Abstract
We report the volatile composition of the body scent of male C57BL/6J mice in comparison to the volatile composition of their urine. From a total of 67 components, nitromethane, propanoic acid, dimethyldisulfide, 1-octene, 1-hexanol, hexanoic acid, indole, α- and β-farnesene, and one unidentified component were observed only in the volatiles from the body of mice. On the other hand, 3-penten-2-one, 3-methyl-2-buten-1-ol, 3-methyl-cyclopentanone, p-xylene, 3-hepten-2-one, 2,3-dehydro-exo-brevicomin, benzylmethylketone, and 13 unidentified components were only found in urine volatiles. All other substances were present in the volatiles of both mice and their urine. Aliphatic aldehydes from pentanal to decanal were prominent mouse odor components. Because receptors for these aldehydes have been extensively characterized in the main olfactory organ, these components may be important for mice in recognizing their conspecifics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Social signaling between mice involves volatile and nonvolatile components present in secretions from the skin, the reproductive tract, and in urine. Volatile cues recognized from a distance interact with the main olfactory system. In contrast, the vomeronasal system requires direct contact and sniffing for activation (Luo et al., 2003) and it can therefore process chemical cues independent of their volatility. A number of low molecular mass volatile compounds have been characterized that elicit specific behavioral responses. These compounds are perceived either through the main or the accessory olfactory organ (reviewed in Brennan and Keverne, 2004; Stowers and Marton, 2005). However, compared to the explosive increase in knowledge on the molecular and cell biology of the olfactory system following the cloning of the receptor genes (summarized by Julius and Katz, 2004), considerably less effort has been directed toward the analysis of the complex mixtures of volatile and nonvolatile chemicals that are used by mice for recognition of kin or territory, in reproduction, or for behavioral responses such as fear and aggression. This situation motivated us to compare the volatiles in the mouse body scent vs. urine-derived volatiles.
Body scent, which may be important for recognition of mice from a distance, has so far not been analyzed. In contrast, there are several reports of the composition of urine odor. Miyashita and Robinson (1980) identified 94 volatiles collected by a cryogenic trapping procedure from urine of male Swiss Webster mice. In the study of Schwende et al. (1986), volatiles were flushed from urine of male BALB/c mice by helium gas and collected on Tenax-filled tubes. Subsequent gas chromatography/mass spectroscopy (GC/MS) led to the characterization of 61 components, among them several substances considered to be mouse pheromones (reviewed by Novotny, 2003). Singer et al. (1997) and Willse et al. (2005) extracted urine pools collected from male C57BL/6 mice with ether, and analyzed the concentrated extract by GC/MS, eventually finding 50 volatile components. Finally, Lin et al. (2005) combined solid phase microextraction (SPME) of urine volatiles from male BALB/c mice with GC/MS and reported 131 components. This variability in the number of detected volatiles reflects differences in odor collection procedures, as well as the choice of the mouse strains and the diet used for raising the animals. In addition, there is surprisingly limited agreement in the chemical identity of the components described in these reports.
In this study, we present the odor composition of the body scent of mice in comparison to the volatile composition of urine odor. This comparison revealed both odor-source-specific and common components, and raises questions regarding the formation and function of mouse volatiles.
Methods and Materials
Mice
Male C57BL/6J mice were raised under specific-pathogen-free conditions at the Max-Planck-Institut für Immunbiologie (Freiburg, Germany), or purchased from Charles River Wiga Deutschland GmbH (Sulzfeld, Germany). Germ-free male C57BL/6 mice and corresponding controls raised under specific-pathogen-free conditions were provided by Dr. B. Jilge (Tierforschungszentrum, Universität Ulm, Ulm, Germany).
To standardize the volatile components with respect to diet, randomly picked animals were kept singly in cages for 3–4 wk in ventilated hoods and fed on “Diät C 1000” (Altromin, Gesellschaft für Tierernährung GmbH, Lage, Germany) composed of casein, corn starch, saccharose, cellulose, vitamins, mineral substances and trace elements. At the time of body scent and urine collection, the mice were 12–13 wk old. The germ-free and corresponding control mice (three animals each) were used for mouse volatile and urine collection directly upon arrival.
