Abstract
The evolution of floral scent as a plant reproductive signal is assumed to be driven by pollinator behavior, with little attention paid to other potential selective forces such as herbivores. I tested 10 out of the 13 compounds emitted by dioecious Cirsium arvense, Canada thistle, including 2-phenylethanol, methyl salicylate, p-anisaldehyde, benzaldehyde, benzyl alcohol, phenylacetaldehyde, linalool, furanoid linalool oxides (E and Z), and dimethyl salicylate. Single compounds (and one isomer) set out in scent-baited water-bowl traps trapped over 10 species of pollinators and 16 species of floral herbivores. The two dominant components of the fragrance blend of C. arvense, benzaldehyde and phenylacetaldehyde, trapped both pollinators and florivores. Other compounds attracted either pollinators or florivores. Florivores of C. arvense appear to use floral scent compounds as kairomones; by advertising to pollinators, C. arvense also attracts its own enemies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insects navigate complicated odor landscapes, identifying the scent of a host plant while filtering out irrelevant chemical signals (Metcalf, 1987; Masson and Mustaparta, 1990). Since the diversification of the angiosperms and many of their pollinators during the Cretaceous period, selection by beneficial insects has been acting on floral scent. Most research on floral volatile evolution has considered pollinators as the primary biotic selective agent (Dobson, 1994; Raguso, 2001). However, it has been proposed that the original role of plant volatiles was as deterrents against herbivores (Pellmyr and Thien, 1986). Mounting evidence suggests that floral herbivores, which include both florivores and predispersal seed predators, are an important factor in selection on fragrance and other floral characters. Florivores can affect fitness directly (through tissue destruction) and indirectly (by deterring pollinators) (Strauss, 1997; Krupnick and Weis, 1999; Mothershead and Marquis, 2000; Adler et al., 2001). Ultimately, these losses can affect seedling recruitment (Louda and Potvin, 1995; Kelly and Dyer, 2002). Studies have demonstrated the role of florivores in the evolution of floral morphology (Brody, 1997; Kudoh and Whigham, 1998; Campbell et al., 2002; Ehrlèn, 2002), flowering phenology (Eriksson, 1995; Pilson, 2000; Mahoro, 2002), flower number (Ohashi and Yahara, 2000), and floral scent (Galen, 1983; Baldwin et al., 1997). If detrimental insects use floral scent to locate their hosts, then they too will contribute selective pressures that affect the evolution of floral scent. Similar scent compounds that attract both pollinators and detrimental insects may be subject to opposing selection pressures: pressure to be both apparent to pollinators and inconspicuous to florivores.
Of the studies that have investigated the attraction of herbivores to floral volatiles, many have focused on agricultural pests (Dobson, 1994). Studies have been carried out both on insects that feed on floral tissue and on those that oviposit into a flower head where their larvae feed (Cantelo and Jacobson, 1979; Wiesenborn and Baker, 1990; Haynes et al., 1991; Roseland et al., 1992; Tingle and Mitchell, 1992; Smart and Blight, 1997; Metcalf et al., 1998; Smart and Blight, 2000). Canada thistle, Cirsium arvense (Asteraceae), is an invasive species in the eastern United States, and native to Europe, Western Asia, and North Africa; it was probably introduced into Canada in the early 17th century (Moore, 1975). I chose to study an exotic invasive species because of its potential to demonstrate both coadapted interactions with introduced insects, as well as novel, interactions with native herbivores.
Cirsium is pollinated by a wide range of generalists, including insects from several different orders (Proctor et al., 1996). In this population, I found the most abundant pollinator on C. arvense to be the nonnative (but coadapted, as it is native to Europe) honeybee (Apis mellifera), with high visitation rates by other bees, especially Halictus and Lasioglossum ssp. (Halictidae; Theis, 2003). Other pollinators included hover flies (Diptera: Syrphidae) and common diurnal Lepidoptera, including Vanessa atalanta and Pieris rapae. A field experiment determined that nocturnal pollination is not relevant in the study population (Theis, 2003). Florivorous insects from several orders feed on C. arvense flower heads, including beetles (Mordellidae, Phalacridae, Meloidae, Cantharidae) and grasshoppers (Acrididae, Tettigoniidae) (Theis, 2003). Many species of Cirsium and other closely related groups are considered noxious weeds in the United States, and a number of biocontrol agents have been introduced to control C. arvense, including root, leaf, and flower feeders (McEvoy and Coombs, 1999). Dasineura gibsoni, an introduced cecidomyiid fly, and two introduced weevils were found on C. arvense; one weevil, Larinus planus, was quite common, whereas Rhinocyllus conicus was rare. With a generalist pollination system and a large number of both introduced and native florivores, it is an ideal choice for studies on potential balancing or diversifying selection on floral scent.
