Abstract
Feeding larvae of Chrysomela lapponica (Coleoptera: Chrysomelidae) acquire characteristic O-glucosides from the leaves of their food plants. The glucosides are selectively channeled from the gut to the defensive gland. Subsequent enzymatic transformations generate a blend of different defensive compounds, e.g., salicylaldehyde and two series of 2-methylbutyl and isobutyryl esters. By using systematically modified and hydrolysis-resistant thioglucosides as structural mimics of the plant-derived glucosides, e.g., salicin and its o-, m-, and p-isomers 1, 2, and 3; o-, m-, and p-cresols 5, 6, 7; along with thioglucosides of 2-phenylethanol 9 and (3Z)-hexenol 10, we demonstrated that the larvae of C. lapponica are able to sequester a broad range of structurally different thioglucosides with comparable efficiency. This sharply contrasts with the sequestration habitus previously observed in Chrysomela populi and Phratora vitellinae, which secrete almost pure salicylaldehyde and posses a highly specific transport mechanism for salicin (Kuhn et al., Proc. Natl. Acad. Sci. USA 101:13808–13813, 2004). Also, neither C. lapponica nor C. populi sequester in their gland the thioglucoside of 8-hydroxygeraniol, the mimic of the glucoside specifically transported by larvae secreting iridoid monoterpenes (Phaedon cochleariae, Gastrophysa viridula). Accordingly, leaf beetle larvae possess selective membrane carriers in their gut and their defensive systems that match the orientation of the functional groups of glucosides from their food plants probably by embedding the substrate in a network of hydrogen bonds inside the membrane carriers. The synthesis and the spectroscopic properties of the test compounds along with a comparative evaluation of the transport capabilities of larvae of C. populi and C. lapponica are described.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sequestration of plant toxins or pretoxins is widespread among insects (pyrrolizidines: Hartmann and Witte, 1995; cardenolides: Malcolm and Brower, 1989; iridoid glycosides: Dyer and Bowers, 1996; cyanogenic glycosides: Nahrstedt, 1996; glucosinolates: Nishida, 2002), but only little is known on the functional molecular basis of the uptake process. Leaf beetles offer excellent opportunities to explore this process, as sequestered compounds are often stored and concentrated in easily accessible reservoirs, in which final transformations occur. Larvae of chrysomeline leaf beetles possess pairs of dorsal defensive glands in the mesothorax and metathorax and the abdominal segments one to seven. When the larvae are disturbed, the reservoirs are everted by blood pressure and small droplets of a defensive secretion appear on top. As soon as the danger is over, the reservoirs are pulled back within the body cavity by muscle fibers and the remaining secretion is sucked back into the reservoir. Although this basic structure is fairly constant across taxa (Hollande, 1911; Garb, 1915; Paterson, 1930; Berti, 1968), the secreted toxins vary both in structure and biosynthetic origin (Fig. 1).
Several species secrete iridoid monoterpenes, e.g., chrysomelidial (Meinwald et al., 1977; Blum et al., 1978; Pasteels et al., 1982; Lorenz et al., 1993; Veith et al., 1994), which are generally synthesized de novo by the insect (Oldham et al., 1996; Søe et al., 2004). Species that specialize on Salicaceae, i.e., P. vitellinae and Chrysomela spp., derive salicylaldehyde from plant phenolglucosides, such as salicin and salicortin (Pavan, 1953, Pasteels et al., 1983). Particularly diverse is the defensive chemistry of the larvae of Chrysomela lapponica feeding on willow and poplar. Prominent series of compounds are isobutyrates or 2-methylbutyrates of 2-phenylethanol, (3Z)-hexenol, or benzylalkohol, respectively (Hilker and Schulz, 1994; Schulz et al., 1997; Termonia and Pasteels, 1999). The advantage of using plant-derived, glycosidically bound precursors is obvious. The compounds are often abundant in the leaves of the food plant, they are mostly non toxic per se, but can be readily cleaved into toxic aglycons or compounds that can be converted into toxic principles by common enzymatic activities. The glycosides are polar precursors that cannot pass membranes without the involvement of functional transport systems, and leaf beetle larvae have evolved selective transport systems that allow a channeling of certain plant-derived glucosides through the gut membrane into the defensive gland via hemolymph transport. For example, larvae of Chrysomela populi and Phratora vitellinae specifically import salicin (Feld et al., 2001; Kuhn et al., 2004). Final transformations of the glucosides into the actual defensive compounds occur within the gland (Pasteels et al., 1990; Daloze and Pasteels, 1994; Oldham et al., 1996). As illustrated in Fig. 2, the glucosides are assumed to be selected from the food by selective carrier systems located in the gut membrane that load the glucosides into the epithelial cells, followed by export to the hemolymph. A second system shuttles the glucoside from the hemolymph to the reservoir, and here all subsequent steps of toxification can occur with strict separation from the body tissue. Besides import systems for plant-derived compounds, export systems should also exist that keep the hemolymph level of the imported molecules low to prevent an uncontrolled pretoxin accumulation in the insect body. Altogether, a network of selective and well-balanced import and export systems that facilitate the sequestration of glycosides could safely guide polar glucosides from the gut to the defensive gland.
(a) Transport pathways of plant-derived glucosides in the larvae of leaf beetles. Shown is the passage of glucosides from the gut into the defensive gland. At least two transport systems, from the gut to hemolymph (transport system I) and from hemolymph to the reservoir (transport system II), are necessary for sequestration. A third system may exist to excrete excess or wrong glucosides from the hemolymph to the feces. (b) Sequestration and transformation of salicin by larvae of C. populi. (c) Sequestration and transformation of, e.g., the glucoside of 2-phenylethanol by larvae of C. lapponica. After cleavage of the glucoside (a: glucosidase), the aglycon salicylalcohol (b: oxidase) is converted into salicylaldehyde (modified from Kuhn et al., 2004)
C. lapponica is remarkable in that it is distributed into isolated populations adapted to different host plants. Larvae from Bavaria and Czech Republic feed on Betula and secrete complex mixtures of compounds, including series of isobutyrates and 2-methylbutyrates (Hilker and Schulz, 1994; Gross and Hilker, 1995), whereas larvae from Finland feeding on Salix borealis, a plant rich in phenolglucosides, have been reported to produce nearly pure salicylaldehyde (Gross and Hilker, 1995).
The current work addresses and compares the molecular basis and the selectivity of the sequestration processes in the two species C. populi and C. lapponica. Because the plant-derived O-glucosides are rather unstable compounds that are easily cleaved by glucosidases in the insect gut, we selected the hydrolysis-resistant S-glucosides instead of the natural O-glucosides to study the mode of their transport from the gut to the defensive gland. The glycomimics are stable against the glucosidases in the gut and the gland, and they accumulate in the reservoir where they can be easily quantified by high-performance liquid chromatography–mass spectrometry (HPLC–MS; Feld et al., 2001). We report on the synthesis of thioanalogues of salicin and several other analogues that were designed to provide information on the selectivity of the uptake of the plant-derived glucoside. Finally, we demonstrate that the larvae of C. lapponica possess transport systems with a higher substrate tolerance, albeit with a lower overall import rate for individual compounds than those previously observed in other leaf beetle larvae.
