Abstract
Mammalian herbivores, particularly browsers and folivores, encounter and consume a range of plant chemical defenses [plant secondary metabolites (PSMs)] on a regular basis. The physiological regulation of PSM ingestion and the resulting behavioral responses of mammalian herbivores directly affect their feeding decisions and the subsequent foraging strategies that they adopt. Generalist mammalian herbivores are hypothesized to consume a generalized diet because of physiological limitations of their detoxification systems. The consumption of a generalized diet is proposed to enable toxin (PSM) dilution through the use of multiple detoxification pathways. We tested the predictions of the detoxification–limitation hypothesis by offering two chemically different plant species, Eucalyptus regnans and E. globulus, to a generalist mammalian folivore, the common brushtail possum (Trichosurus vulpecula), as single- and mixed-species diets. By feeding more efficiently, brushtail possums benefited more, through increased intake, on the mixed-species diet than on either of the single-species diets. We argue that frequently switching between chemically diverse foliage reduces the physiological constraints imposed by a PSM-rich diet and enables more efficient feeding. The behavioral responses of brushtail possums were consistent with the proposed physiological constraints of a chemically defended diet, offering support for predictions of the detoxification–limitation hypothesis. We suggest that feeding behavior of herbivores may be a useful indicator of the physiological constraints imposed by a chemically defended diet.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
By consuming plants, herbivores encounter a range of plant physical and chemical defenses (Feeny, 1970; Bryant and Kuropat, 1980). Plant chemical defenses, or plant secondary metabolites (PSMs), predominantly occur throughout woody plants as highly diverse compounds, both structurally and functionally. Ingestion of PSMs can incur physiological costs that include digestibility reduction (Robbins et al., 1987), toxicity (Pfister et al., 1997), and acidosis (Foley, 1992). To exploit woody plants, mammalian browsers (e.g., roe deer, Capreolus capreolus; Tixier and Duncan, 1996) and folivores (e.g., common brushtail possum, Trichosurus vulpecula; Kerle, 1984) must be able to process and deal with these chemical defenses. Mammalian browsers and folivores (hereafter referred to as herbivores) are indeed able to subsist on a chemically defended diet through a range of physiological and behavioral adaptations (Cork and Foley, 1991; McArthur et al., 1991).
Following ingestion and absorption, most PSMs must be detoxified to reduce the detrimental effects that they pose. Mammalian herbivores have evolved a series of detoxification pathways that convert absorbed PSMs into more hydrophilic and polar compounds through oxidation, reduction, or hydrolysis (phase I reactions), making them easier to excrete and/or preparing them for conjugation with an endogenous molecule (phase II reactions), again enhancing excretion (Sipes and Gandolfi, 1991). However, there are several limitations of these detoxification processes. Detoxification pathways rely on a series of rate-limited reactions, which, in turn, govern how much a herbivore can ingest per unit of time (Freeland and Janzen, 1974). Mammalian herbivores have been shown to regulate PSM ingestion at or below a proposed threshold level (Pfister et al., 1997; Lawler et al., 1998; Boyle and McLean, 2004). The inability to tightly regulate ingestion below a particular threshold may result in toxicosis, internal malaise, and, if severe enough, may even be fatal to the animal (Freeland and Janzen, 1974). Whereas most PSMs must be detoxified to aid in their excretion, the reactions involved can generate strong organic acids (Sipes and Gandolfi, 1991), which themselves may cause disturbances to a herbivore's acid–base status and, subsequently, disruptions to physiological and biochemical processes (Chilcott and Hume, 1984; Foley, 1992). All acid metabolites must, therefore, be buffered and excreted from the body (Foley et al., 1995). Detoxification of PSMs, through associated metabolic and excretory pathways, is an energetically expensive process (Cork and Foley, 1991; Freeland, 1991).
The capacity to which herbivores efficiently detoxify PSMs governs their subsequent feeding strategies. Only when a herbivore is able to minimize the energetic costs of detoxification (Sorensen et al., 2005), and efficiently metabolize and excrete ingested PSMs (Boyle et al., 1999), is it able to specialize on a relatively homogenous diet. A generalized feeding strategy is by far the most common feeding approach adopted by mammalian browsers and folivores (Freeland, 1991). The consumption of a generalized diet is hypothesized to ensure adequate nutritional balance (Westoby, 1978). Acting over a much shorter timeframe, the detoxification–limitation hypothesis proposes that a generalized diet of chemically diverse plants enables toxin dilution, through the use of multiple detoxification pathways (Freeland and Janzen, 1974). Consistent with this hypothesis, research has shown that generalist folivores perform better on mixed than single forage diets through increased intake (Dearing and Cork, 1999; Wiggins et al., 2003). Explicit testing of the detoxification–limitation hypothesis is difficult because of limited knowledge of the mechanisms by which herbivores detoxify specific PSMs (Marsh et al., 2005).
