Abstract
The production of salicylihalamide A by the marine sponge Haliclona sp. was investigated. Samples of the two morphologies (green and brown) were collected from four locations covering approximately 1,200 km of coastline. Temporal variation between winter and summer was also examined at Bremer Bay. Chemical profiling by using liquid chromatography coupled with ultra violet detection and mass spectrometry showed that salicylihalamide A was produced only by the green morphology. Salicylihalamide A concentration was significantly correlated to water temperature but not to the size or depth of the sponge. Salicylihalamide A concentration was found to differ significantly among locations (Bremer Bay 13.5 μg g−1, Hamelin Bay 11 μg g−1, Rottnest Island 9.9 μg g−1, and Jurien Bay 8.5 μg g−1) partially accounted for by the influence of water temperature. A difference between seasons was also observed in Bremer Bay (summer concentration of 13.5 μg g−1 vs. winter concentration of 8.2 μg g−1). Environmental and physiological factors appear to be important in the production of salicylihalamide A by the green morphology. Additionally, the brown morphology does not produce salicylihalamide A, thus adding to the evidence that this morphology may be a different species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sponges continue to be a rich source of marine natural products (Munro et al. 1999; McClintock and Baker 2001; Paul et al. 2006). Biologically active substances have been found in all orders of demosponges (McClintock and Baker 2001), a large number of which have potential for development into bioproducts (Sipkema et al. 2005; Paul et al. 2006). The temperate marine sponge Haliclona sp. (Demospongiae; Haplosclerida; Chalinidae) has attracted considerable attention because of its production of the potent cytotoxic natural product salicylihalamide A (Fig. 1). Salicylihalamide A represents a class of compounds that has a unique chemical scaffold different from that of known antitumor compounds (Erickson et al. 1997). Its mode of action is via the inhibition of mammalian vacuole ATPase activity, which is implicated in pathological processes such as osteoporosis, renal disease, HIV infection, and tumor metastasis (Boyd et al. 2001). Salicylihalamide A is currently undergoing preclinical trials; however, its success as a drug is hindered by lack of a reliable supply (Newman and Cragg 2004). Whereas salicylihalamide A has been synthesized (Wu et al. 2000), this is currently not an economically viable option for supply. The greatest potential for securing commercial quantities of salicylihalamide A is through harvesting either wild or cultured biomass (Munro et al. 1999), highlighting the importance of developing an understanding of the natural concentrations of the compound (Munro et al. 1999).
New populations of Haliclona sp. have been discovered since the sponge was initially collected in 1989 at Rottnest Island (Erickson et al. 1997). Closer inspection of these new populations has resulted in the identification of two morphologies that have similar spicule and skeletal characteristics (unpublished data). The first morphology (green Haliclona) has a deep green coloration, an amorphous shape, and varying numbers of oscules. The oscules are chimney-like in shape and extend outwards at least 20 mm from the main sponge. The sponge mesohyl is open with large internal canals present. The second morphology (brown Haliclona) has a brown coloration, a mound shaped body, and small, slightly raised oscules that extend less than 5 mm above the surface. The oscules are numerous, and the sponge has a compact mesohyl with few internal canals. Closer inspection of the original voucher sample showed that salicylihalamide A was isolated from the green morphology. The brown morphology has not yet been examined. Understanding the natural concentrations of secondary metabolites provides information on both the biology and ecology of the source organism, such as the relationships between populations and individuals (Turon et al. 1996; Lopez-Legentil et al. 2005), the physiology of the source organism (Turon et al. 1996; Duckworth and Battershill 2001), and environmental influences (Thompson et al. 1987; Maida et al. 1993; Page et al. 2005). Limited ecological information exists for both morphologies of Haliclona sp., with most literature restricted to the chemistry of the green Haliclona.