Methods and Materials
General
Reactions were performed under Ar. Solvents were dried according to standard methods. IR: Bruker Equinox 55 FTIR Spectrophotometer. 1H and 13C nuclear magnetic resonance (NMR): Avance DRX 500 spectrometer; CDCl3 as solvent, if not stated otherwise. Chemical shifts of 1H and 13C NMR are given in ppm (δ) downfield relative to TMS as internal standard. Gas chromatography (GC)–MS (70 eV): Finnigan Magnum, equipped with a fused silica capillary, coated with DB5 (30 m × 0.25 mm); helium served as carrier gas. HR–MS: high-resolution GC–MS was performed on a Micromass MasSpec (Micromass, Manchester, UK) double-focusing magnetic sector mass spectrometer (geometry EBE) via solid probe. Mass spectra were measured in electron impact (EI) mode at 70 eV, with a source temperature of 200°C, an acceleration voltage of 8 kV, and a resolution of 5000. The mass range was scanned between m/z 35 and m/z 300 at 1 scan sec−1. Perfluorokerosene (PFK, Aldrich, D-82041 Deisenhofen, Germany) served for calibration. Silica gel: Si 60 (0.200–0.063 mm, E. Merck, Darmstadt, Germany) was used for chromatography. Thin layer chromatography was performed with silica gel plates from Merck (60 F256).
Collection and Rearing of Insects
Egg-laying C. lapponica were collected near Saint-Veran (Queyras, France) on Salix breviserrata. Neonate larvae were reared in Jena at 18°C with a photophase of 16 hr on S. caprea leaves until the third instar. S. caprea was selected because of its very low content in phenolglucoside in its leaves. As a consequence, larval secretion is not dominated by salicylaldehyde, allowing an easier detection of the other compounds.
Administration of Thioglucosides to Feeding Larvae
Leaves from S. caprea were painted on the upper side with 0.5 ml of MeOH/H2O (1:1, v/v, 25 μmol ml−1) containing the test compound at a concentration of 25 mM. After evaporation of the solvent, third instars (four larvae per ca. 35 cm−2 leaf segment) were allowed to feed on the treated leaves for 48 hr, followed by sampling of defensive secretion and hemolymph. The defensive secretion was withdrawn with a small capillary from the everted glands of four larvae. The entire operation was conducted under a binocular while the larvae were gently squeezed by tweezers to provoke extrusion of the dorsal reservoirs. To avoid contamination with material from the leaf surface adhering to the larval body, the secretion was collected only from the tip of the everted glands. Hemolymph was obtained most conveniently from larvae that were previously killed by cooling to −20°C for 30 min. The surface of the larvae was cleaned by brief dipping into water (three times), followed by clipping of a single leg. The emerging hemolymph was collected with a small capillary. The collected secretion or hemolymph was determined by weighing the capillaries before and after collection. Weighing was repeated three times to minimize errors. Defensive secretions or hemolymph were transferred into MeOH/H2O (1:1, v/v, 40 μl). Following centrifugation, the supernatant was directly analyzed by liquid chromatography (LC)–MS.
Analysis of Thioglucosides by LC–MS
To monitor and quantify the polar thioglucosides in the defensive secretion or the hemolymph, a combination of reversed-phase HPLC and mass spectrometry was used. Analyses were carried out using a Thermoquest LCQ (Thermoquest, D-63329 Egelsbach, Germany) in the APCI mode (vaporizer temperature: 560°C) connected to an Agilent HP1100 HPLC system equipped with an RP18 column, GROM-SIL ODS-3 CP (125 × 2 mm, 3 μm; Grom Chromatography GmbH, D-72108 Rottenburg-Hailfingen, Germany), and GROM-SIL ODS-7 ph (125 × 2 mm, 4 μm). Samples were analyzed by using gradient elution at 0.25 ml min−1 (solvent A: H2O + 0.5% CH3COOH; solvent B: MeCN + 0.5% CH3COOH) according to the following protocol: starting with 2% B, then after 20 min to 100% B maintained for 8 min, and 2% B for 5 min before recycling. UV detection before the mass spectrometer allowed a convenient and reliable quantification of the thioglucosides (λ max = 224 nm, 254 nm). Standard curves were recorded for 1 to 11 and used for calibration (Figs. 4 and 5). Abundance was expressed as nanomoles of compound per milligram of sample (undiluted secretion or hemolymph). The threshold of detection was set to 1 nmol mg−1 thioglucoside in secretion. Quantification by UV was particularly valuable for the quantification of acylated thioglucosides in the defensive secretion of larvae of C. lapponica. Acylated thioglucosides displayed significant differences in their response based on mass spectrometry; calibration was achieved by using synthetic references (unpublished).
Statistics
Data are presented as mean values ± standard deviation (Sachs, 1999). Outliers according to Grubbs test were eliminated. To obtain valid data, feeding experiments were repeated several times. C. populi: compounds 1 and 4 (N = 10); 11 (N = 7); 8 (N = 6); 10, 5, and 2 (N = 5); 9 and 3 (N = 4); 7 and 6 (N = 3). C. lapponica: compounds 10 and 9 (N = 10); 5, 2, 1, and 4 (N = 9); 11 (N = 8). In all experiments, post hoc multiple comparisons (Tamhane’s T2 test, SPSS) were carried out to evaluate significant differences (P < 0.05) between secretion samples. The hemolymph samples were not treated statistically owing to the low concentrations at the limit of the analytical approach.
Synthesis of Thioglucosides. Königs–Knorr Approach to Acetylated Aromatic Thioglucosides: General Procedure
A solution of 2,3,4,6-tetra-O-acetyl-α-d-glucopyranosyl bromide (0.824 g, 2.0 mmol), triethylamine (560 μl, 4 mmol), and the respective aromatic thiol (2.0 mmol) in acetonitrile (4.0 ml) was stirred under argon for 2 hr at room temperature. Trichloromethane (50 ml) was added and the organic layer was thoroughly washed with water (3 × 10 ml). Removal of solvent (CHCl3) afforded the per-acetylated thioglucoside as a light-brown oil. The product was dissolved in a small amount of warm ethanol and filtered, affording white crystals upon cooling. Spectroscopic data for the acetylated thioglucoside intermediates 12 to 21 are given in the Supplementary Material.
2-Methoxycarbonyl-phenyl-2′,3′,4′,6′-tetra-O-acetyl-1′-thio-β-d-glucopyranoside (12)
Prepared from 2-mercapto-benzoic acid methyl ester (1.5 ml, 8.9 mmol), triethylamine (3.0 ml), and 2,3,4,6-tetra-O-acetyl-α-d-glucopyranosyl bromide (4.08 g, 9.92 mmol) as described. Yield: 4.48 (8.99 mmol, 91%).
3-Methoxycarbonyl-phenyl-2′,3′,4′,6′-tetra-O-acetyl-1′-thio-β-d-glucopyranoside (13)
Prepared from 3-mercapto-benzoic acid methyl ester (336 mg, 2.0 mmol), triethylamine (560 μl), and 2,3,4,6-tetra-O-acetyl-α-d-glucopyranosyl bromide (824 mg, 2.0 mmol) as described. Yield: 917 mg (1.84 mmol, 93%).
4-Methoxycarbonyl-phenyl-2′,3′,4′,6′-tetra-O-acetyl-1′-thio-β-d-glucopyranoside (14)
Prepared from 4-mercapto-benzoic acid methyl ester (0.336 g, 2.0 mmol), triethylamine (560 μl) and 2,3,4,6-tetra-O-acetyl-α-d-glucopyranosyl bromide (0.824 g, 2.0 mmol) as described. Yield: 0.625 g (1.25 mmol, 63%).
Phenyl-2′,3′,4′,6′-tetra-O-acetyl-1′-thio-β-d-glucopyranoside (15)
Prepared from thiophenol (615 μl, 6.0 mmol), triethylamine (1.68 ml), and 2,3,4,6-tetra-O-acetyl-α-d-glucopyranosyl bromide (2.472 g, 6.0 mmol). Yield: 2.421 g (5.5 mmol, 92%).