Predictions of the detoxification–limitation hypothesis offer insight and understanding into the foraging decisions made by generalist mammalian herbivores. Consumption of a PSM-rich diet imposes direct physiological constraints to the herbivore. These constraints, in turn, should govern how generalists behaviorally respond to their diet, through the selection and mixing of different items. We have recently demonstrated that a folivore's rate of intake, and the frequency of switching between two chemically different plant species, was essential for maximizing intake (Wiggins et al., 2006). These feeding strategies were hypothesized to be directly related to the folivore's blood plasma PSM concentrations, a direct physiological constraint to the herbivore. A herbivore's feeding behavior is likely to be pivotal in how it responds to its dietary physiological constraints and could be used as an indicator of such constraints. Thus, the simultaneous investigation of three factors (intake, physiological responses, and behavioral responses) of herbivores to a mixed diet should enable us to evaluate predictions of the detoxification–limitation hypothesis. We know of no previous studies that have directly integrated these three components concurrently. Such integration is essential for the development of a more thorough understanding of the feeding decisions, and ultimately the foraging strategies, adopted by generalist mammalian herbivores.
We set out to test predictions of the detoxification–limitation hypothesis, in an attempt to explain why generalist mammalian herbivores are able to perform better on a chemically diverse diet than on a single species diet. We offered two chemically different plant species, Eucalyptus regnans and E. globulus (Wiggins et al., 2006), to a generalist mammalian folivore, the common brushtail possum (T. vulpecula), as single- and mixed-species diets. The four specific aims of this study were to (1) demonstrate differences in chemistry between the two eucalypt species; (2) determine whether intake was greater on the mixed- than single-species diets; (3) compare urinary characteristics between diet treatments as a crude indicator of the physiological status of the animals (e.g., pH, titratable ions, ammonia, urea); and (4) quantify differences in feeding behavior relative to maximizing intake.
Methods and Materials
Animals and Diet
Six adult brushtail possums (T. vulpecula), three males and three females [3.34 ± 0.59 kg (body weight mean ± SD)], were collected from Hobart (Tasmania, Australia), housed in a covered outdoor enclosure in individual mesh cages (4.3 × 1.7 × 2.5 m) at the School of Zoology, University of Tasmania, and provided with a nest box and logs for above-ground runways. Animals were maintained on a basal diet that was 18% dry matter (DM), consisting of [as % fresh matter (FM)] 46% apple, 35% silver beet, 10% carrot, 5% lucerne (ground to pass through a 1-mm sieve), and 4% raw sugar. Fruit and vegetable constituents were mixed in a food processor, then combined with dry ingredients. Food was provided at levels sufficient for maintenance (McArthur et al., 2000) and so was freshwater daily.
Feeding Trial
Possums were initially offered 50% of maintenance requirements of a basal diet and E. regnans and E. globulus sapling foliage ad libitum for 5 d, 1 wk before the trial. They received 120% basal diet two nights directly before the trial. Foliage was collected from Geeveston, Tasmania, and stored with stems in freshwater in a 5°C cool room. The feeding trial consisted of three treatment diets fed to possums in a balanced crossover design (Ratkowsky et al., 1993). The treatment diets were (1) E. regnans and (2) E. globulus single-species diets and (3) E. regnans and E. globulus mixed diet. Foliage was fed ad libitum in 300-g bunches, provided fresh each day. Each treatment was run for 4 consecutive nights, and immediately followed by 4 “rest” nights where possums were offered 120% basal diet to ensure they maintained body weight during the trial. Foliage intake was measured daily and expressed as grams dry matter per kilogram of body mass for each possum [g DM (kg BM)−1].
Foliage Properties
Two control bunches of foliage per species per night were pooled and subsampled for physical and chemical analyses to confirm that foliar secondary chemical properties differed. Foliage was assayed for DM, leaf toughness, nitrogen, fiber, oils, and phenolics.
Dry Matter
Subsamples of foliage were oven-dried at 55°C for 48 hr to determine percentage dry matter (% DM).
Leaf Toughness
Five leaves per species per night were sampled at three sections of the leaf (upper, middle, and lower) by using a dual tension gauge portable penetrometer (Chatillon, John Chatillon & Sons, Inc., NY, USA). Units are an index only, expressed as total grams of force required to puncture the thickness of foliage using a 1-g, 0.60-mm pin (Sands and Brancatini, 1991).
