the uropygial secretion of the green woodhoopoe, Phoeniculus purpureus. J. Chem. Ecol. 30:1603–1611.
Gassett, J. W., Wiesler, D. P., Baker, A. G., Osborn, D. A., Miller, K. V., Marchinton, R. L., and Novotny, M. 1997. Volatile compounds from the forehead region of male white-tailed deer (Odocoileus virginianus). J. Chem. Ecol. 23:569–578.
Grubb, T. C. 1972. Smell and foraging in shearwaters and petrels. Nature 237:404–405.
Hagelin, J. C., Jones, I. J., and Rasmussen, L. E. L. 2003. A tangerine-scented social odour in a monogamous seabird. Proc. R. Soc. Lond. B 270:1323–1329.
Jacob, J., and Hoerschelmann, H. 1982. Chemotaxonomische Untersuchungen Zur Systematik Der Rohrennasen (Procellariiformes). J. Ornithol. 123:63–84.
Jacob, J., and Ziswiler, V. 1982. The Uropygial Gland, pp. 199–324, in D. S. Farner, J. R. King, K. C. Parkes (eds). Avian Biology, vol. 6. Academic, New York.
Jacob, J., Balthazart, J., and Schoffeniels, E. 1979. Sex differences in the chemical composition of uropygial gland waxes in domestic ducks. Biochem. Syst. Ecol. 7:149–153.
Ma, W. D., Wiesler, D., and Novotny, M. V. 1999. Urinary volatile profiles of the deermouse (Peromyscus maniculatus) pertaining to gender and age. J. Chem. Ecol. 25:417–431.
Manning, C. J., Wakeland, E. K., and Potts, W. K. 1992. Communal nesting patterns in mice implicate MHC genes in kin recognition. Nature 360:581–583.
Maynard Smith, J., and Harper, D. 2003. Animal Signals. Oxford University Press, Oxford.
Mclafferty, F. W. 1998. Wiley/NBS Registry of Mass Spectral Data, 4th ed. Wiley, New York, USA.
Mougeot, F., and Bretagnolle, V. 2000. Predation as a cost of sexual communication in nocturnal seabirds: an experimental approach using acoustic signals. Anim. Behav. 60:647–656.
Nevitt, G. A. and Bonadonna, F. 2005a. Seeing the world trough the nose of a bird: New developments in the sensory ecology of procellariiform seabirds. Marine Ecol. Prog. Ser. 287:292–295.
Nevitt, G. A. and Bonadonna, F. 2005b. Sensitivity to dimethyl sulphide suggests a mechanism for olfactory navigation by seabirds. Biol. Lett. 1:303–305.
Roper, T. J. 1999. Olfaction in birds. Adv. Study Behav. 28:247–332.
Soini, H. A., Schrock, S. E., Bruce, K. E., Wiesler, D., Ketterson, E. D., and Novotny, M. V. 2007. Seasonal variation in volatile compound profiles of preen gland secretions of the dark-eyed junco (Junco hyemalis). J. Chem. Ecol. 33:183–198.
Stephen, S. 1998. NIST98 Mass Spectral Library. The NIST Mass Spectroscopy Data Center Gaithersburg, MD.
Stettenheim, P. 1972. The integument of birds, pp.1–63, in D. S. Farner, J. R. King (eds). Avian Biology. Academic, New York.
Warham, J. 1996. The Behaviour, Population Biology and Physiology of the Petrels, p. 613. Academic, London.
Wyatt, T. D. 2003. Pheromones and Animal Behaviour. Cambridge University Press, Cambridge.
Zelano, B., and Edwards, S. E. 2002. An Mhc component to kin recognition and mate choice in birds: predictions, progress, and prospects. Am. Nat. 160:225–238.
Acknowledgments
This study was supported by the Institut Polaire Français Paul Emile Victor (IPEV, Program no. 354) and performed in adherence to the IPEV/CNRS ethical guidelines. We thank B Buatois for the help with the gas chromatography; Dr. G Nevitt, C Bajzak, and Dr. S Caro for the help in the field; Prof. F S Dobson, Dr. P D’Ettorre, and Dr. M-C Anstett and two anonymous reviewers for the critical review of an earlier draft of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bonadonna, F., Miguel, E., Grosbois, V. et al. Individual Odor Recognition in Birds: An Endogenous Olfactory Signature on Petrels’ Feathers?. J Chem Ecol 33, 1819–1829 (2007). https://doi.org/10.1007/s10886-007-9345-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-007-9345-7