Spatial variation in concentrations of secondary metabolites has been documented in several cnidarians (Harvell et al. 1993; Maida et al. 1993; Kelman et al. 2000; Marti et al. 2005), bryozoans (Mendola 2003; Marti et al. 2005), tunicates (Lopez-Legentil et al. 2005; Marti et al. 2005), and sponges (Thompson et al. 1987; Becerro et al. 1995; Page et al. 2005). Fewer studies have examined temporal variation (Turon et al. 1996; Duckworth and Battershill 2001; Marti et al. 2005; Page et al. 2005). Chemical variability has been attributed to several factors such as hydrodynamic characteristics (Duckworth et al. 2004; Page et al. 2005), depth (Thompson et al. 1987; Harvell et al. 1993; Page et al. 2005), competition (Maida et al. 1993; Turon et al. 1996), size (Maida et al. 1993; Becerro et al. 1995), and habitat (Becerro et al. 1995).
The primary goal here was to determine whether both morphologies of Haliclona sp. produce salicylihalamide A. Secondary to this was to describe the spatial and temporal patterns of salicylihalamide A production in the recently discovered Haliclona sp. populations at Hamelin Bay, Jurien Bay, and Bremer Bay, and the ecological implications. This is the first study of the variability of a bioactive compound in a marine sponge from the west coast of Australia.
Materials and Methods
Collection
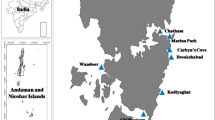
Collections were made haphazardly by SCUBA at each of the four locations (Bremer Bay—34.45°S, 119.38°E; Hamelin Bay—34.20°S, 115.04°E; Rottnest Island—32.00°S, 115.52°E; and Jurien Bay—30.275°S, 115.02°E) along the south western coast of Western Australia (Fig. 2) during the Australian summer (December–February). Winter (June–August) samples were collected only from Bremer Bay because of unfavorable diving conditions at the three other locations. Sponge size was determined before sampling by using an underwater stereo camera to capture a series of stereo images of each sponge (Abdo et al. 2006b). Each set of images was processed in the laboratory to obtain a volume estimate for each sponge (Abdo et al. 2006b). Samples (approximately 9 cm3 in size; N = 5, except Rottnest Island where N = 6) of both the green and brown morphologies were excised and placed in individual vials and frozen at −20°C. The depth of each sponge, the number of necrotic areas, and the water temperature were also recorded.
Chemical Extraction and Analysis
The concentration of salicylihalamide A was determined by using high performance liquid chromatography (HPLC) coupled with ultra violet (UV) detection and mass spectrometry (MS). Each frozen sample (approximately 8 cm3) was cut into small pieces, placed within preweighed and labeled vials, and freeze-dried for 48 h. The dry weight was recorded (883.29 ± 31.97 mg [mean±s.e.]), and each sample was extracted exhaustively ×3 by addition of 10 ml of ethanol and sonicated for 30 min during each extraction. The supernatant was filtered under vacuum, concentrated in vacuo, freeze-dried, and weighed (67.63 ± 3.45 mg [mean±s.e.]). Samples were dissolved in methanol to give a concentration of 10 mg ml−1. A 160-μl aliquot of each sample was combined with 40 μl (equivalent to 0.5 mg ml−1) of the internal standard, coumarin (C9H6O2). For each sample, 40 μl (equivalent to 8 μg μl−1 of crude extract) were injected onto a Gemini 3 μ C18 110 Å 50 × 4.6-mm column (25°C) at a flow rate of 1 ml min−1 with a gradient of 50% acetonitrile (ACN): water to 100% ACN over 40 min by using an Agilent 1100 HPLC system (comprising a degasser, binary pump, PDA [for ultra violet detection] and a Gilson 215 Liquid Handler auto sampler/collector). The eluant was directed to a Bruker Daltonics Esquire3000plus mass spectrometer with an Apollo source operating at 40 eV to measure low resolution mass spectral data.
The concentration of salicylihalamide A was quantified by calculating the integral of the peak area at the retention time of 12 min and λ = 285 nm (in relation to the integral of the coumarin peak area at 5 min and λ = 285 nm) for each sample and converted to a concentration (μg g−1 of dry weight) by reference to a standard curve.
Data Analysis
Pearson’s correlation coefficient (r; Zar 1999) was used to examine correlations between the concentration of salicylihalamide A and water temperature, size, and depth of the sponge. Correlations between water temperature, sponge size, and depth were also examined to ensure that each variable did not influence the other.