Nitrogen
Foliage was oven-dried at 55°C for 48 hr and ground to pass through a 1-mm sieve by using a cyclone grinder, then further oven-dried at 70°C for 24 hr. Following this, a sulfuric acid and hydrogen peroxide digest was performed following methods of Lowther (1980). Digested samples were colorimetrically analyzed for nitrogen (QuikChem reference 10-107-06-2E, Lachat Instruments, WI, USA) on a continuous flow injector analyzer (QuikChem 800, Lachat Instruments). Results are expressed as % DM.
Fiber
Ground samples were analyzed for plant cell-wall components of neutral detergent fiber, acid detergent fiber, and lignin following methods in the ANKOM200/220 Technology Operator's Manual (1997). Results are expressed as % DM.
Total Oils
Fresh-frozen foliage was thawed prior to oil and phenolic assays. Oils were extracted following methods modified from Jones et al. (2002). Briefly, 1 g of foliage was cut into 1-cm2 pieces and soaked in 10 ml dichloromethane for 1 hr. Extracts were analyzed by combined gas chromatography–mass spectrometry (GC-MS) as detailed in O'Reilly-Wapstra et al. (2004). Total ion currents were determined for the sum of all oil components (total oils) and the heptadecane internal standard. Results for total oils were standardized by dividing by the internal standard. Results are expressed as mg “cineole equivalents” per g DM.
Total Phenolics
One gram of foliage was cut into 1-cm2 pieces, homogenized with a Polytron Homogenizer (POLYTRON® MR2100, Kinematica AG, Switzerland) in 20 ml acidified (pH 1) methanol (Close et al., 2001), and then boiled for 1.5 min. Samples were extracted overnight at 5°C in the dark, centrifuged at 10,000 rpm for 7 min, and analyzed by high-performance liquid chromatography, detailed in O'Reilly-Wapstra et al. (2004). Differences between major classes of phenolic compounds, the hydrolyzable tannins, and the formylated phloroglucinol compounds (FPCs) only were identified. Representative chromatograms of oils and phenolics were used as a visual reference to demonstrate differences in PSM composition between E. regnans and E. globulus.
Urine Collection
On night 4 of each treatment, four of the six possums (because of logistic constraints) were placed in individual metabolism cages (60 × 60 × 45 cm) for 24 hr for urine collection at the School of Pharmacy, University of Tasmania. Possums were fed with their allocated treatment diets of fresh foliage overnight. Urine was collected into containers held in liquid nitrogen and frozen immediately, then stored at −20°C until later analysis. Animals were acclimatized to the metabolism cages for a 24-hr period 1 week before commencement of the feeding trial. Urinary properties of pH, titratable ions, ammonia, and urea were measured for N = 3 possums because of low urinary output of one animal during the collection period. Ammonia and urea methods were modified methods of Faulkner and King (1970). The titratable ion variable was based on the concept of titratable acids (Foley, 1992).
pH
Urine samples were thawed and pH was measured with a TPS digital pH meter.
Titratable Ions
Either 0.5 M HCl (for basic urine solutions) or 0.01 M NaOH (for acidic urine solutions) was used to titrate urine samples to pH 7.4 (neutral). Titrations were performed at 20°C, and results are expressed as mmol l−1 to pH 7.4.
Ammonia
Tubes were set up with 0.1, 0.4, and 1.0 ml of 1:10 dilutions of urine and made up to 1.0 ml with deionized H2O. Tubes containing a standard ammonium sulfate solution [800 nmol NH4 + ml−1; derived from 53 ml of 0.1% ammonium sulfate (Ajax Chemicals, Sydney, Australia) solution diluted in 1 l deionized H2O] were also set up. To each tube, 5 ml of phenol–nitroprusside reagent [10.0 g phenol (May & Barker Ltd., Dagenham, England) and 0.05 g sodium nitroprusside (May & Baker Ltd.) dissolved in 1 l deionized H2O] were added and mixed by vortex. Following this, 5 ml of NaOH–hypochlorate reagent [5.0 g NaOH (Sigma, St. Louis, MO, USA) and 44 ml of 4% sodium hypochlorite (Aldrich, Milwaukee, WI, USA) dissolved in 1 l deionized H2O] were added to tubes and mixed by vortex. Tubes were incubated at 37°C for 30 min, and absorbance was read at 570 nm against the reagent blank. Results are expressed as μmol d−1.