Collection of Volatiles
Sampling of volatiles was performed under conditions that minimized the introduction of contaminants, e.g., from plastic materials. Mouse odor was collected from a horizontal stainless steel cylinder (length 14 cm, ID 9.8 cm) closed at one end, and supplied with a copper-ring-sealed ultrahigh vacuum flange on the other end (Fig. 1). Synthetic air (hydrocarbon-free; Messer-Griesheim, Sulzbach, Germany) was blown via a Mass Flow Controller GFC171 (ANALYT-MTC, Müllheim, Germany) at a rate of 200 ml/min through a charcoal filter (Small Cartridge ACCOSORB, Messer-Griesheim) attached to an inlet at the closed end of the cylinder into a stainless steel pipe (length 12 cm, 5 mm diameter) running parallel to the inner wall of the cylinder. The mouse volatiles were collected from the circulating air into freshly conditioned adsorption tubes (Tenax TA stainless steel prepacked sample tubes, Cat. No. N930-7005, Perkin-Elmer Instruments, Rodgau, Germany) directly connected to an outlet located opposite the inlet. Similarly, urine volatiles were collected at a flow rate of 100 ml air/min from custom-made, round-bottom glass dishes fitting into smaller stainless steel cylinders (length 11 cm, ID 3.8 cm) of otherwise similar design. By arranging a second Tenax or Carbotrap 300 tube (Cat. No. N930-7000) in tandem with the Tenax tube, it was shown that only minor amounts of highly volatile and polar compounds such as triethylamine (#1 in Table 1) were not completely adsorbed in the first tube.
Urine was collected at room temperature overnight from groups of three mice placed in metabolic cages (Tecniplast, Hohenpeiβenberg, Germany) and then stored at −20°C. During urine collection, the animals were supplied with water but no food. The urine samples were normalized to a creatinine concentration of 0.12 mg/ml by the addition of water. Volatiles were collected from 1 ml adjusted urine for 4 hr; during this time, about half of the sample evaporated. Between collections from different urine samples, the glass dishes and steel cylinders were rinsed with water and heated to 120°C for at least 1 hr.
Three mice were stimulated to urinate on paper tissue and the mice were then placed into the steel cylinder. Volatiles were collected for 1 hr. The vessel was then opened, inspected for the presence of urine, and the mice were again stimulated to urinate. Volatile body odor components were then collected for another hour in a fresh sample tube. Between collections with the next group of mice, the cylinder was rinsed with water and heated as described above. We had to eliminate some early mouse odor measurements due to urine contamination, which was subsequently prevented by the optimized procedure.
Analysis of Volatiles by Gas Chromatography/Mass Spectrometry
The volatiles were desorbed from the sample tubes in a Thermal Desorption System (Perkin-Elmer ATD400) and then separated by gas chromatography on an Agilent J&W DB-5 column (60 m × 0.25 mm, 0.25 μm; temperature program: 35°C for 5 min; 35–220°C at 3.75°C/min; 220–265°C at 7.5°C/min; 265°C for 3 min) in a Hewlett-Packard GC interfaced to an HP 5973 Mass Selective Detector.
Chromatograms were evaluated for 72 peaks in the following way. A data bank was set up from the mass spectra of all detectable peaks in two urine and three mouse odor chromatograms, which was then used to evaluate all other chromatograms for corresponding components. This procedure allowed reliable sorting of major components but was less successful for minor components. Because the retention times varied by 0.1–0.2 min for different chromatograms, assignments of the respective components required the individual comparison of retention time and mass spectra for all peaks in all chromatograms. Components were tentatively identified by comparison with the NIST 98 Mass Spectral Library (National Institute of Standards and Technology, Gaithersburg, MD, USA). Identities of some components were confirmed by comparisons of retention times and mass spectrum with those of commercially available or donated standards as detailed in Table 1. 6-Hydroxy-6-methyl-3-heptanone was synthesized according to the procedure described by Novotny et al. (1999a), using pyridinium chlorochromate (18 hr at room temperature in CH2Cl2) instead of CrO3/aq. H2SO4 as the oxidant.
Results
Collection and Analysis of Volatiles
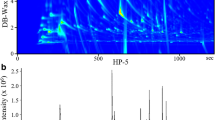
Using stainless steel vessels and highly purified air, we were able to eliminate most contaminants during volatile analysis (compare spectra in the upper panels of Fig. 2). Three remaining contaminants derived from the absorption tubes (peaks #9, 29, and 70, cf. Table 1) were useful landmarks in the chromatograms, and their abundance showed little variation. Typical chromatograms for mouse body and urine volatiles are shown in the lower panels of Fig. 2. Because the composition of urine volatiles may depend on the chow used for feeding (Brown et al., 1996), mice were kept individually on a synthetic diet for several weeks before the experiments. To obtain sufficient amounts of material for analysis in an adequate time, we always collected the body scent or the urine from groups of three randomly picked mice. We did not observe aggressive acts between these mice in the metabolic cage during urine collection. Although we cannot exclude aggressive behavior during mouse scent collection in the closed steel cylinder, we consider this unlikely because hair release caused by biting was not observed.