2-Methyl-phenyl-2′,3′,4′,6′-tetra-O-acetyl-1′-thio-β-d-glucopyranoside (16)
Prepared from 2-methyl-benzenethiol (0.242 g, 2.0 mmol), triethylamine (560 μl), and 2,3,4,6-tetra-O-acetyl-α-d-glucopyranosyl bromide (0.824 g, 2.0 mmol). Yield: 0.772 g (1.7 mmol, 85%).
3-Methyl-phenyl -2′,3′,4′,6′-tetra-O-acetyl-1′-thio-β-d-glucopyranoside (17)
Prepared from 3-methyl-benzenethiol (0.248 g, 2.0 mmol), triethylamine (560 μl), and 2,3,4,6-tetra-O-acetyl-α-d-glucopyranosyl bromide (0.824 g, 2.0 mmol) as described. Yield: 0.757 g (1.67 mmol, 83%).
4-Methyl-phenyl -2′,3′,4′,6′-tetra-O-acetyl-1′-thio-β-d-glucopyranoside (18)
Prepared from 4-methyl-benzenethiol (0.246 g, 2.0 mmol), triethylamine (560 μl), and 2,3,4,6-tetra-O-acetyl-α-d-glucopyranosyl bromide (0.824 g, 2.0 mmol) as described. Yield: 0.764 g (1.68 mmol, 84%).
2-Methoxycarbonyl-phenyl-2′,3′,4′,6′-tetra-O-acetyl-1′-thio-β-d-galactopyranoside (19)
Prepared from 2-mercapto-benzoic acid methyl ester (300 μl, 2.0 mmol), triethylamine (560 μl), and 2,3,4,6-tetra-O-acetyl-α-d-galactopyranosyl bromide (824 mg, 2 mmol) as described. The crude product was purified instantly by flash-chromatography (flash silica 32–63 μm, 60 Å pore size), using a solvent mixture of petroleum ether (100–0%) and diethyl ether (0–100%). The product eluted at a solvent ratio of ca 1:4 and was monitored by TLC (silica gel 60, F254). Yield: 0.499 g (1.0 mmol, 50%).
Mitsunobu-Type Synthesis of Acetylated Thioglucosides 20 and 21: General Procedure
To a solution of carefully dried ADDP (0.504 g, 2.0 mmol) in dry tetrahydrofurane (40 ml) was added with cooling (0°C) a solution of trimethylphosphane (2.0 ml, 1 M in tetrahydrofurane). The mixture was stirred at room temperature until the color turned from orange to light yellow. Then, 2,3,4,6-tetra-O-acetyl-thio-α-d-glucopyranose (0.472 g, 1.3 mmol) and a solution of the respective aglycon (1.0 mmol) in tetrahydrofurane (20 ml) were added with stirring at room temperature. A white precipitate occurred and after 2 hr the solvent was removed. The residue was dissolved in the minimum amount of ethyl acetate and the hydrazide was precipitated with petroleum ether. Solids were removed by filtration and the solvent was evaporated under reduced pressure. The crude product was dissolved in dichloromethane (50 ml) and washed with water (2 × 25 ml) and a saturated solution of NaHCO3 (25 ml) solution. The product was purified by flash chromatography on silica gel using a binary solvent mixture (ethyl acetate/petroleum ether, 1:1) for elution.
2-Phenethyl-2′,3′,4′,6′-tetra-O-acetyl-1′-thio-β-d-glucopyranoside (20)
Prepared from 2,3,4,6-tetra-O-acetyl-thio-α-d-glucopyranose (1.42 g, 3.9 mmol) and 2-phenylethanol (0.366 g, 3.0 mmol) as described. Yield: 0.37 g (1.65 mmol, 55%).
(3Z)-Hex-3-enyl-2′,3′,4′,6′-tetra-O-acetyl-1′-thio-β-d-glucopyranoside (21)
Prepared from 2,3,4,6-tetra-O-acetyl-thio-α-d-glucopyranose (0.472 g, 1.29 mmol) and (3Z)-hex-3-en-1-ol (0.10 g, 1.0 mmol) as described. Yield: 0.366 g (0.82 mmol, 82%).
Reductive Deprotection of the Thioglucosides 12, 13, and 14: General Procedure
A solution of the acetylated thioglucosides 12, 13, or 14 (1.0 mmol) in dry tetrahydrofurane (15 ml) was added slowly to a cooled and well stirred suspension of LiAlH4 [0.12 g, 3.2 mmol in the same solvent (15 ml) kept under argon]. The reaction mixture was heated to reflux for 2 hr. After cooling (0°C), excess LiAlH4 was hydrolyzed by sequential addition of water (0.15 ml), 15% NaOH(aq) (0.15 ml), and water (0.45 ml). The resulting solid was removed by filtration. After removal of the solvent, the crude thioglucoside was purified by medium pressure liquid chromatography (MPLC) on reversed phase (RP 18 LiChroprep 40–63 μm; column 3.2 cm ID × 23 cm length) using a solvent gradient for elution. For elution, a binary mixture of water (60%) and methanol (40%) was ramped within 20 min to pure methanol. Flow rate: 35 ml min−1 at 20 bar. The elution of products was monitored by UV at 254 nm.
2-Hydroxymethyl-phenyl-1′-thio-β-d-glucopyranoside (1)
Prepared from 12 (0.50 g, 1.0 mmol) by reduction with LiAlH4 (0.120 g, 3.16 mmol) as described. Yield: 0.147 g (0.49 mmol, 50%). M.p.: 199.1°C. UV λ max [nm]: 205 1H NMR (CD3OD) δ [ppm]: 7.74 (dd, J = 7.6, 1.3, 1H), 7.46 (dd, J = 7.6, 1H), 7.32 (td, J = 7.5, 1.4, 1H), 7.27 (td, J = 7.5, 1.5, 1H), 4.88 (d, J = 12.8, 1H), 4.74 (d, J = 12.8, 1H), 4.49 (d, J = 9.8, 1H), 3.85 (dd, J = 11.9, 2.1, 1H), 3.65 (dd, J = 12.2, 5.5, 1H), 3.36 (dd, J = 8.7, 1H), 3.30–3.22 (m, 2H), 3.16 (dd, J = 9.6, 8.7). 13C NMR (CD3OD) δ [ppm]: 144.9, 135.8, 132.8, 129.8, 129.5, 129.2, 89.8, 82.2, 79.7, 73.8, 71.5, 63.8, 63.0. IR (KBr) ν [cm−1]: >3200, 3100, 3063, 3052, 2943, 2887, 1587, 1473, 1459, 1445, 1429, 1365, 1332, 1246, 1213, 1180, 1103, 1086, 1070, 1037, 1000, 751. MS (70 eV) m/z (%): 302(6); 145(25); 140(82); 123(40); 122(100); 121(51); 87(28); 85(42); 77(33); 73(41); 57(29). HR–MS m/z [M+]: calcd. for C13H18O6S 303.082410; found 303.082832.