Urea
Tubes were set up with 0.1, 0.4, and 1.0 ml of 1:500 dilutions of urine and made up to 1.0 ml with deionized H2O. Tubes containing a standard urea solution [250 nmol ml−1; derived from 30.0 mg urea (AnalaR®, Kilsyth, Australia) diluted in 2 l deionized H2O] were also set up. To each tube, 0.1 ml of urease suspension {100.0 mg urease [Sigma] dissolved in 100 ml phosphate–EDTA buffer [made from 2.0 g Na2EDTA (BDH Chemicals Ltd., Poole, England) and 5.7 g anhydrous Na2HPO4 (Ajax Chemicals, Auburn, Australia) dissolved in 100 ml deionized H2O, adjusted to pH 6.5 with 1 M HCl (Ajax Chemicals), then made up to 200 ml with deionized H2O]}, stored on ice, were added and mixed by vortex, then incubated at 37°C for 15 min. Following this, 5 ml of phenol–nitroprusside reagent were added to tubes and mixed, followed by 5 ml of NaOH–hypochlorate reagent (see Ammonia in Methods and Materials), with tubes mixed by vortex. Tubes were incubated at 37°C for 30 min, and absorbance was read at 570 nm against the reagent blank. Results are expressed as μmol d−1.
Feeding Behavior
Feeding behavior was measured for all six possums from nights 1–3. Animals were filmed nightly from 18:00 until 08:00 hr using individual B&W Bullet CCD Cameras (equipped with a Samsung™ sensor, JayCar, Hobart, Tasmania), one camera per cage. Each camera was connected to a Panasonic® Video Cassette Recorder (Series NV-FJ630). An 80-W red flood lamp (Osram Par 38, manufactured in EC) was positioned in each cage for enhanced visibility. Video footage was recorded directly onto BASF E-300 cassette tapes by using extended long-play recording speed. Data from video footage were summarized using The Observer® (v 4.1, Noldus Information Technology, The Netherlands, 2002). Behavioral variables were calculated using The Observer® and Microsoft® Excel (Microsoft Corporation, 1997). These variables were (1) time from first to last feeding bout (incorporating nonfeeding activity); (2) total feeding time; (3) rate of intake; (4) number of feeding bouts; (5) time per feeding bout; (6) intake per feeding bout; (7) total number of switches; and (8) the frequency of feeding bouts within which switching occurred. A single feeding bout was defined as the time from the possum's first bite to the end of its last chew, with at least 1 min of nonfeeding directly following this last chew (Wiggins et al., 2006). Total number of switches was a count of switches between plant species, which could occur both during and between feeding bouts. The frequency of feeding bouts within which switching occurred was calculated by dividing the total number of “switching bouts” per night by the total number of feeding bouts for that night. Switching behavior could only be measured on the mixed diet. Results for all intake and behavioral variables are expressed as mean values ± standard errors (SE).
Statistical Analyses
The average of 3 d data for each treatment for all six possums was used in the analyses. Dependent variables of intake and aspects of feeding behavior were tested against the independent variables of possum, treatment, period, and carryover, using the general linear model procedure (PROC GLM) in SAS (SAS v6.12, SAS Institute Inc. 1989), following the methods of Ratkowsky et al. (1993). The Wilk-Shapiro statistic, normal probability plots, and standardized residual plots all indicated normality of the data. When an effect was significant, pairwise comparisons of the least-squares means were made by using the Tukey-Kramer adjustment. Data for three possums from d 4 were used for urine analysis. Dependent variables of intake, pH, titratable ions, ammonia, and urea were tested against the independent variables of possum, period, and treatment (PROC GLM, SAS Institute Inc. 1989). Differences between foliar physical and chemical properties of E. regnans and E. globulus were also tested (PROC GLM, SAS Institute Inc. 1989).
Results
Foliage Chemistry
Leaf percentage DM, leaf toughness, and nitrogen were similar between E. regnans and E. globulus foliage (Table 1). Of the primary constituents, only fiber differed between the two species (Table 1). Of the secondary metabolites, E. regnans contained higher levels of total oils than E. globulus (Table 1). Of the 15 major oil compounds identified, only one compound, bicyclogermacrene, was present in both plant species, with amounts similarly low in both species (Fig. 1). There were also major differences in phenolic profiles between plant species. The FPCs peaking between 9 and 11 min in E. globulus are not present in E. regnans (Fig. 2), whereas differences in the early major peaks indicate the presence of different hydrolyzable tannins in each species (peaks substantially differ from 1 to 4 min and from 4 to 6 min between the foliage species).