The influence of any significant correlations of water temperature, depth, and sponge size with salicylihalamide A concentration was removed with a one-way analysis of covariance (ANCOVA; Zar 1999) to determine if variation in salicylihalamide A concentration occurred among locations (fixed factor, four levels). A two sample t-test was used to examine for variation in the concentration of salicylihalamide A between summer and winter (Zar 1999). For all analyses, data were checked for normality and homoscedascity before analysis, and Bonferonni corrections were applied to account for multiple comparisons (Zar 1999). To determine if the health of the sponge influences the concentration of salicylihalamide A, a two sample t-test was used to compare the salicylihalamide A concentration of healthy (necrotic tissue absent) and unhealthy (necrotic tissue present) sponges.
Results
Differences Between Morphologies
Salicylihalamide A was produced by all samples of the green Haliclona collected from the four locations and during both summer and winter seasons at Bremer Bay. The mean concentration of salicylihalamide A within green samples was 10.7 μg g−1 (±1.1 μg g−1). The mean yield of salicylihalamide A from the crude extract was 2.83% (±0.28%). Salicylihalamide A could not be detected by either UV or MS in any of the brown samples collected from any location or season (Fig. 3).
HPLC chromatographs (λ = 285 nm) illustrating the difference in the chemical profiles of a green Haliclona samples and b brown Haliclona samples. Salicylihalamide A (at retention time of 12 min) was detected in the green morphology only. Coumarin (C9H6O2) was used as an internal standard (at a retention time of 5 min)
Temperature, Volume, and Depth Correlations
Water temperature showed an increasing trend in green Haliclona from southern to northern locations (Bremer Bay 19.5°C [±0.3°C] < Hamelin Bay 20.1°C [±0.2°C] < Rottnest Island 21.2°C [±0.5°C] < Jurien Bay 22.2°C [±0.4°C]). Salicylihalamide A concentration showed a large, negative correlation (r = −0.703, \(P_{{\alpha = 0.017}} < 0.01\)) to water temperature in summer (Fig. 4). However, no significant correlation was observed between water temperature and sponge size (r = −0.396, \(P_{{\alpha = 0.017}} = 0.075\)) or water temperature and sponge depth (r = 0.383, \(P_{{\alpha = 0.017}} = 0.087\)). Interestingly, the difference in mean concentration in the summer between Bremer Bay and Jurien Bay was 5.0 μg g−1 with an associated 3°C temperature difference, but the seasonal difference in concentration (5.3 μg g−1) at Bremer Bay was associated with a 1°C temperature change.
Green Haliclona sponges ranged in size from 180 to 4,287.5 cm−3 with no correlation between sponge volume and salicylihalamide A concentration (r = −0.358, \(P_{{\alpha = 0.017}} = 0.111\)). No correlation was found between sponge size and the depth (r = −0.002, \(P_{{\alpha = 0.017}} = 0.993\)). Sponges were collected from depths ranging from 4 to 18 m, and no correlation was observed between depth and concentration of salicylihalamide A (r = 0.234, \(P_{{\alpha = 0.017}} = 0.306\)).
Spatial and Temporal Differences
Variability among individuals within a location ranged from 9.0 to 15.9 μg g−1 at Bremer Bay; 6.1 to 15.4 μg g−1 at Hamelin Bay; 5.1 to 20.6 μg g−1 at Rottnest Island; and 5.0 to 13.8 μg g−1 at Jurien Bay. Water temperature was the only covariate utilized in the one-way ANCOVA. Salicylihalamide A concentration was influenced by water temperature (Table 1). After accounting for variation because of water temperature, salicylihalamide A differed significantly among all locations (Table 1 and Fig. 5a) except for Hamelin Bay and Bremer Bay (Table 2).
A significant difference (t = 3.18, df = 10, \(P_{{\alpha = 0.025}} = 0.010\)) in the concentration of salicylihalamide A was also found between summer (ranging from 9.0 to 16 μg g−1) and winter (ranging from 4.6 to 12.7 μg g−1) at Bremer Bay (Fig. 5b).