3-Hydroxymethyl-phenyl-1′-thio-β-d-glucopyranoside (2)
Prepared from 13 (0.50 g, 1.0 mmol) by reduction with LiAlH4 (0.120 g, 3.16 mmol) as described. Yield: 0.119 g (0.40 mmol, 40%). UV λ max [nm]: 201, 251. 1H NMR (D2O) δ [ppm]: 7.60 (s, 1H), 7.55 (d, J = 7.8, 1H), 7.30 (dd, J = 7.9, 7.7, 1H), 7.24 (d, J = 7.8, 1H), 4.69 (d, J = 9.8, 1H), 4.52 (s, 2H), 3.78 (dd, J = 12.5, 2.1, 1H), 3.60 (dd, J = 12.5, 5.8, 1H), 3.42 (t, J = 9.2, 1H), 3.38 (ddd, J = 9.8, 6.0, 2.1, 1H), 3.30 (t, J = 9.4, 1H), 3.24 (t, J = 9.4, 1H). 13C NMR (D2O) δ [ppm]: 142.7, 133.6, 131.9, 131.4, 130.7, 128.2, 88.4, 81.2, 78.5, 73.0, 70.6, 64.7, 62.1. IR (KBr) ν [cm−1]: >3100, 2925, 2853, 1269, 1120, 1016. MS (70 eV) m/z (%): 302(2), 162 (6), 145(14), 141(8), 140(100), 139(5), 127(9), 123(7), 109(5), 107(7), 97(5), 91(9), 87(14), 85(20), 79(7), 77(11), 73(17). HR–MS m/z [M+]: calcd. for C13H18O6S 302.082410; found 302.082123.
4-Hydroxymethyl-phenyl-1′-thio-β-d-glucopyranoside (3)
Prepared from 14 (0.475 g, 0.95 mmol) by reduction with LiAlH4 (0.120 g, 3.16 mmol) as described. Yield: 0.165 g (0.55 mmol, 57%). M.p.: 77°C. UV λ max [nm]: 201, 252. 1H NMR (D2O) δ [ppm]: 7.46 (d, J = 8.2, 2H), 7.27 (d, J = 8.2, 2H), 4.67 (d, J = 10.1, 1H), 4.52 (s, 2H), 3.78 (dd, J = 12.4, 1.7, 1H), 3.60 (dd, J = 12.4, 5.6, 1H), 3.41 (t, J = 9.0, 1H), 3.37 (ddd, J = 10.0, 5.9, 1.0, 1H), 3.29 (t, J = 9.6, 1H), 3.23 (dd, J = 9.5, 9.6, 1H). 13C NMR (D2O) δ [ppm]: 142.9, 134.5, 133.9, 130.8, 89.9, 82.6, 79.9, 74.4, 72.0, 66.0, 63.5. IR (KBr) ν [cm−1]: >3100, 2893, 2449, 2363, 1489, 1405, 1275, 1035, 860, 800. MS (70 eV) m/z (%): 302(3), 145(14), 142(5), 141(8), 140(100), 139(5), 127(10), 123(9), 122(7), 111(8), 109(4), 107(13), 97(6), 91(8), 87(12), 85(21), 79(8), 78(4), 77(12), 73(17), 71(3), 69(9), 61(10), 60(9), 57(6). HR–MS m/z [M+]: calcd. for C13H18O6S 302.082410; found 302.082756.
2-Hydroxymethyl-phenyl-1′-thio-β-d-galactopyranoside (4)
Prepared from 19 (0.556 g, 1.0 mmol) by reduction with LiAlH4 (0.120 g, 3.16 mmol) as described. The product was separated from the solid by 12-hr continuous extraction with diethyl ether. After removal (rotavapor) of the solvent (tetrahydrofuran), the crude thiogalactoside was purified by MPLC on reversed phase (RP 18, LiChroprep 40–63 μm; column 3.2 cm ID × 23 cm length) using a mixture of water (60–0% in 20 min) and methanol (40–100% in 20 min) for elution. Flow rate: ca. 35 ml/min (ca. 20 bar). The eluting product was monitored by UV at 254 nm. Yield: 0.165 g (0.55 mmol, 55%). M.p.: 158°C. UV λ max [nm]: 201, 250. 1H NMR (CD3OD) δ [ppm]: 7.78 (dd, J = 7.7, 1.3, 1H), 7.49 (dd, J = 7.5, 1.3, 1H), 7.35 (td, J = 7.5, 1.3, 1H), 7.30 (td, J = 7.5, 1.5, 1H), 4.89 (d, J = 13.1, 1H), 4.79 (d, J = 12.9, 1H), 4.51 (d, J = 9.8, 1H), 3.91 (dd, J = 3.6, 0.8, 1H), 3.79 (dd, J = 11.5, 6.8, 1H), 3.73 (dd, J = 11.3, 5.2, 1H), 3.60 (t, J = 9.4, 1H), 3.56 (dd, J = 5.4, 1.0, 1H), 3.55 (dd, J = 5.8, 0.6, 1H), 3.51 (dd, J = 9.2, 3.2, 1H). 13C NMR (CD3OD) δ [ppm]: 144.8, 135.2, 133.6, 129.6, 129.2, 90.8, 80.7, 76.3, 71.1, 70.5, 63.8, 62.7. IR (KBr) ν [cm−1]: >3100, 2940, 2917, 2886, 1417, 1130, 1085, 1045, 1009, 950, 860, 820, 802, 758. MS (EI) m/z (%): 302(15), 140(71), 123(33), 122(100), 121(41), 91(46), 85(28), 77(22),73(55), 69(16), 61(21), 57(24). HR–MS m/z [M+]: calcd. for C13H18O6S 303.082410; found 303.082154.
Base-Catalyzed Deacetylation of Thioglucosides 15, 16, 17, and 18: General Procedure
A solution of the acetylated thioglucosides 15, 16, 17, or 18 (0.73 mmol) in methanol (10 ml) is treated with sodium methoxide (0.01 ml of a 30% soln., 3.0 mmol) and stirred for 12 hr at room temperature. After neutralization with amberlite IRC50, the product is purified by MPLC on reversed phase (RP-18, Büchi column no. 17982) using a binary solvent of MeOH/H2O (3:2) for isocratic elution or high-speed countercurrent chromatography (Triple Coil, P.C., Inc., CHCl3/MeOH/H2O (7:13:8), S f = 0.8).
Phenyl-1′-thio-β-d-glucopyranoside (8)
Prepared from 15 (2.42 g, 5.50 mmol) as described. Yield: 0.94 g (3.45 mmol, 63%). Mp: 113°C. UV λ max [nm]: 204, 3. 1H NMR (CD3OD) δ [ppm]: 7.58 (dd, J = 8.2, 1.2, 2H), 7.32 (dd, J = 8.5, 7.0, 2H), 7.27 (tt, J = 7.2, 1.1, 1H), 4.50 (d, J = 9.8, 1H), 3.89 (dd, J = 12.2, 2.1, 1H), 3.57 (dd, J = 12.2, 5.3, 1H), 3.41 (t, J = 8.7, 1H), 3.35 (ddd, J = 9.6, 5.5, 2.1, 1H), 3.32 (t, J = 9.3, 1H), 3.25 (dd, J = 9.6, 8.7, 1H). 13C NMR (CD3OD) δ [ppm]: 135.5, 132.9, 130.1, 128.6, 89.6, 82.3, 79.9, 74.0, 71.6, 63.1. IR (KBr) ν [cm−1]: >3100, 2972, 2931, 2900, 2528, 1582, 1482, 1438, 1384, 1271, 1123, 1045, 876, 819, 738, 687. MS (70 eV) m/z (%): 272(3), 145(18), 127(11), 111(10), 110(100), 109(12), 97(6), 91(7), 87(14), 85(23), 77(7), 73(18), 69(10), 66(5), 65(5), 61(11), 60(5), 61(11), 60(5),57(11). HR–MS m/z [M+]: calcd. for C12H16O5S 272.071846; found 272.071877.