Representative chromatograms of (a) E. regnans and (b) E. globulus oils derived from combined gas chromatography–mass spectrometry. Compounds are labeled with assigned letters, which stand for (a) α-phellandrene; (b) β-phellandrene; (c) piperitol; (d) piperitone; (e) bicyclogermacrene; (f) hedycaryol/elemol conversion; (g) β-eudesmol; (h) n-heptadecane (internal standard); (i) eudesmyl acetate; (j) α-pinene; (k) limonene; (l) 1,8-cineole; (m) terpinyl acetate; (n) aromadendrene; and (o) globulol.
Intake
Possums consumed substantially more foliage on the mixed- than on the single-species diets (F 2,17 = 14.99, P = 0.001; Fig. 3).
Urinary Properties
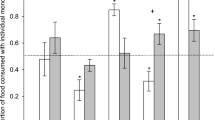
Possums produced a slightly acidic urine on E. globulus foliage, alkaline on E. regnans foliage, and urine at relatively neutral pH on the mixed diet (F 2,8 = 9.20, P = 0.032; Fig. 4). Urine from the E. regnans single-species diet required the greatest titration to return it to neutral pH (F 2,8 = 7.68, P = 0.043). Urinary properties of ammonia (F 2,8 = 0.95, P = 0.458) and urea (F 2,8 = 1.42, P = 0.343) were not significantly different between diets (Fig. 3).
Urinary properties for brushtail possums fed E. regnans and E. globulus foliage across treatment diets. (a) pH; (b) titratable ions (mmol l−1); (c) ammonia (μmol d−1); and (d) urea (μmol d−1). Values are least-squares means for three possums with SE bars. Letters that differ are significantly different (α = 0.05 after Tukey–Kramer adjustment for multiple comparisons).
Feeding Behavior
Possums spent longer feeding on the mixed and E. globulus single-species diets than on the E. regnans single-species diet (F 2,17 = 20.15, P = 0.002; Fig. 5a). They ate faster on the mixed diet than the respective single-species diets (F 2,17 = 4.53, P = 0.049; Fig. 5b). Possums did not alter the number of feeding bouts per night (mean 11.8 ± 1.3, P = 0.209), but their time per feeding bout (F 2,17 = 7.55, P = 0.014; Fig. 5c) and intake per feeding bout (F 2,17 = 8.09, P = 0.008; Fig. 5d) were least on E. regnans and generally greater on the mixed diet. There was no difference between the time from the first to last feeding bout in response to treatment diets (mean 7.0 ± 0.2 hr, P = 0.113).
(a) Total feeding time (minutes); (b) rate of intake [g DM (kg BM)−1 min−1]; (c) time per feeding bout (min bout−1); and (d) intake per feeding bout [g DM (kg BM)−1 bout−1] of E. regnans and E. globulus foliage by brushtail possums across diet treatments. Values are least-squares means with SE bars for total intake. Letters that differ are significantly different (α = 0.05 after Tukey–Kramer adjustment for multiple comparisons).
On the mixed diet, possums switched between foliage both within and between feeding bouts 10.6 ± 0.9 times per night. Of these, 46% of switches (4.4 ± 0.4) occurred within feeding bouts, whereas 54% (6.3 ± 0.4) occurred between feeding bouts. Of the within-bout switching figure, possums switched more often after 50% of their total feeding bouts had passed (3.0 ± 0.2 times) than before (1.5 ± 0.2 times; mean difference 1.2 ± 0.2, P = 0.001).
Discussion
Foliar Chemical Properties
Foliar secondary chemical constituents of total oils and total phenolics differed substantially between E. regnans and E. globulus, as demonstrated by the minimal overlap in the major oils identified for either species and the absence of known herbivore feeding deterrents, the FPCs (O'Reilly-Wapstra et al., 2004), in E. regnans. There were also differences in the types of hydrolyzable tannins present in either species. This result confirms major differences between the foliar secondary chemistry in the two species, as previously demonstrated (Wiggins et al., 2006).