Health Influences
Only five green Haliclona individuals had visible signs of necrosis, and no significant difference (t = 0.75, df = 19, P = 0.462) was observed between healthy and unhealthy sponges.
Discussion
Green Haliclona individuals contained salicylihalamide A at all locations and in both seasons at Bremer Bay. The yield from crude extracts in this study (2.83%) was comparable to the 2.55% reported by Erickson et al. (1997) in the initial extraction of Haliclona sp. Salicylihalamide A was not detected in any of the brown individuals from the same sites. Given that both color morphologies have similar spicule and skeletal characteristics, this difference in chemical profile may be a useful characteristic in taxonomic classification (Thompson et al. 1987).
Salicylihalamide A concentration was significantly influenced by water temperature, thus probably accounting for some variation among locations. The concentration was also significantly higher in summer than winter in sponges from Bremer Bay. This variation of salicylihalamide A among locations compares favorably to previous studies where large variability in bioactive compounds has been observed as a result of spatial variation (Thompson et al. 1987; Becerro et al. 1995; Page et al. 2005). Page et al. (2005) found that spatial differences corresponded to a variation in the bioactive compounds produced by Mycale hentschelli and that the variation was a result of the environmental differences between locations. This was also evident here, as we found a strong decreasing trend in salicylihalamide A concentration with increasing water temperature. A large environmental gradient occurs along the south west Australian coastline with a 0.5°C rise in water temperature for every degree of latitude (Creswell and Golding 1980), thus resulting in a temperature difference of approximately 3°C between the most northern (Jurien Bay) and southern (Bremer Bay) locations in this study.
Whereas water temperature is an important contributor in the production of salicylihalamide A, other biological or physiological factors, however, also appear to be influencing its production. Salicylihalamide A concentration differed among locations even when the influence of water temperature was removed. The difference in mean salicylihalamide A concentration between Bremer Bay and Jurien Bay during summer (∼5.3 μg g−1) was similar to the difference in mean concentration between seasons at Bremer Bay (∼5 μg g−1). However, the difference in water temperature between seasons at Bremer Bay is only a third of the temperature difference between locations during summer (1 vs. 3°C).
Organism size influences the level of bioactivity in sponges (Becerro et al. 1995), with smaller individuals having higher levels of bioactivity presumably as a result of their need for a competitive advantage in obtaining space (Becerro et al. 1995). Becerro et al. (1995) suggested that the sponge Crambe crambe may have reached a stand-off with its competitors by reaching an optimal area–perimeter relationship that results in a lower energetic demand for border defense (Becerro et al. 1995; and references within). The production of salicylihalamide A did not correlate to the size of the sponge.
Variation of salicylihalamide A concentrations among locations, beyond the influence of water temperature, may be explained by changes in skeletal spicule mass relative to salicylihalamide A concentration (O’Neal and Pawlik 2002). Differences in spicule or skeletal mass because of environmental influences, such as wave exposure, could be driving the variation in salicylihalamide A concentration.
The temporal variation in secondary metabolite production seen in this study is consistent with that observed for other species (Turon et al. 1996; Duckworth and Battershill 2003; Page et al. 2005). Both Page et al. (2005) and Duckworth and Battershill (2003) found that concentration of bioactive compounds was higher in summer than winter. It was suggested that seasonal variation can occur in response to increased fouling of the sponge surface (Duckworth and Battershill 2001) and to an increase in competitors (Turon et al. 1996) during the summer months. The potential ecological role of salicylihalamide A as an antifoulant is considered unlikely as the green Haliclona inhabits low light (irradiance < 6 μE m−2 s−1; Abdo et al. 2006a) overhang habitats and has not been observed to be overgrown by algae (personal observation). Depth-related variability in bioactive compounds has also been related to increased fouling by algae (Thompson et al. 1987); however, there was no correlation observed here between the concentration of salicylihalamide A and depth, further indicating that fouling is unlikely to be influencing production.