2-Methyl-phenyl-1′-thio-β-d-glucopyranoside (5)
Prepared from 16 (0.450 g, 0.99 mmol) as described. Yield: 0.278 g (0.97 mmol, 98). UV λ max [nm]: 210,3. 1H NMR (CD3OD) δ [ppm]: 7.52 (dd, J = 7.2, 1.7, 1H), 7.21 (dd, J = 7.8, 1.4, 1H), 7.18 (td, J = 7.2, 1.9, 1H), 7.15 (td, J = 7.4, 1.8, 1H), 4.69 (d, J = 10.1, 1H), 3.78 (dd, J = 12.5, 2.1, 1H), 3.60 (dd, J = 12.5, 5.5, 1H), 3.35 (t, J = 8.7, 1H), 3.48 (ddd, J = 9.6, 5.5, 2.1, 1H), 3.45 (d, J = 6.1, 1H), 3.43 (dd, J = 8.5, 7.6, 1H), 2.42 (s, 3H). 13C NMR (CD3OD) δ [ppm]: 140.0, 135.2, 132.7, 131.1, 128.2, 127.7, 89.3, 82.0, 79.7, 74.2, 71.4, 62.9, 21.1. IR (KBr) ν [cm−1]: >3100, 2925, 1589, 1466, 1363, 1272, 1228, 1108, 1045, 921, 884, 825, 737, 680. MS (70 eV) m/z (%): 286(3), 145(12), 124(100), 97(6), 91(21), 87(13), 85(22), 73(15), 69(9), 61(10), 57(12). HR–MS m/z [M+]: calcd. for C13H18O5S 286.087496; found 286.087761.
3-Methyl-phenyl-1′-thio-β-d-glucopyranoside (6)
Prepared from 17 (0.476 g, 0.99 mmol) as described. Yield: 0.297 g (1.04 mmol, 99%). M.p.: 91°C. UV λ max [nm]: 203, 250. 1H NMR (CD3OD) δ [ppm]: 7.36 ( s, 1H), 7.30 (d, J = 7.6, 1H), 7.13 (t, J = 7.6, 1H), 7.02 (d, J = 7.6, 1H), 4.54 (d, J = 10.1, 1H), 3.82 (dd, J = 12.2, 1.8, 1H), 3.62 (dd, J = 12.2, 5.5, 1H), 3.35 (t, J = 8.7, 1H), 3.28 (ddd, J = 9.2, 5.9, 1.9, 1H), 3.25 (dd, J = 9.3, 8.7, 1H), 3.18 (dd, J = 9.8, 8.9, 1H), 2.22 (s, 3H). 13C NMR (CD3OD) δ [ppm]: 140.1, 135.3, 133.4, 130.0, 129.3, 89.7, 82.3, 79.9, 74.1, 71.7, 63.2, 21.6. IR (KBr) ν [cm−1]: >3100, 2908, 1586, 1473, 1419, 1375, 1280, 1214, 1123, 1067, 890, 828, 772, 688. MS (70 eV) m/z (%): 286(4), 145(10), 127(8), 126(5), 125(10), 124(100), 123(6), 97(5), 91(21), 87(10), 85(18), 73(13), 69(7), 61(9), 57(9). HR–MS m/z [M+]: calcd. for C13H18O5S 286.087496; found 286.087410.
4-Methyl-phenyl-1′-thio-β-d-glucopyranoside (7)
Prepared from 18 (0.459 g, 1.01 mmol) as described. Yield: 0.274 g (0.96 mmol, 96%). M.p.: 148°C. UV λ max [nm]: 201, 250. 1H NMR (CD3OD) δ [ppm]: 7.37 (d, J = 8.2, 2H), 7.14 (d, J = 8.2, 2H), 4.59 (d, J = 9.8, 1H), 3.77 (dd, J = 12.5, 2.1, 1H), 3.59 (dd, J = 12.4, 5.6, 1H), 3.40 (t, J = 8.9, 1H), 3.33 (ddd, J = 9.8, 5.5, 2.1 1H), 3.27 (t, J = 9.6, 1H), 3.21 (q, J = 9.0, 1H), 2.22 (s, 3H). 13C NMR (CD3OD) δ [ppm]: 139.0, 133.8, 131.4, 130.8, 89.9, 82.2, 79.9, 73.9, 71.6, 63.1, 21.4. IR (KBr) ν [cm−1]: >3200, 3021, 2912, 2878, 2641, 1488, 1438, 1388, 1322, 1239, 1211, 1178, 1079, 1045, 868, 807. MS (70 eV) m/z (%): 286(6), 145(8), 127(6), 124(100), 123(10), 97(5), 91(22), 87(10), 85(15), 73(13), 69(7), 61(8), 57(9). HR–MS m/z [M+]: calcd. for C13H18O5S 286.087496; found 287.087404.
2-Phenyl-ethyl-1′-thio-β-d-glucopyranoside (9)
Prepared from 20 (0.773 g, 1.65 mmol) as described. Yield: 0.476 g (1.58 mmol, 96%). UV λ max [nm]: 201, 259. 1H NMR (CD3OD) δ [ppm]: 7.24–7.17 (m, 4H), 7.17 (tt, J = 6.8, 1.8, 1H), 4.37 (d, J = 9.5, 1H), 3.87 (dd, J = 11.9, 2.1 1H), 3.62 (dd, J = 11.9, 5.5, 1H), 3.37–3.37 (m, 1H), 3.31 (t, J = 8.7, 1H), 3.27 (ddd, J = 9.5, 6.0, 2.4, 1H), 3.21 (dd, J = 9.8, 8.5, 1H), 2.99–2.84 (m, 4H). 13C NMR (D2O) δ [ppm]: 141.2, 129.3, 129.2, 127.1, 85.6, 80.4, 77.8, 73.0, 70.1, 61.5, 36.2, 31.6. IR (KBr) ν [cm−1]: > 3100, 3026, 2923, 1499, 1453, 1350, 1273, 1050, 885, 822, 698. MS (70 eV) m/z (%): 300(3), 282(3), 264(2), 251(2), 223(3), 163(10), 162(10), 145(58), 138(57), 127(37), 116(56), 105(100), 104(61), 91(58), 87(43), 85(69), 73(61), 69(23), 61(31), 60(30), 57(35). HR–MS m/z [M+]: calcd. for C14H20O5S 300.103146; found 300.102707.
(3Z)-Hex-3-enyl-1′-thio-β-d-glucopyranoside (10)
Prepared from 21 (0.325 g, 0.73 mmol) as described. Yield: 0.133 g (0.48 mmol, 67%). Mp: 65°C. 1H NMR (CD3OD) δ [ppm]: 5.80–5.55 (m, 2H), 4.67 (dd, J = 9.8, 4.5, 1H), 4.03 (d, J = 12.5, 1H), 3.88 (dd, J = 12.2, 4.3, 1H), 3.65–3.57 (m, 3H), 3.48 (td, J = 9.5, 1.6, 1H), 2.94–2.91(m, 2H), 2.57–2.46 (m, 2H), 2.25–2.12 (m, 2H), 1.11 (t, J = 7.5, 3H). 13C NMR (CDCl3) δ [ppm]: 134.2, 127.4, 85.7, 80.2, 77.7, 72.8, 69.9, 61.3, 30.2, 27.7, 20.7, 14.1. IR (KBr) ν [cm−1]: 3416, 2942, 2832, 1637, 1458, 1261, 1112, 1032, 804. MS (70 eV) m/z (%): 278(2), 234(1), 229(1), 205(2), 198(4), 187(3), 169(5), 163(13), 162(18), 145(78), 127(53), 117(18), 116(43), 115(100), 103(13), 99(14), 97(21), 91(27), 87(92), 85(90), 83(18), 82(22), 81(12), 73(65), 71(18), 69(34), 67(17), 61(39), 60(30), 57(41), 55(43). HR–MS m/z [M+]: calcd. for C12H22O5S 278.118796; found 278.118609.