Herbivore Urinary Status
Consumption of each diet treatment generated different urinary characteristics in the common brushtail possum (T. vulpecula). Brushtail possums produced highly alkaline urine on the E. regnans single-species diet, slightly acidic urine on the E. globulus single-species diet, and relatively neutral urine on the mixed diet. Previous studies have shown that the consumption of Eucalyptus diets generally creates acidic urine to varying degrees, as a result of high buffering activity (as indicated by levels of ammonia excretion) because of organic acid production from the metabolism of dietary terpenes (Hamm and Simon, 1987; Foley, 1992; Foley et al., 1995). Interestingly, the consumption of E. regnans did not generate an acid urine. In fact, it was highly alkaline and showed similarities with urine of both brushtail possums and desert woodrats (Neotoma stephensi and N. albigula) consuming an artificial maintenance diet of fruit and vegetable matter (Dearing et al., 2000; Wiggins et al., unpublished data). The generation of alkaline urine may explain why the urinary properties of pH, ammonia, and urea indicated minimal disturbances to the acid–base status of possums feeding on E. regnans, as these measurements have only previously been used to demonstrate changes in the physiological properties of acid urine (e.g., Foley, 1992). Therefore, with the examination of acid and alkaline urine, titratable ions appear to be the most relevant indicator of herbivore physiological status. What the urinary results do suggest, more importantly, is that possums deal with dietary PSMs in E. regnans differently from E. globulus (confirming our premise for using these two species to test the effects of mixed diets).
Intake and Behavioral Responses
Brushtail possums ate more on a mixed diet of E. regnans and E. globulus foliage than on their respective single-species diets. This result is consistent with previous research (Dearing and Cork, 1999; Wiggins et al., 2003, 2006) and offers support for the predictions of the detoxification–limitation hypothesis (Freeland and Janzen, 1974). Marsh et al. (2006) recently demonstrated that brushtail possums were only able to eat more on a mixed PSM diet, compared with their respective single-PSM diets, when individual PSMs were metabolized by using different (major) detoxification pathways. Brushtail possums were unable to eat more on a mixed diet when the PSMs were metabolized with competing detoxification pathways (Marsh et al., 2006). The fact that intake of the mixed diet in our trial was greater than either single-species diet indicates that the chemical properties of E. regnans and E. globulus foliage were sufficiently diverse to impose different physiological (detoxification) constraints to brushtail possums.
Brushtail possums ate more on the mixed-species than either of the single-species diets by eating faster, while tending to spend more time and eating more per feeding bout. They frequently switched between both species of foliage on the mixed diet, with the number of within-bout switches increasing in the latter half of their feeding bouts. By frequently switching between foliage on the mixed diet, possums may have been able to overcome the short-term intake constraints because of the toxic effects of each species. Wiggins et al. (2006) proposed that diet switching was a direct behavioral response to the detoxification limitations of a PSM-rich diet. That is, when intake on one species is constrained because of saturation of specific detoxification pathways, possums can continue feeding on the other species. They demonstrated that brushtail possums were only able to maximize their intake when able to frequently switch between dietary species during their nightly feeding activities: when foliage time and availability constraints were imposed on possums, they were unable to maintain intake (Wiggins et al., 2006). Results of this current study expand on this hypothesis through a more detailed investigation of the frequency and occurrence of dietary switching.
By switching between foliage more frequently during the latter half of their feeding bouts, we suggest that brushtail possums were responding to the accumulation of PSMs and/or detoxification metabolites over their nightly feeding timeframe. Brushtail possums have been shown to regulate dietary 1,8-cineole ingestion so as not to exceed a critical threshold level over a 3-hr feeding timeframe, but cineole metabolites accumulated throughout that sampling period (Boyle et al., 2005). Cessation of feeding on one plant species may have been a direct response to blood PSM levels that were nearing, or had indeed reached, a specific critical threshold level. By immediately switching to the next available plant species, possums may have been attempting to regulate PSM ingestion of E. regnans while continuing to feed on E. globulus. Increasing the frequency of this switching behavior during the latter stages of their feeding activities presumably reflects that detoxification limitations were at their most critical stages for both species of foliage. This result strengthens the argument that diet switching in a generalist mammalian folivore is a behavioral response to detoxification limitations of a PSM-rich diet (Wiggins et al., 2006).
The ability to frequently switch between foliage, which was not an option on the single-species diets, enabled possums to feed more efficiently, through a faster feeding rate and greater feeding bout size and length. These behavioral responses to a physiologically constraining diet represent an important facet that helps explain why, and how, generalist mammalian herbivores are able to benefit from a chemically diverse diet.
In summary, the common brushtail possum was able to benefit from the consumption of a chemically diverse diet, even over a short feeding timeframe. Possums fed more efficiently on a mixed- than on a single-species diet and achieved increased intake. We propose that diet switching is a behavioral strategy adopted by T. vulpecula as a direct response to the physiological limitations of detoxification pathways, enabling them to efficiently consume a chemically diverse diet.