Our major concern was whether the collected mouse body volatiles were contaminated by urine components. We observed that the typical urine component 2,3-dehydro-exo-brevicomin (peak #57, cf. Table 1), which binds to the major urinary proteins (MUPs, Bacchini et al., 1992; Novotny et al., 1999b), was only detected in those chromatograms where the mice had urinated in the stainless steel vessel. When we stimulated the mice to urinate before insertion into the vessel, we consistently prohibited obvious urination in the vessel and did not detect 2,3-dehydro-exo-brevicomin in the spectra of mouse volatiles. However, small amounts of urine may be visually undetectable because marks may quickly dry in the air flux in the cylinder. Assuming that two abundant MUP ligands, 6-hydroxy-6-methyl-3-heptanone (peak #27) and 2-sec-butyl-4,5-dihydrothiazole (peak #62), are released solely in the urine and not excreted by MUP-producing glands (e.g., the submaxillary gland), a very rough estimate for the contribution of urine volatiles to the mouse body scent can be obtained from the ratio of the relative peak areas in the CG/MS spectra. The ratio of the medians for the mouse body and urine scents is 0.025 and 0.024 for peaks #27 and 62, respectively (Fig. 4 and Supplementary Material), suggesting that the mouse body volatiles may be contaminated by 2.5% of the urine volatiles collected from 1 ml urine (corresponding to a volume of 25 μl). This limitation must be taken into account when comparing the mouse and urine scent data. However, for less abundant urine volatiles such as 2,3-dehydro-exo-brevicomin, this contamination falls below the detection level in the mouse body scent.
In view of the large variability in the abundance of many components, it was important to document the reproducibility of successive mouse odor and duplicate urine odor measurements. As an example, Fig. 3A depicts the normalized abundance of n-aliphatic aldehydes from C5 to C10 in mouse body odor. Volatiles were collected from groups of three C57BL/6J mice for 1 hr (e.g., F3) and then for another hour (e.g., F4). With some exceptions, the agreement between successive samplings was reasonably good (compare equally marked pairs). However, for different groups of mice, aldehyde abundance varied by a factor of 5–10 (compare differentially marked pairs). Similar variability was observed in volatiles collected from urine samples normalized to a constant creatinine concentration (Fig. 3B). The four candidate pheromones chosen as examples differed in abundance by a factor of up to 10 when comparing urine samples from different groups of mice (compare differentially marked pairs). However, collection of volatiles from the same urine preparation was consistently reproducible (compare equally marked pairs). We concluded from these experiments that under our standardized conditions, variability in abundance was primarily attributable to the odor source rather than to the procedures used for collection and analysis of volatiles.
Representative examples for demonstrating the reproducibility of the odor collection experiments. (A) Normalized peak areas of the total ion current for C5–C10 aldehydes. Odor was collected from three C57BL/6J mice for 1 hr (F3) and directly thereafter for another hour (F4) or from another three C57BL/6J mice (F5 and F6). A third group of this strain was used for collecting the samples L03 and L04, and 3 d later for samples L07and L08. Average values of the peak area for the total ion current of the eight measurements were 0.16 for pentanal, 1.12 for hexanal, 0.59 for heptanal, 0.78 for octanal, 2.98 for nonanal, and 0.17 for decanal. (B) Normalized peak areas of the total ion current for pheromones. Note that bis-(methylthio)methane is not considered a pheromone, but overlaps with the 2-heptanone peak in the chromatogram. Double determinations (D15/D16, I5/I6, and M07/M08) from three urine samples collected from three animals each are shown. Average values of the peak areas for the total ion current of the eight measurements were 0.57 for heptanone + bis-(methylthio)methane, 3.87 for 2-sec-butyl-4,5-dihydrothiazole, 1.02 for 2,3-dehydro-exo-brevicomin, and 112.19 for 6-hydroxy-6-methyl-3-heptanone.