Results
Analysis of the Defensive Secretion of C. lapponica Larvae Fed on S. caprea (Queyras, France)
Chrysomela lapponica were collected near Saint-Veran, Queyras, France, and their larvae were fed on S. caprea. The total ion chromatogram of their exocrine secretion is shown in Fig. 3. For comparison, the secretion of C. populi is shown as an insert. Although both species feed on Salix spp., generally rich in different phenolglucosides, only the secretion of C. lapponica shows a large number of compounds ultimately derived from glucosides of the host plant. A list of the identified compounds of C. lapponica comprising salicylaldehyde and of two series of isobutyrates and 2-methylbutyrates of 2-phenylethanol, benzylalcohol, hydroxylinalool, and (3Z)-hexenol is given in the legend of Fig. 3. A more detailed analysis will be reported elsewhere.
Total ion chromatogram of the low boiling fraction of the exocrine defensive secretions of larvae of C. lapponica (Queyras, France) feeding on S. caprea; inset: C. populi feeding on P. canadensis. Identification of compounds: 1, salicylaldehyde; 2, (3Z)-hexenyl isobutyrate; 3, (3Z)-hexenyl 2-methylbutryrate; 4, benzyl isobutyrate; 5, benzyl 2-methylbutyrate; 6, 2-phenylethyl isobutyrate; 7, 2-phenylethyl 2-methylbutyrate; 8, 8-(isobutyryloxy)-linalool; 9, 8-(2-methylbutyryloxy)-linalool; 10, 2-phenylethyl benzoate
Synthesis of Thioglucosides
To understand the selection criteria for the selective sequestration of salicin by larvae of C. populi, and the broad range import of plant-derived glucosides by C. lapponica (see Fig. 3), we chose isomeric thioglucosides of salicin, and other aliphatic or aromatic thioglucosides, which occur in different host plants, as test compounds. Thioglucosides offer a unique structural similarity to the natural substrates retaining the ability to adopt the same bioactive conformations as natural carbohydrates and conjugates (Nicotra, 1998). At the same time, they exhibit exceptional chemical and biological stability, making them to ideal tools to study transport phenomena of glucosides (Scheme 1).
The test compounds 1 to 9 cover thioglucosides of the o-, m-, and p-isomers of salicin, as well as those of thiocresols, which lack the hydroxyl group of the C1 substituent of salicin. Compound 4 maintains all features of 1, except that the glucose moiety is replaced by galactose, epimeric to glucose at C-4. The thioglucoside 8 lacks the hydroxymethyl group of salicin. Compounds 9, 10, and 11 represent stable S-analogues of natural O-glucosides found in Salix or birch leaves that are sequestered by larvae of C. lapponica (Table 1 in Schulz et al., 1997). Compound 11 was demonstrated to be specifically transported by larvae secreting iridoid monoterpenes (Feld et al., 2001; Kuhn et al., 2004). Compounds 1 to 11 were previously tested on C. populi (Kuhn et al., 2004; see Fig. 4) and the thioglucosides 1, 2, 4, 6, 9, 10, and 11 were used in the present study to evaluate the selectivity of the transports system(s) of C. lapponica.
Survey on the accumulation of the thioglucosides 1 to 10 in the defensive secretion of feeding larvae of C. populi; modified from Kuhn et al., 2004
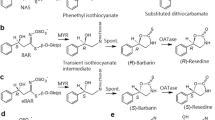
The synthesis of most of the aromatic thioglucosides 12 to 18 followed a uniform protocol based on alkylation of acetobromoglucose with o-, m-, p-mercaptobenzoates or o-, m-, p-mercaptocresols as nucleophiles (Scheme 2). The isomeric thiosalicins 1, 2, 3, and 8 were obtained after reductive removal of the acetate moieties with LiAlH4. The hydride reagent simultaneously reduced the benzoate ester to the required -CH2OH substituent. The thioglucosides of the isomeric cresols 6, 7, and 8 were liberated from the acetylated precursors by treatment with methoxide in methanol. The basic cleavage was the method of choice, if only the acetates had to be removed, because the work-up proved to be more efficient than after reduction with LiAlH4.
The galactoside 4 was also obtained via the Königs–Knorr protocol by using peracetylated α-bromogalactose and o-mercaptobenzoate as the nucleophile.
After reductive removal of the acetates with simultaneous reduction of the benzoate, the galactoside 4 was obtained in 57% yield (Scheme 3). The synthesis of the thioglucosides 9 and 10 required a different approach, because the thionucleophile corresponding to (Z)-hex-3-enol or phenylethanol was not readily available. In this case, the alcohols were condensed with 2,3,4,6-tetra-O-acetyl-β-d-thioglucopyranose according to a modified Mitsunobu protocol (Falconer et al., 1999). The acetates were saponified in high yield with sodium methoxide in methanol (Scheme 4).
Purification of all glucosides was best achieved by MPLC on reversed phase (RP 18) by using methanol/water for elution or by high-speed counter-current chromatography as described in Methods and Materials.
Analysis of the Transport Characteristics of the Thioglucosides by Larvae of C. lapponica and C. populi
Third instars of C. populi were allowed to feed on leaves of Populus canadensis pretreated with aqueous solutions of the different thioglucoside 1 to 11. After 48 hr, their defensive secretions and their hemolymph were collected and analyzed by LC–MS in the atmospheric pressure chemical ionization mode after minimal sample preparation (see Methods and Materials). Although the results of the feeding experiments with C. populi were already published (Kuhn et al., 2004), the data are repeated in Fig. 4 to allow a comparative analysis of the transport modalities of the two systems of C. populi and C. lapponica. Because all compounds were applied at the same concentration and because the leaves were consumed to a comparable extent, the accumulation of the thioglucosides in the defensive secretion can be taken as a measure for the efficiency of the transport process.
Larvae of C. populi sequestered only the thiosalicin 1 with high efficiency, and the compound accumulated to a final level of 1100 nmol mg−1 in the defensive secretion. The concentration in the hemolymph did not exceed ca. 1 to 2 nmol mg−1. Unlike thiosalicin 1, the m- and the p-isomers 2 and 3 were not notably transported and did not accumulate in the secretion (final level 10–20 nmol mg−1). Surprisingly, the thioglucoside of the cresol 5, lacking only the hydroxyl group of the salicin side chain, was also not significantly enriched in the gland reservoir. Within the limits of error, the transport efficiency was roughly the same for all three regioisomeric cresol thioglucosides 5, 6, and 7, and was comparable with the transport rate of the nonnatural regioisomers of thiosalicin (1). The thioglucoside of 8-hydroxygeraniol 11, the precursor for iridoid biosynthesis, was not significantly imported by larvae of C. populi.