I quantified the chemical composition of both staminate and pistillate C. arvense plants and used scent-emitting traps to determine the olfactory preferences of both pollinators and florivores to C. arvense fragrance components. If beneficial and detrimental insects use different fragrance components, then directional selection should minimize the compounds attractive to the detrimental insects.
Methods and Materials
Taxa and Field Sites
Cirsium arvense (L.) Scopoli, Canada thistle, (Asteraceae), is an erect perennial whose height at reproduction ranges from 0.3 to 2.0 m. Unique among thistles, C. arvense has dioecious flower heads of pink-purple disk flowers, which bloom from July through September (Nuzzo, 1997). I conducted this study at the U.S. Fish and Wildlife Service Wallkill River National Wildlife Refuge. Located on the border of Sussex, NJ, and Warwick, NY, USA (74°31′W, 41°17′N), the site is a former sod farm. It is dominated by mixed communities of native and exotic invasive plants in open fields, including Asclepias syriaca, Ambrosia artemisiifolia, Solidago sp., Lythrum salicaria, and Carduus nutans.
Emission Rates
Volatiles were collected from C. arvense by using dynamic headspace sampling in the field. Intact flower heads were enclosed within a nylon resin oven bag (Reynolds Consumer Products, Richmond, VA, USA). Ambient air flowed into the bag across the flower head and into Porapak® Q (80–100 mesh) packed cartridges at a flow rate of ca. 200 ml/min, via either an Air Check 52 or Air Check 2000 diaphragm pump (SKC Inc., Eighty Four, PA, USA). Cartridges were eluted with 3 ml hexane, and an internal standard of 3 μl of 0.01% anisole in hexane was added. Samples were concentrated to 75 μl with N2. To ascertain the fragrance production of cut flower heads (N = 2 staminate, 3 pistillate), scent was collected for 4 hr before cutting and 4 hr immediately after cutting. Emission rates from the traps were measured for 10 min by using the same protocol described above, but in a growth chamber with controlled light and temperature (24°C) to mimic average field conditions (N = 3).
Fragrance Analysis
Combined capillary gas chromatography-mass spectrometry (GC-MS), with a Shimadzu GC-17A equipped with a Shimadzu QP5000 quadrupole electron impact MS as a detector (Shimadzu Scientific Instruments, Inc., Columbia, MD, USA), was used to analyze the fragrance. A 1-μl aliquot was injected splitless onto a polar column (EC WAX) (30 m × 0.25 mm; Alltech Associates, Deerfield, IL, USA) at an initial temperature of 60°C for 3 min, which was increased by 10°C/min until 260°C, where it was held for 7 min (Theis and Raguso, 2005). Compounds were identified by using retention time (from previously injected standards) and mass spectral libraries [Wiley (1995) and NIST (1998)], with greater than 120,000 mass spectra. Quantification was achieved by relating the mass ion of each scent compound to the mass ion of the internal standard with serial dilution curves of the scent compounds run as external standards.
Scent-Baited Traps
I used scent-emitting insect traps, during July and August 2002, to attract both pollinators and florivores by using 10 of the 13 floral compounds identified from the scent blend of C. arvense (Andersson et al., 2002; Theis and Raguso, 2005). Each trap consisted of a clear bowl (8-in. plastic bowls; Hummert International, Topeka, KS, USA) fixed to a tier of a tomato cage at a similar height level with that of the blooming plants. A second bowl filled with soapy water (Alconox liquid detergent 1 ml/5 l; Alconox, White Plains, NY, USA) was set inside the fixed bowl. Suspended above the soapy water by a wire was a microcentrifuge tube filled with either scent or an unbaited control tube. A hole was cut into the microcentrifuge tube, and a wick of single-stranded embroidery floss was submerged in the chemical and projected 7 mm above the top of the tube, in order to emit scent. All wicks were renewed (cut and pulled another 7 mm) once a day. Scents were refilled at least once a week. As a visual display, a cut staminate or pistillate C. arvense flower head (set into a water-pik) was suspended above water bowls. Flower heads also were renewed daily.