This integration of dietary chemical properties, animal physiological, and behavioral data has enabled us to test predictions of the detoxification–limitation hypothesis. Herbivore feeding behavior (e.g., diet switching) may be a useful indicator of the physiological constraints (e.g., detoxification limitations) imposed by a PSM-rich diet. Understanding the underlying processes that govern the intake of generalist herbivores is important: the way in which a herbivore is physiologically constrained by its diet, and the resulting behavioral responses, will directly impact on the herbivore's foraging decisions, and subsequently their foraging energetics.
References
ANKOM220/220 Fiber Analyzer Operator's Manual. 1997. ANKOM Technology, Fairport, NY, USA.
Boyle, R. and McLean, S. 2004. Constraint of feeding by chronic ingestion of 1,8-cineole in the brushtail possum (Trichosurus vulpecula). J. Chem. Ecol. 30:757–775.
Boyle, R., McLean, S., Foley, W. J., and Davies, N. W. 1999. Comparative metabolism of dietary terpene, p-cymene, in generalist and specialist folivorous marsupials. J. Chem. Ecol. 25:2109–2126.
Boyle, R., McLean, S., Brandon, S., and Wiggins, N. L. 2005. Rapid absorption of dietary 1,8-cineole results in critical blood concentration of cineole and immediate cessation of eating in the common brushtail possum (Trichosurus vulpecula). J. Chem. Ecol. 31:2775–2790.
Bryant, J. P. and Kuropat, P. J. 1980. Selection of winter forage by subarctic browsing vertebrates: the role of plant chemistry. Ann. Rev. Ecolog. Syst. 11:261–285.
Chilcott, M. J. and Hume, I. D. 1984. Digestion of Eucalyptus andrewsii foliage by the common ringtail possum, Pseudocheirus peregrinus. Aust. J. Zool. 32:605–613.
Close, D. C., Davies, N. W., and Beadle, C. L. 2001. Temporal variation of tannins (galloylglucoses), flavonols and anthocyanins in leaves of Eucalyptus nitens seedlings: implications for light attenuation and antioxidant activities. Aust. J. Plant Physiol. 28:269–278.
Cork, S. J. and Foley, W. J. 1991. Digestive and metabolic strategies of arboreal mammalian folivores in relation to chemical defenses in temperate and tropical forests, pp. 133–166, in R. T. Palo and C. T. Robbins (eds.). Plant Defenses Against Mammalian Herbivory. CRC Press, Boca Raton, FL.
Dearing, D. M. and Cork, S. 1999. Role of detoxification of plant secondary compounds on diet breadth in a mammalian herbivore, Trichosurus vulpecula. J. Chem. Ecol. 25:1205–1219.
Dearing, D. M., Mangione, A. M., and Karasov, W. H. 2000. Diet breadth of mammalian herbivores: nutrient versus detoxification constraints. Oecologia 123:397–405.
Faulkner, W. R. and King, J. W. 1970. Renal function, pp. 975–1014, in N. W. Tietz (ed.). Fundamentals of Clinical Chemistry. W. B. Saunders Company, London.
Feeny, P. 1970. Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars ecology. Ecology 51:565–581.
Foley, W. J. 1992. Nitrogen and energy retention and acid–base status in the common ringtail possum (Pseudocheirus peregrinus): evidence of the effects of absorbed allelochemicals. Physiol. Zool. 65:403–421.
Foley, W. J., McLean, S., and Cork, S. J. 1995. Consequences of biotransformation of plant secondary metabolites on acid–base metabolism in mammals—a final common pathway? J. Chem. Ecol. 21:721–743.
Freeland, W. J. 1991. Plant secondary metabolites: biochemical coevolution with herbivores, pp. 61–81, in R. T. Palo and C. T. Robbins (eds.). Plant Defenses Against Mammalian Herbivory. CRC Press, Boca Raton, FL.
Freeland, W. J. and Janzen, D. H. 1974. Strategies in herbivory by mammals: the role of plant secondary compounds. Am. Nat. 108:269–289.
Hamm, L. L. and Simon, E. E. 1987. Roles and mechanisms of urinary buffer excretion. Am. J. Physiol. 253:595–605.
Jones, T. H., Potts, B. M., Vaillancourt, R. E., and Davies, N. W. 2002. Genetic resistance of Eucalyptus globulus to autumn gum moth defoliation and the role of cuticular waxes. Can. J. For. Res. 32:1961–1969.
Kerle, J. A. 1984. Variation in the ecology of Trichosurus: its adaptive significance, pp. 115–128, in A. P. Smith and I. D. Hume (eds.). Possums and Gliders. Surrey Beatty & Sons, Sydney.