When larvae of C. lapponica were allowed to feed on leaves of S. caprea pretreated with the thioglucosides 1, 2, 4, 6, 9, 10, or 11, sequestration into the glandular reservoir was observed for all compounds except for the thiogalactoside 4 (Fig. 5). The latter compound was not imported by the feeding larvae of C. lapponica and only to a very low extent by other previously studied chrysomeline larvae (Kuhn et al., 2004). Consistent with the low concentration of the thioglucosides in the hemolymph of C. populi, in the larvae of C. lapponica, only a very low titer of the sequestered thioglucosides was also observed and hampered a reliable quantification. The major difference observed in C. populi was in the final concentrations of the imported thioglucosides in the reservoir. Although larvae of C. populi accumulated thiosalicin 1 to about 1100 nmol mg−1 in their defensive secretion, 1 and other test compounds did not exceed 200–450 nmol mg−1 in the secretion of C. lapponica. Moreover, the import pattern did not show a clear preference for a single compound. Another remarkable difference in C. populi was the observation that a fraction of the imported thioglucosides was acylated at the conjugating sugar or even at the alcohol moiety if additional hydroxy groups were present in the aglycon, e.g., in 1, 2, and 11. The total concentration of the imported thioglucosides was, therefore, corrected for the acylated fraction by using synthetic references for calibration (unpublished data).
In larvae of C. lapponica, the missing hydroxyl group in the thioglucoside 6 does not affect the import of the compound; the import of 1 and 6 is achieved with similar efficiency. Moreover, the structurally unrelated thioglucosides of 2-phenylethanol 9 and (3Z)-hexenol 10 are also readily sequestered and accumulate to the same level as thiosalicin 1. On the other hand, the import of the terpenoid-type thioglucoside 11 was low in larvae of C. lapponica from Queyras, but not from other locations (unpublished results).
Discussion
Selective transport systems that facilitate the sequestration of secondary metabolites from plants have been postulated or demonstrated several times (Rothschild, 1973; Blum, 1981; Hartmann, 1999). For example, transport systems are required for the uptake of pyrrolizidine-N-oxides from Asteraceae by Oreina cacaliae (Hartmann et al., 1999) or for the sequestration of the highly polar cardenolide glycosides (Duffey and Scudder, 1972). Irrespective of the enormous importance of the phenomenon of sequestration of plant-derived metabolites, none of the involved transport systems has, as yet, been identified and characterized at the molecular level. The current work, together with similar experiments with other leaf beetle larvae (Feld et al., 2001; Kuhn et al., 2004), provides the first information on the different molecular and stereochemical features that govern the uptake processes of salicin from Populus leaves by larvae of C. populi, and also other plant-derived glucosides by larvae of C. lapponica from Queyras (France). In both cases, thioglucosides could be successfully used as substitutes of the naturally occurring O-glucosides. Owing to their stability against glucosidases, which are omnipresent in the defensive secretions of leaf beetle larvae (Pasteels et al., 1990), the compounds are not cleaved during the gut passage and accumulate unchanged in the defensive gland. As illustrated in Fig. 1, a first transport system has to be located in the membrane of the gut, facilitating the uptake of the precursors into the hemolymph. A second transport system has to be associated with the membranes of the defensive system. The latter selectively imports the glycosides from the circulating hemolymph. The concentration of the most efficiently transported glycoside 1 in the hemolymph of C. populi never exceeded 1–2 nmol mg−1, whereas in the reservoir up to 1100 nmol mg−1 were measured. Comparable observations were made for C. lapponica, but the maximum concentrations of the thioglucosides in the reservoir were much lower (up to 450 nmol mg−1 secretion).
The sequestration strategy of the larvae of C. populi and C. lapponica is remarkably different. Although the import system of C. populi is specialized for salicin, finally converted to salicylaldehyde, larvae of C. lapponica import a broad range of glucosides that are converted into a blend of defensive compounds (see Fig. 5). In addition to salicylaldehyde, the secretion of C. lapponica contains significant amounts of isobutyl and 2-methylbutyl esters, of e.g., (3Z)-hexenol and 2-phenylethanol. This coincides with the observation that the thioglucosides of both alcohols are readily sequestered from food (Fig. 3). Apparently, the import of a broader range of glucosides is facilitated in these larvae by a reduced selectivity of the transport systems. This becomes particularly obvious by the comparable import rate of the thioglucosides 1, 5, 9, and 10. The lack of the hydroxyl group in the thiocresol 5 does not affect the import rate, and this may suggest that this functional group is not involved in interactions with the transport system of C. lapponica. This is very different in larvae of C. populi. Their transport system responds to this structural modification by ca. 90% reduction of the import of 5 when compared to 1 (Fig. 3). Apparently, the transport system of C. populi interacts with all hydroxyl groups of the substrate salicin by formation of hydrogen bridges. In the case of C. lapponica, the recognition of the aglycon is less strict and shows a larger tolerance to structural changes. In particular, the presence or absence of hydroxy groups within the aglycon is not critical in the case of C. lapponica.
A mechanistic model postulating hydrogen bridges between the functional groups of the glucoside and the transport system would also explain why the galactoside fails to be sequestered (Figs. 3 and 4). In this case, the hydroxyl group at C(4) of the sugar moiety has the wrong orientation and is not able to interact with the transport system. Because both insect species rely on the import of glucosides, this structural element is of equal importance to both. A selective interaction with the hydroxyl group of the salicin aglycon by larvae of C. populi also accounts for the differences between the m-, and p-isomers 2 and 3. Because these compounds are less efficiently accumulated than the o-, m-, and p-cresols 5, 6, and 7 (Fig. 4), a hydroxyl group out of place may even prevent the correct positioning of the substrate in the transport system.
The high accumulation in the reservoir is only possible if the membrane carrier in the glandular system is coupled to an active energy source (Hartmann et al., 1999). With respect to a low hemolymph titer of 1, the transport system in the gut must not operate against a steep gradient. Here, the concentration in the gut should be generally higher due to a constant supply of the compound from the ingested food.
An interesting aspect concerns the identity of the two required transport systems, the first in the gut and the second in the membrane of the defensive gland (see Fig. 1). The current feeding experiments give only an overall view on the selectivity of the passage of compounds through both systems. Individual analyses of the gut-associated and gland-based transport systems are needed.
Another important aspect of this work is the finding that the transport systems, once evolutionary adapted to plant-derived glucosides, apparently remain quite stable within related groups of insects. For example, larvae of the iridoid-producing Phaedon cochleariae, Gastrophysa viridula, and Hydrothassa marginella are able to import the (thio)glucoside of 8-hydroxygeraniol from pretreated leaves, but fail to enrich any aromatic glucoside (Kuhn et al., 2004). Inversely, larvae of C. populi and Phratora laticollis sequester salicin, but not the glucoside of 8-hydroxygeraniol. In all cases, the high preference for a single plant-derived glucoside coincides with the composition of the defensive secretion that is based on a single or a few compounds. The more evolved C. lapponica apparently use transport systems of moderate selectivity that allow the import of a broader range of plant-derived glucosides. After removal of the sugar moiety, the aglycons may be oxidized (e.g., to salicylaldehyde) or acylated to give the isobutyl or 2-methylbutyl esters. Whether the import of a broad range of phytogenic glucosides is achieved by a single system with low selectivity or by a number of highly selective transport systems remains to be clarified. This and related questions on the existence of a network of interacting import and export systems within the insect have to await the isolation of the corresponding transport proteins and their genes allowing a thorough phylogenetic analysis.
References
Berti, N. 1968. Quelces observations sur la biologique de Phaedonia circumcincta Sahl at description des stades larvaire et nymphal. Bull. Soc. Entomol. Fr. 73:114–127.
Blum, M. S. 1981. Sequestration of plant natural products in nonsecretory structures. in Chemical Defenses of Arthropods. Academic Press, New York, pp 421–438.
Blum, M. S., Wallace, J. B., Duffield, R. M., Brand, J. M., Fales, H. M., and Sokoloski, E. A. 1978. Chrysomelidial in defensive secretion of leaf beetle Gastrophysa cyanea Melsheimer. J. Chem. Ecol. 4:47–53.