Composition of Volatiles
Taken together, we evaluated 22 chromatograms derived from 9 different groups of mice for body odor and 26 chromatograms for urine odor involving 11 urine samples from 11 different groups of C57BL/6J mice (Table 1 and Supplementary Material). A few measurements were performed with germ-free mice and controls raised under specific-pathogen-free conditions. All chromatograms were evaluated for the 67 components plus the three contaminants listed in Table 1. These 67 components were therefore characteristic of the total ensemble of samples analyzed in this study. The entire data set can be inspected in the Supplementary Material. Table 1 also indicates the fraction from all measurements where a given volatile could be detected.
Prominent in the list were acids (#6, 13, 24, 30, 32, 48), ketones (#2, 6, 8, 11, 14, 18, 27, 31, 38, 43, 51, 63), aldehydes (#12, 26, 40, 46, 54, 61, 65), and alcohols (#2, 7, 10, 17, 21, 22, 33, 37, 49, 59), as well as components considered to be mouse pheromones (#27, 38, 57, 62, 68, 69; Novotny, 2003). The structures of about one third of the components remain to be identified. Nitromethane (#4), propanoic acid (#13), dimethyldisulfide (#19), 1-octene (#25), 1-hexanol (#37), hexanoic acid (#48), indol (#67), α- and β-farnesene (#68 and 69), and one unidentified component (#64) were only observed in the volatiles from mouse body scent. On the other hand, 3-penten-2-one (#18), 3-methyl-2-buten-1-ol (#22), 3-methyl-cyclopentanone (#31), p-xylene (#36), 3-hepten-2-one (#43), 2,3-dehydro-exo-brevicomin (#57), benzylmethylketone (#63), and 13 unidentified compounds (#7, 23, 34, 35, 39, 47, 50, 52, 55, 56, 58, 60, and 66) were only found in urine volatiles. All other substances were present in the volatiles of both mice and their urine.
Although we did only a few experiments with mice fed on a conventional chow under germ-free or, as a control, specific-pathogen-free conditions, some observations are noteworthy. Mouse odor components (#4, 6, 12, 13, 20, 24, 26, 27, 30, 32, 33, 36, 40, 46, 48, 51, 54, 61, and 65) and urine odor components (#2, 6, 11, 12, 14, 15, 16, 18, 26, 27, 28, 39, 40, 41, 42, 43, 44, 45, 46, 52, 53, 54, 57, 61, 62, and 65) were clearly present in germ-free animals. This set includes the aliphatic aldehydes, which were therefore derived from the mice rather than their bacterial flora. One compound exclusively detected in germ-free mouse scent was 2-furanmethanol (#33). A more detailed comparison of germ-free and specific-pathogen-free mice would require a larger data set.
Figure 4 shows the medians and the quartile ranges on a logarithmic scale for all those components that were detected in more than 80% of the measurements (18 mouse body odor and 29 urine components). Small quartile ranges, e.g., for urine peaks #2, 11, 43, 44, and 46, indicated a good reproducibility in the detection of these volatiles. On the other hand, components such as mouse volatiles #10, 14, and 27 were highly variable in abundance. Inspection of the data after normalization to the medians suggested that the abundance of urine volatiles was less variable than the mouse volatiles (not shown).
Relative abundance of selected volatiles collected from the body of mice (A) or their urine (B). The peak area of the total ion current (TIC) is plotted on a logarithmic scale. The numbers refer to those listed in Table 1. Lengths of the horizontal bars represent the interquartile range of the measurements; median is indicated by a vertical bar within the horizontal bars
Discussion
To our knowledge, the present study represents the first report on the composition of mouse body scent. Although most volatiles are present in both body and urine odors, several components were specific to one or the other scent. We find it intriguing that the mouse odor reproducibly contained aliphatic aldehydes from C5 to C10 as prominent components, because odorant receptors for these aldehydes are found in abundance in the main olfactory epithelium and they have been characterized in detail (Zhao et al., 1998; Krautwurst et al., 1998; Araneda et al., 2000, 2004; Kaluza and Breer, 2000). Behavioral experiments using these aldehydes may show that they are important to mice in recognizing their conspecifics. Interestingly, short-chain aldehydes have also been found in human odor (Bernier et al., 2000; Haze et al., 2001; Curran et al., 2005). These aldehydes can be produced through oxidative degradation of monounsaturated fatty acids such as palmitoleic acid and vaccenic acid (Haze et al., 2001), which are secreted by sebaceous glands. In the rat, these aldehydes have been detected in extracts from the preputial gland (Sioni et al., 2005).