Lawler, I. R., Foley, W. J., Eschler, B. M., Pass, D. M., and Handasyde, K. 1998. Intraspecific variation in Eucalyptus secondary metabolites determines food intake by folivorous marsupials. Oecologia 116:160–169.
Lowther, J. R. 1980. Use of a single sulphuric acid–hydrogen peroxide digest for the analysis of Pinus radiata needles. Commun. Soil Sci. Plant 11:175–188.
McArthur, C., Hagerman, A. E., and Robbins, C. T. 1991. Physiological strategies of mammalian herbivores against plant defenses, pp. 103–114, in R. T. Palo and C. T. Robbins (eds). Plant Defenses Against Mammalian Herbivory. CRC Press, Boca Raton, FL.
McArthur, C., Goodwin, A., and Turner, S. 2000. Preferences, selection and damage to seedlings under changing availability by two marsupial herbivores. For. Ecol. Manag. 139:157–173.
Marsh, K. J., Wallis, I. R., and Foley, W. J. 2005. Detoxification rates constrain feeding in common brushtail possums (Trichosurus vulpecula). Ecology 86:2946–2954.
Marsh, K. J., Wallis, I. R., McLean, S., Sorensen, J. S., and Foley, W. J. 2006. Conflicting demands on detoxification pathways influence how common brushtail possums choose their diets. Ecology (in press).
O'Reilly-Wapstra, J. M., McArthur, C., and Potts, B. M. 2004. Linking plant genotype, plant defensive chemistry and mammal browsing in a Eucalyptus species. Funct. Ecol. 18:677–684.
Pfister, J. A., Provenza, F. D., Manners, G. D., Gardner, D. R., and Ralphs, M. H. 1997. Tall larkspur ingestion: can cattle regulate intake below toxic levels? J. Chem. Ecol. 23:759–777.
Ratkowsky, D. A., Evans, M. A., and Alldredge, J. R. (eds.). 1993. Cross-over Experiments: Design, Analysis, and Application. Marcel Dekker, New York.
Robbins, C. T., Hanley, T. A., Hagerman, A. E., Hjeljord, O., Baker, D. L., Schwartz, C. C., and Mautz, W. W. 1987. Role of tannins in defending plants against ruminants: reduction in protein availability. Ecology 68:98–107.
Sands, D. P. A. and Brancatini, V. A. 1991. A portable penetrometer for measuring leaf toughness in insect herbivory studies. Proc. Entomol. Soc. Wash. 93:786–788.
SAS Institute. 1989. SAS Software, v 6.12. SAS Institute, Cary, NC, USA.
Sipes, I. G. and Gandolfi, A. G. 1991. Biotransformation of toxicants, pp. 88–126, in M. O. Amdur, J. Doull and C. D. Klaassen (eds.). Casarett and Doull's Toxicology. The Basic Science of Poisons. Pergamon Press, New York.
Sorensen, J. S., McLister, J. D., and Dearing, D. M. 2005. Plant secondary metabolites compromise the energy budgets of specialist and generalist mammalian herbivores. Ecology 86:125–131.
Tixier, H. and Duncan, P. 1996. Are European roe deer browsers? A review of variations in the composition of their diets. Rev. Ecol. 51:3–17.
Westoby, M. 1978. What are the biological bases of varied diets? Am. Nat. 112:627–631.
Wiggins, N. L., McArthur, C., McLean, S., and Boyle, R. 2003. Effects of two plant secondary metabolites, cineole and gallic acid, on nightly feeding patterns of the common brushtail possum. J. Chem. Ecol. 29:1447–1464.
Wiggins, N. L., McArthur, C., and Davies, N. W. 2006. Diet switching in a generalist mammalian folivore: fundamental to maximising intake. Oecologia 147:650–657.
Acknowledgements
We thank Hugh Fitzgerald and Kit Williams for technical assistance, Julien Wiggins for help with experimental procedures, and Sue Brandon and Ann Wilkinson for analytical assistance. This research was approved by the University of Tasmania's Animal Ethics Committee (Permit Number A6700) and Parks and Wildlife Service (Permit Number FA 02119). Research was funded by an Australian Postgraduate Award and the CRC for Sustainable Production Forestry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wiggins, N.L., McArthur, C., Davies, N.W. et al. Behavioral Responses of a Generalist Mammalian Folivore to the Physiological Constraints of a Chemically Defended Diet. J Chem Ecol 32, 1133–1147 (2006). https://doi.org/10.1007/s10886-006-9076-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-006-9076-1