Two field experiments were run simultaneously for 4 wk, spanning the peak of C. arvense's flowering season. One experiment had six treatments, including three pure compounds (phenylacetaldehyde, methyl salicylate, 2-phenylethanol), racemic linalool, one blend of two linalool oxide furanoid isomers, and a dry control tube. The second experiment consisted of seven treatments: two pure compounds (dimethyl salicylate, benzyl alcohol), two compounds diluted by 1/2 with mineral oil (p-anisaldehyde, benzaldehyde), one control tube containing mineral oil, and two cut flower heads (one staminate, the other pistillate). The cut flower heads were not used until the second week of the experiment. I did not test the pyranoid linalool oxide isomers or benzyl benzoate in the traps. The treatments were arranged in a circular array surrounding a patch of C. arvense. Each circle contained two replicates of each treatment, and the arrangement of compounds was randomized once a week. Traps were placed 3 m apart and a replicate circle was set up at least 10 m away, for a total of four replicate traps. Trapped insects were identified daily and collected every 2 d.
Chemicals
The synthetic compounds were obtained from Sigma-Aldrich (St. Louis, MO, USA; 2-phenylethanol 100%, methyl salicylate 99%, p-anisaldehyde 99%, benzaldehyde 99%, benzyl alcohol 99%, phenylacetaldehyde >90%, (±)-linalool 97%, furanoid linalool oxide mixture of isomers >97%) and from Quest International (Ashford Kent, UK; dimethyl salicylate).
Statistical Analyses
Trap catch was not normally distributed for any insect species. For groups with more than 24 trapped individuals, I used Systat 10.0 to test for differences in trap catch between each treatment and the control within each circle (replicates were lumped) by using the Wilcoxon's signed-ranks test for paired comparisons (Sokal and Rohlf, 1995).
Results
Trapped Species
In two field experiments, I tested the attraction of pollinators and florivores to 10 components of the C. arvense blend (eight compounds and one isomeric mixture). Emission rates from the traps ranged from 343 to 769 μg/hr (Table 1). For comparison, the average total emission rate from C. arvense is 19 μg/hr for staminate flower heads and 4 μg/hr for pistillate. I captured a total of 15 species of florivores and nine species of pollinators, seven of which were bees, including three genera of Halictidae (Table 2). Butterflies pollinate C. arvense, although infrequently in this population, but were not captured within water bowl traps.
Pollinator Attractants
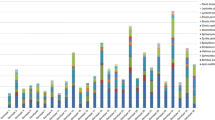
A pollinator assemblage, summed for all species of pollinators with greater than 10 representative individuals, was tested for fragrance preference. Three compounds were more attractive to pollinators than the control: benzaldehyde, phenylacetaldehyde, and p-anisaldehyde (Wilcoxon's signed-ranks test, P < 0.05; Table 2, Fig. 1). Of these, p-ansialdehyde and phenylacetaldehyde were by far the most attractive compounds, trapping on average 48% and 37% of all pollinators within each of the respective experimental arrays. Only honeybees and Lasioglossum were caught in sufficient numbers to be able to detect significant differences in trap preference for individual pollinator genera; both were trapped by p-anisaldehyde. Phenylacetaldehyde and linalool were also attractive to honeybees.
The average number of trapped pollinators and florivores in each scent trap contrasted with the abundance of each of these compounds in the floral blend of staminate Cirsium arvense, in parentheses next to compound name. Trap catch was normalized by total trap catch and control trap catch. Significance tested by Wilcoxon's signed-ranks test paired comparisons between treatments and control *P < 0.05.
Florivore Attractants
Florivores were attracted to floral scent compounds from C. arvense. I summed the data for all florivores (that had greater than 10 individuals trapped) and determined that 26% of all florivores were trapped by benzaldehyde (Wilcoxon's signed-ranks test, P < 0.05; Table 2, Fig. 1). In contrast, more florivores were trapped by the control than by linalool. Specific florivores also demonstrated significant preferences, both positive and negative. Ants (Formicidae) were attracted to phenylacetaldehyde, and mordellids were attracted to benzyl alcohol and to the furanoid linalool oxide isomers. In the first 2 wk of the experiment, mordellids were frequently trapped by dimethyl salicylate, but this preference was not consistent throughout the experiment, and it is not significant. The tettigoniid grasshoppers (predominantly nymphs) were never trapped by linalool or 2-phenylethanol.
Visual Display
Cut flower heads also trapped both pollinators and florivores. Cut flower heads emitted less than half the total emission of intact flower heads (approximately 44% for staminate flower heads and 35% for pistillate flower heads). In spite of fragrance reduction, staminate and pistillate cut flower heads were attractive to pollinators (Wilcoxon's signed-ranks test, P < 0.05; Table 2). Summed data for all pollinators was strongly influenced by Lasioglossum, which were more often trapped by cut staminate flower heads, and by honeybees, which were more frequently trapped by cut pistillate flower heads, although these preferences were not significant. Florivores and particularly the tettigoniids were attracted to cut staminate flower heads.