Haliclona sp. is a brooding sponge that produces well developed parenchymella larvae and is at the peak of its reproductive period during the summer sampling period of this study. Seasonal changes in bioactive compound production have been related to reproduction in octocorals. Coll (1992) showed that the bioactive compounds were present only during the reproductive season and were confined to the reproductive products. However, Turon et al. (1996) proposed that variation in bioactivity of C. crambe decreased with increasing reproductive investment. Future research that investigates whether eggs, embryos, and larvae of green Haliclona possess salicylihalamide A would help answer this. It would also be beneficial to sample at all locations during winter and at finer temporal scales, as Page et al. (2005) found site-specific temporal differences in the bioactive compounds of Mycale hentscheli.
This study has implications for the future development of salicylihalamide A as a pharmaceutical and for management of green Haliclona as an exploitable resource. We revealed a high yield population in Bremer Bay during summer. The consistent production of salicylihalamide A in individuals, regardless of depth or sponge size, suggests that this population could be a target for wild harvest. Caution must be taken, however, if considering wild harvesting as a supply avenue, as salicylihalamide A occurs in relatively low levels within the sponge (0.012% g−1 tissue dry weight), and large amounts of biomass would be required for commercial supply.
References
Abdo, D. A., Battershill, C. N., and Harvey, E. S. 2006a. Manipulation of environmental variables and the effect on the growth of Haliclona sp.: implications for open water aquaculture. Mar. Biol. Res. 2:326–332.
Abdo, D. A., Seager, J. W., Harvey, E. S., Mcdonald, J. I., Kendrick, G. A., and SHortis, M. R. 2006b. Efficiently measuring complex sessile epibenthic organisms using a novel photogrammetric technique. J. Exp. Mar. Biol. Ecol. 339:121–133.
Becerro, M. A., Turon, X., and Uriz, M. J. 1995. Natural variation of toxicity in encrusting sponge Crambe crambe (Schmidt) in relation to size and environment. J. Chem. Ecol. 21:1931–1946.
Boyd, M. R., Farina, C., Belfiore, P., Gagliardi, S., Kim, J. W., Hayakawa, Y., Beutler, J. A., Mckee, T. C., Bowman, B. J., and Bowman, E. J. 2001. Discovery of a novel antitumour benolactone enamide class that selectively inhibits mammalian vacuolar-type (H+)-ATPases. J. Pharmacol. Exp. Ther. 297:114–120.
Coll, J. C. 1992. The chemistry and chemical ecology of octocorals (Coelenterata, Anthozoa, Octocorallia). Chem. Rev. 92:895–899.
Creswell, G. R. and Golding, T. J. 1980. Observations of a south flowing current in the southeastern Indian Ocean. Deep-Sea Res. 27A:449–466.
Duckworth, A. R. and Battershill, C. N. 2001. Population dynamics and chemical ecology of New Zealand Demospongiae Latrunculia sp. nov. and Polymastia croceus (Poecilosclerida: Latrunculiidae: Polymastiidae). N. Z. J. Mar. Freshw. Res. 35:935–949.
Duckworth, A. and Battershill, C. 2003. Sponge aquaculture for the production of biologically active metabolites: the influence of farming protocols and environment. Aquaculture 221:1–4.
Duckworth, A. R., Battershill, C. N., and Schiel, D. R. 2004. Effects of depth and water flow on growth, survival and bioactivity of two temperatex sponges cultured in different seasons. Aquaculture 242:237–250.
Erickson, K. L., Beutler, J. A., Cardellina, J. H., and Boyd, M. R. 1997. Salicylihalamides A and B, novel cytotoxic macrolides from the marine sponge Haliclona sp. J. Org. Chem. 62:8188–8192.
Harvell, D. C., Fenical, W., Roussis, V., Ruesink, J. L., Griggs, C. C., and Greene, C. H. 1993. Local and geographic variation in the defensive chemistry of a West Indian gorgonian coral (Briareum asbestinum). Mar. Ecol. Prog. Ser. 93:165–173.
Kelman, D., Benayahu, Y., and Kashman, Y. 2000. Variation in the secondary metabolite concentrations in yellow and grey morphs of the red sea soft coral Parerythropodium fulvum fulvum: possible ecological implications. J. Chem. Ecol. 26:1123–1133.