Another remarkable observation is that the abundance of some components, e.g., 1-methoxy-2-propanol (#10), 6-hydroxy-6-methyl-3-heptanone (#27), phenol (#49), and methylphenol (#59) varied over a range of 10- to 100-fold in different experiments (Fig. 4 and Supplementary Material). This finding suggests the occasional discharge of these components, either spontaneously or in response to environmental or social cues. Triggers for the release of these components and their source remain to be defined.
It is puzzling that there is little overlap between the volatile components identified by different investigators in mouse urine. Therefore, reported volatile composition may strongly depend on the method used for odor collection and analysis as well as the type of mouse strain and diet used in the experiments. In Table 1, those compounds that have previously been identified are marked. However, many volatiles found in this study have not been described previously and vice versa (Miyashita and Robinson, 1980; Schwende et al., 1986; Singer et al., 1997; Montag et al., 2001; compare also Willse et al., 2005; Lin et al., 2005). We used a synthetic diet for feeding the mice, which should reduce the complexity of volatiles, and we have tried to rigorously exclude contaminants during odor collection. In addition, control experiments showed that the absorption tubes used for odor collection were efficient. These efforts toward a standardization of the methodology were successful, in that the set of observed components was generally reproducible for the body and urine scents of C57BL/6 mice, but there was a large variation in abundance such that minor components frequently were not detected (Table 1 and Supplementary Material).
We confirmed the presence of putative pheromones known to be present in male urine (2-sec-butyl-4,5-dihydrothiazole, 2-heptanone, 2,3-dehydro-exo-brevicomin, and 6-hydroxy-6-methyl-3-heptanone). Two further pheromones, α- and β-farnesene, which are secreted by the preputial gland (Novotny et al., 1990), were detected in some mouse samples but not in urine samples. We also did not detect the recently identified semiochemical (methylthio)methanethiol, a component of very low abundance (Lin et al., 2005).
In view of the importance of the mouse as a model for mammalian behavior and for the analysis of recognition and processing of olfactory signals, a better standardization for the collection and analysis of volatiles released by the animals and their urine appears desirable.
References
Araneda, R. C., Kini, A. D., and Firestein, S. 2000. The molecular receptive range of an odorant receptor. Nat. Neurosci. 3:1248–1255.
Araneda, R. C., Peterlin, Z., Zhang, X., Chesler, A., and Firestein, S. 2004. A pharmacological profile of the aldehyde receptor repertoire in rat olfactory epithelium. J. Physiol. 555.3:743–756.
Bacchini, A., Gaetani, E., and Cavaggioni, A. 1992. Pheromone binding proteins of the mouse, Mus musculus. Experientia 48:419–421.
Bernier, U. R., Kline, D. L., Barnard, D. R., Schreck, C. E., and Yost, R. A. 2000. Analysis of human skin emanations by gas chromatography/mass spectrometry. 2. Identification of volatile compounds that are candidate attractants for the yellow fever mosquito (Aedes aegypti). Anal. Chem. 72:747–756.
Brennan, P. A. and Keverne, E. B. 2004. Something in the air? New insights into mammalian pheromones. Curr. Biol. 14:R81–R89.
Brown, R. E., Schellinck, H. M., and West, A. M. 1996. The influence of dietary and genetic cues on the ability of rats to discriminate between the urinary odors of MHC-congenic mice. Physiol. Behav. 60:365–372.
Curran, A. M., Rabin, S. I., Prada, P. A., and Furton, K. G. 2005. Comparison of the volatile organic compounds present in human odor using SPME-GC/MS. J. Chem. Ecol. 31:1607–1619.
Haze, S., Gozu, Y., Nakamura, S., Kohno, Y., Sawano, K., Ohta, H., and Yamazaki, K. 2001. 2-Nonenal newly found in human body odor tends to increase with aging. J. Invest. Dermatol. 116:520–524.
Julius, D. and Katz, L. C. 2004. A Nobel for smell. Cell 119:747–752.
Kaluza, J. F. and Breer, H. 2000. Responsiveness of olfactory neurons to distinct aliphatic aldehydes. J. Exp. Biol. 203:927–933.
Krautwurst, D., Yau, K. W., and Reed, R. R. 1998. Identification of ligands for olfactory receptors by functional expression of a receptor library. Cell 95:917–926.