Discussion
Floral Scent Traps Both Pollinators and Florivores
The fragrance emitted by C. arvense attracts both pollinators and florivores (Table 2, Fig. 1). Some compounds significantly attracted only pollinators (i.e., p-anisaldehyde), while others significantly attracted only particular species of florivores (i.e., benzyl alcohol). Some compounds were attractive to both pollinators and florivores (benzaldehyde and phenylacetaldehyde). Attractive compounds were not necessarily the most abundant components of the C. arvense fragrance blend (Fig. 1). For example, p-anisaldehyde is 0.5% of the total blend, but a highly attractive compound for pollinators. Although trapping experiments using blends would convey a more complete picture of selection on the fragrance phenotype, pure compounds, nonetheless, should be informative. From studies on the neurophysiology of honeybees, Galizia and Menzel (2001) have demonstrated that a mix of two compounds results in a signal in the insect brain that is not novel, but rather additive of the component parts with some degree of deviation, both positive and negative (inhibition). There could be synergistic effects of compounds in the blend that result in an increase in attraction, reducing our ability to identify the intensity of selection on any one of these compounds, but not changing the direction of selection. Overall, these results demonstrate that the fragrance emitted from C. arvense is perceived and utilized not just by beneficial insects, but by detrimental insects as well. As a result, components of the fragrance blend should be shaped by opposing selection pressures.
Diversity of Pollinators in Odor Traps
The dominant pollinators of C. arvense were caught in the traps, including species from five families in two insect orders (Table 2). The largest numbers of individuals were caught from the family Halictidae, a sample dominated by Lasioglossum (Dialictus) bees. Also trapped were honeybees, the dominant pollinators of C. arvense at this study site, as indicated in a census (Theis, 2003). Lepidopteran pollinators were excluded from analysis for two reasons: the water bowl traps did not attract butterflies, and nocturnal pollination does not occur in this population (Theis, 2003). Therefore, the moths that were trapped in these experiments, while indicative of the general attraction to aromatic floral compounds (see Cantelo and Jacobson, 1979; Plepys et al., 2002), are omitted from further discussion.
Diversity of Florivores in Odor Traps
A large diversity of dominant florivores were caught in the traps, including species from 14 families in five insect orders. All of the insects defined as florivores were seen feeding on C. arvense, and many are recognized as highly detrimental, including the Japanese beetle (Popilla japonica), the tarnished plant bug (Lygus lineolaris), and blister beetles (Epicauta sp.) (Borrer et al., 1992). Two of the florivore groups in this system have been implicated as selective forces driving the evolution of floral scent in other plant species: Negro bugs (Corimelaena sp.) on Nicotiana (Baldwin et al., 1997) and ants (Formicidae) on Polemonium (Galen, 1983). Nevertheless, the classification of these insects as detrimental is only putative. This applies particularly for the Cantharidae, which may be predatory on other florivores, resulting in a net positive effect. Many of the florivores that are attracted to C. arvense floral odors are specialists on Asteraceae (Borrer et al., 1992). Some insects, such as L. planus, were frequently found on C. arvense, but were infrequently captured in the traps, including control traps. It is possible that these insects are attracted by scent, but the trapping apparatus does not efficiently trap them. With such a high diversity of florivores on C. arvense, none of which is completely specialized or devastating, selection pressure from this group is likely to be somewhat diffuse.
Repellent Compounds
Only florivores were significantly less attracted to some components of the floral scent blend (e.g., linalool) than to the unbaited control. A trap with the full blend minus the putative repellent could establish repellence for individual components. Repellent compounds have infrequently been reported in the literature (Dobson, 1994; Theis and Raguso, 2005), perhaps because florivores may adapt to perceive a compound as attractive if there is a reward associated with the cue.
Selection on Floral Scent Biosynthesis
Selection acting on quantitative variation in the emission of specific components of the floral scent blend may result in selection on the biochemical pathways from which those compounds are produced. The 13 compounds emitted by the flowers of C. arvense are products of two biosynthetic pathways. Phenylacetaldehyde, benzaldehyde, and p-anisaldehyde are all products of the shikimate pathway (Dey and Harborne, 1997). In the shikimate pathway, phenylalanine ammonia lyase (PAL) is the “branch-point” enzyme between primary and secondary metabolism (Dixon and Paiva, 1995). The monoterpenoids, linalool and the linalool oxides, are produced in the DOXP/MEP pathway localized to plastids (Lichtenthaler et al., 1997; Raguso and Pichersky, 1999). Multiple enzymes may be at work in producing the diversity of volatiles, but single terpene synthase enzymes are sometimes responsible for the synthesis of several major and minor volatile products (Bohlmann et al., 1998). If selection acting on one compound affects the production of a different compound in the blend, a number of outcomes could arise. For example, honeybees are attracted by linalool, whereas florivores may be repelled. With this simple model, I would expect that linalool should increase in the blend. However, the mordellid beetles are attracted by the furanoid linalool oxide isomers (Table 2), and it is possible that positive selection acting on linalool could be constrained by negative selection on the linalool oxides, which are produced from linalool (Raguso and Pichersky, 1999). If the pollinator attractant increases, perhaps its associated oxides would also increase, resulting in the attraction of florivores. Only a few of the enzymes involved in the production of floral scent compounds have as yet been identified (Dudareva et al., 2003). However, understanding the selection pressures on the components of the floral scent blend will require knowledge of enzymatic pathways in order to obtain a full picture of selection pressure on the blend.