Lopez-Legentil, S., Dieckmann, R., Bontemps-Subielos, N., Turon, X., and Banaigs, B. 2005. Qualitative variation of alkaloids in color morphs of Cystodytes (Ascidiacea). Biochem. Syst. Ecol. 33:1107–1119.
Maida, M., Carroll, A. R., and Coll, J. C. 1993. Variability of terpene content in the soft coral Sinularia flexibilis (Coelentrata: Octocorallia), and its ecological implications. J. Chem. Ecol. 19:2285–2296.
Marti, R., Uriz, M. J., and Turon, X. 2005. Spatial and temporal variation of natural toxicity in cnidarians, bryozoans and tunicates in Mediterranean caves. Sci. Mar. 69:485–492.
McClintock, J. B. and Baker, B. J. (Editors). 2001. Marine Chemical Ecology. CRC Marine Science Series. CRC Press, Boca Raton, FL, p. 610.
Mendola, D. 2003. Aquaculture of three phyla of marine invertebrates to yield bioactive metabolites: process developments and economics. Biomol. Eng. 20:441–458.
Munro, M. H. G., Blunt, J. W., Dumdei, E. J., Hickford, S. J. H., Lill, R. E., Li, S., Battershill, C. N., and Duckworth, A. R. 1999. The discovery and development of marine compounds with pharmaceutical potential. J. Biotechnol. 70:15–25.
Newman, D. J. and Cragg, G. M. 2004. Advanced preclinical and clinical trials of natural products and related compounds from marine sources. Curr. Med. Chem. 11:1693–1713.
O’neal, W. and Pawlik, J. R. 2002. A reappraisal of the chemical and physical defenses of Caribbean gorgonian corals against predatory fishes. Mar. Ecol. Prog. Ser. 240:117–126.
Page, M., West, L., Northcote, P., Battershill, C., and Kelly, M. 2005. Spatial and temporal variability of cytotoxic metabolites in populations of the New Zealand sponge Mycale hentscheli. J. Chem. Ecol. 31:1161–1174.
Paul, V. J., Puglisi, M. P., and Ritson-Williams, R. 2006. Marine chemical ecology. Nat. Prod. Rep. 23:153–180.
Sipkema, D., Osinga, R., Schatton, W., Mendola, D., Tramper, J., and Wijffels, R. H. 2005. Large-scale production of pharmaceuticals by marine sponges: sea, cell, or synthesis? Biotechnol. Bioeng. 90:201–222.
Thompson, J. E., Murphy, P. T., Bergquist, P. R., and Evans, E. A. 1987. Environmentally induced variation in diterpene composition of the marine sponge Rhopaloeides odorabile. Biochem. Syst. Ecol. 15:595–606.
Turon, X., Becerro, M. A., and Uriz, M. J. 1996. Seasonal patterns of toxicity in benthic invertebrates: the encrusting sponge Crambe crambe (Poeciloscledida). Oikos 75:33–40.
Wu, Y., Esser, L., and De Brabander, J. K. 2000. Revision of the absolute configuration of Salicylihalamide A through asymmetric total synthesis. Angew. Chem. Int. Ed. 39:4308–4310.
Zar, J. H. 1999. Biostatistical Analysis. Prentice Hall, New Jersey.
Acknowledgments
The authors are indebted to Linda Heap, Caine Delacy, Dave Gull, and Craig Lebens (Bremer Bay Diving Services) for help during field collections. We also thank the anonymous reviewers for improving this manuscript, and Drs. G. Kendrick, J. McDonald, J. Fromont, and S. Whalan for discussion and comments on the research. Funding for this work was provided by a University of Western Australia (UWA) Small Grant to E. H. and C. B. and UWA University Postgraduate Award to D. A. This research was carried out under Fisheries WA Regulation 179 permit and Fisheries WA who are thanked for assistance with permitting.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdo, D.A., Motti, C.A., Battershill, C.N. et al. Temperature and Spatiotemporal Variability of Salicylihalamide A in the Sponge Haliclona sp.. J Chem Ecol 33, 1635–1645 (2007). https://doi.org/10.1007/s10886-007-9326-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-007-9326-x