Daloze, D. and Pasteels, J. M. 1994. Isolation of 8-hydroxygeraniol-8-O-b-d-glucoside, a probable intermediate in biosynthesis of iridoid monoterpenes, from defensive secretions of Plagiodera versicolora and Gastrophysa viridula (Coleoptera, Chrysomelidae). J. Chem. Ecol. 20:2089–2097.
Duffey, S. and Scudder, G. C. E. 1972. Cardiac glycosides in North American Asclepiadaceae, a chemical basis for unpalatability in brightly coloured Hemiptera and Coleoptera. J. Insect Physiol. 18:63–78.
Dyer, L. A. and Bowers, M. D. 1996. The importance of sequestered iridoid glycosides as a defense against an ant predator. J. Chem. Ecol. 22:1527–1539.
Falconer, R. A., Jablonkai, I., and Toth, I. 1999. Efficient synthesis of thioglycosides via a Mitsunobu condensation. Tetrahedron Lett. 40:8663–8666.
Feld, B., Pasteels, J., and Boland, W. 2001. Phaedon cochleariae and Gastrophysa viridula (Coleoptera: Chrysomelidae) produce defensive iridoid monoterpenes de novo and are able to sequester glycosidically bound terpenoid precursors. Chemoecology 11:191–198.
Garb, G. 1915. The eversible glands of a Chrysomelid larva, Melasoma lapponica. J. Entomol. Zool. 8:88–97.
GROSS J. and HILKER, M. 1995. Chemoecological studies of the exocrine glandular larval secretions of two Chrysomelid species (Coleoptera): Phaedon cochleariae and Chrysomela lapponica. Chemoecology 5/6:185–189.
Hartmann, T. 1999. Chemical ecology of pyrrolizidine alkaloids. Planta 207:483–495.
Hartmann, T. and Witte, L. 1995. in S. W. Pelletier (ed.). Alkaloids: Chemical and Biological Perspectives. Pergamon, Oxford, pp. 155–233.
Hartmann, T., Theuring, C., Schmidt, J., Rahier, M., and Pasteels, J. M. 1999. Biochemical strategy of sequestration of pyrrolizidine alkaloids by adults and larvae of chrysomelid leaf beetles. J. Insect Physiol. 45:1085–1095.
Hilker, M. and Schulz, S. 1994. Composition of larval secretion of Chrysomela lapponica (Coleoptera, Chrysomelidae) and its dependence on host–plant. J. Chem. Ecol. 20:1075–1093.
Hollande, A. C. 1911. L’autohémorrhée ou le rejet du sang chez les insectes (toxicologie du sang). Arch. Anat. Microsc. 13:171–318.
Kuhn, J., Pettersson, E. M., Feld, B. K., Burse, A., Termonia, A., Pasteels, J. M., and Boland, W. 2004. Selective transport systems mediate sequestration of plant glucosides in leaf beetles: a molecular basis for adaptation and evolution. Proc. Natl. Acad. Sci. USA 101:13808–13813.
Lorenz, M., Boland, W., and Dettner, K. 1993. Biosynthesis of iridodials in the defense glands of leaf beetle larvae (Chrysomelinae). Angew. Chem. Int. Ed. 32:912–914.
Malcolm, S. B. and Brower, L. P. 1989. Evolutionary and ecological implications of cardenolide sequestration in the monarch butterfly. Experientia 45:284–295.
Meinwald, J., Jones, T. H., Eisner, T., and Hicks, K. 1977. Defense-mechanisms of arthropods. new methylcyclopentanoid terpenes from larval defensive secretion of a chrysomelid beetle (Plagiodera versicolora). Proc. Natl. Acad. Sci. USA 74:2189–2193.
Nahrstedt, A. 1996, in J. T. Romeo, J. A. Saunders, and P. Barbosa (eds.). Phytochemical diversity and redundancy in ecological interactions. Plenum Press, New York, pp. 217–230.
Nicotra, F. 1998. Modified Carbohydrates and carbohydrate analogues. in G-J. Boons (ed.). Carbohydrate Chemistry. Blackie Academic & Professional, London, pp. 384–429.
Nishida, R. 2002. Sequestration of defensive substances from plants by Lepidoptera [review]. Annu. Rev. Entomol. 47:57–92.
Oldham, N. J., Veith, M., Boland, W., and Dettner, K. 1996. Iridoid monoterpene biosynthesis in insects—evidence for a de novo pathway occurring in the defensive glands of Phaedon armoraciae (Chrysomelidae) leaf beetle larvae. Naturwissenschaften 83:470–473.
Pasteels, J. M., Braekman, J. C., Daloze, D., and Ottinger, R. 1982. Chemical defense in chrysomelid larvae and adults. Tetrahedron 38:1891–1897.
Pasteels, J. M., Rowell-Rahier, M., Braekman, J. C., and Dupont, A. 1983. Salicin from host plant as precursor of salicylaldehyde in defensive secretion of chrysomeline larvae. Physiol. Entomol. 8:307–314.
Pasteels, J. M., Duffey, S., and Rowell-Rahier, M. 1990. Toxins in chrysomelid beetles. Possible evolutionary sequence from de novo synthesis to derivation from food–plant chemicals. J. Chem. Ecol. 16:135–142.
Paterson, N. F. 1930. The bionomics and morphology of the early stage of Paraphaedon tumidulus Germ (Coleoptera, Phytophaga, Chrysomelidae). Proc. Zool. Soc. Lond. 1930:627–676.
Pavan, M. 1953. Studi sugli antibiotici e insetticidi di origine animale I. Sul principo attivo della larva di Melasoma populi L. Arch. Zool. Ital. 38:157–183.
Rothschild, M. 1973. Secondary plant substances and warning colouration in insects. in H. van Emden (ed.). Symposium of the Royal Entomological Society of London. Blackwell Scientific Publications, Oxford, pp 59–83.
Sachs, L. 1999. Angewandte Statistik. Springer, Berlin.
Schulz, S., Gross, J., and Hilker, M. 1997. Origin of the defensive secretion of the leaf beetle Chrysomela lapponica. Tetrahedron 53:9203–9212.
Søe, A. R. B., Bartram, S., Gatto, N., and Boland, W. 2004. Are terpenoids in leaf beetle larvae synthesized de novo or from plant-derived precursors? Isotopes Environ. Health Stud. 40:175–180.
Termonia, A. and Pasteels, J. M. 1999. Larval chemical defence and evolution of host shifts in Chrysomela Leaf Beetles. Chemoecology 9:13–23.
Veith, M., Lorenz, M., Boland, W., Simon, H., and Dettner, K. 1994. Biosynthesis of iridoid monoterpenes in insects—defensive secretions from larvae of leaf beetles (Coleoptera, Chrysomelidae). Tetrahedron 50:6859–6874.
Acknowledgments
We thank Angelika Berg for rearing of the insects, and Dr. A. Burse for proofreading of the manuscript. Financial support by the Fonds der Chemischen Industrie, Frankfurt a.M. and from the Belgian Fund for Join Basic Research is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM1
(doc 14 kb)
Rights and permissions
About this article
Cite this article
Kuhn, J., Pettersson, E.M., Feld, B.K. et al. Sequestration of Plant-Derived Phenolglucosides by Larvae of the Leaf Beetle Chrysomela lapponica: Thioglucosides as Mechanistic Probes. J Chem Ecol 33, 5–24 (2007). https://doi.org/10.1007/s10886-006-9201-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-006-9201-1