Lin, D. Y., Zhang, S.-Z., Block, E., and Katz, L. C. 2005. Encoding social signals in the mouse main olfactory bulb. Nature 434:470–477.
Luo, M., Fee, M. S., and Katz, L. C. 2003. Encoding pheromonal signals in the accessory olfactory bulb of behaving mice. Science 299:1196–1201.
Miyashita, K. and Robinson, A. B. 1980. Identification of compounds in mouse urine vapor by gas chromatography and mass spectrometry. Mech. Ageing Dev. 13:177–184.
Montag, S., Frank, M., Ulmer, H., Wernet, D., GÖpel, W., and Rammensee, H.-G. 2001. “Electronic nose” detects major histocompatibility complex-dependent prerenal and postrenal odor components. Proc. Natl. Acad. Sci. USA 98:9245–9254.
Novotny, M. V. 2003. Pheromones, binding proteins and receptor responses in rodents. Biochem. Soc. Transact. 31:117–122.
Novotny, M., Harvey, S., and Jemiolo, B. 1990. Chemistry of male dominance in the house mouse, Mus domesticus. Experientia 46:109–113.
Novotny, M. V., Jemiolo, B., Wiesler, D., Ma, W., Harvey, S., Xu, F., Xie, T.-M., and Carmack, M. 1999a. A unique urinary constituent, 6-hydroxy-6-methyl-3-heptanone, is a pheromone that accelerates puberty in female mice. Chem. Biol. 6:377–383.
Novotny, M. V., Ma, W., Wiesler, D., and Zidek, L. 1999b. Positive identification of the puberty-accelerating pheromone of the house mouse: The volatile ligands associating with the major urinary protein. Proc. R. Soc. Lond. B 266:2017–2022.
Schwende, F. J., Wiesler, D., Jorgenson, J. W., Carmack, M., and Novotny, M. 1986. Urinary volatile constituents of the house mouse, Mus musculus, and their endocrine dependency. J. Chem. Ecol. 12:277–296.
Singer, A. G., Beauchamp, G. K., and Yamazaki, K. 1997. Volatile signals of the major histocompatibility complex in male mouse urine. Proc. Natl. Acad. Sci. USA 94:2210–2214.
Sioni, H. A., Bruce, K. E., Wiesler, D., David, F., Sandra, P., and Novotny, M. V. 2005. Stirr bar sorptive extraction: A new quantitative and comprehensive sampling technique for determination of chemical signal profiles from biological media. J. Chem. Ecol. 31:377–392.
Stowers, L. and Marton, T. F. 2005. What is a pheromone? Mammalian pheromones reconsidered. Neuron 46:699–702.
Willse, A., Belcher, A. M., Preti, G., Wahl, J. H., Thresher, M., Yang, P., Yamazaki, K., and Beauchamp, G. K. 2005. Identification of major histocompatibility complex-regulated body odorants by statistical analysis of a comparative gas chromatography/mass spectrometry experiment. Anal. Chem. 77:2348-2361.
Zhao, H., Ivic, L., Otaki, J. M., Hashimoto, M., Mikoshiba, K., and Firestein, S. 1998. Functional expression of a mammalian odorant receptor. Science 179:237–242.
Acknowledgments
We thank Dr. H. Mossman (Freiburg) for providing mice, K.Mori (Tokyo), C. Mucignat (Padova), and P. Landolt (Wapato, Wa) for donating standards, and Dr. J. Kopka (Berlin) for help in the interpretation of mass spectra. Helpful suggestions by Dr. M. Wandel and Prof. K.P. Hadeler and technical help by A. Hohneder and B. Pömmerl are gratefully acknowledged. P.O. thanks the Max-Planck-Gesellschaft for generous support.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
The measurements refer to the analysis of mouse body odor or urine volatiles, respectively, for C57BL/6J mice (wild type), germ-free or matching C57BL/6 mice raised under specific-pathogen-free (SPF) conditions. Mice used for the experiments have been serially numbered (mouse #). The spectra have been designated as indicated (spectrum #).
The table shows the relative total ion current for all components defined by their substance number and retention time (see Table 1). The plus (+) refers to the detection of characteristic fragments for the respective component.
Table 1
(PDF 46 kb)
Rights and permissions
About this article
Cite this article
Röck, F., Mueller, S., Weimar, U. et al. Comparative Analysis of Volatile Constituents from Mice and their Urine. J Chem Ecol 32, 1333–1346 (2006). https://doi.org/10.1007/s10886-006-9091-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-006-9091-2