References
Adler, L. S., Karban, R., and Strauss, S. Y. 2001. Direct and indirect effects of alkaloids on plant fitness via herbivory and pollination. Ecology 82:2032–2044.
Andersson, S., Anders, N. L., Groth, I., and Bergström, G. 2002. Floral scents in butterfly-pollinated plants: Possible convergence in chemical composition. Bot. J. Linn. Soc. 140:129–153.
Baldwin, I. T., Preston, C., Euler, M., and Gorham, D. 1997. Patterns and consequences of benzyl acetone floral emissions from Nicotiana attenuata plants. J. Chem. Ecol. 23:2327–2343.
Bohlmann, J., Meyer-Gauen, G., and Croteau, R. 1998. Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc. Natl. Acad. Sci. USA 95:4126–4133.
Borrer, D. J., Triplehorn, C. A., and Johnson, N. F. 1992. An Introduction to the Study of Insects. Harcourt Brace College Publishers, New York.
Brody, A. K. 1997. Effects of pollinators, herbivores, and seed predators on flowering phenology. Ecology 78:1624–1631.
Campbell, D. R., Crawford, N., Brody, A. K., and Forbis, T. A. 2002. Resistance to pre-dispersal seed predators in a natural hybrid zone. Oecologia 131:436–443.
Cantelo, W. W. and Jacobson, M. 1979. Corn silk volatiles attract many pest species of moths. J. Environ. Sci. Health 8:695–707.
Dey, P. M. and Harborne, J. B. 1997. Plant Biochemistry. Academic Press, San Diego.
Dixon, R. A. and Paiva, N. L. 1995. Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085–1097.
Dobson, H. E. M. 1994. Floral volatiles in insect biology, pp. 47–81, in E. A. Bernays (ed.). Insect Plant Interactions, vol 5. CRC Press, Boca Raton.
Dudareva, N., Martin, D., Kish, C. M., Kolosova, N., Gorenstein, N., Faldt, J., Miller, B., and Bohlmann, J. 2003. (E)-beta-ocimene and myrcene synthase genes of floral scent biosynthesis in snapdragon: function and expression of three terpene synthase genes of a new terpene synthase subfamily. Plant Cell 15:1227–1241.
Ehrlèn, J. 2002. Assessing the lifetime consequences of plant–animal interactions for the perennial herb Lathyrus vernus (Fabaceae). Perspect. Plant Ecol. Evol. Syst. 5:145–163.
Eriksson, O. 1995. Asynchronous flowering reduces seed predation in the perennial forest herb Actaea spicata. Acta Oecol.-Int. J. Ecol. 16:195–203.
Galen, C. 1983. The effects of nectar thieving ants on seedset in floral scent morphs of Polemonium viscosum. Oikos 41:245–249.
Galizia, C. G. and Menzel, R. 2001. The role of glomeruli in the neural representation of odours: results from optical recording studies. J. Insect Physiol. 47:115–130.
Haynes, K. F., Zhao, J. Z., and Latif, A. 1991. Identification of floral compounds from Abelia grandiflora that stimulate upwind flight in Cabbage looper moths. J. Chem. Ecol. 17:637–646.
Kelly, C. A. and Dyer, R. J. 2002. Demographic consequences of inflorescence-feeding insects for Liatris cylindracea, an iteroparous perennial. Oecologia 132:350–360.
Krupnick, G. A. and Weis, A. E. 1999. The effect of floral herbivory on male and female reproductive success in Isomeris arborea. Ecology 80:135–149.
Kudoh, H. and Whigham, D. F. 1998. The effect of petal size manipulation on pollinator/seed-predator mediated female reproductive success of Hibiscus moscheutos. Oecologia 117:70–79.
Lichtenthaler, H. K., Rohmer, M., and Schwender, J. 1997. Two independent biochemical pathways for isopentenyl diphosphate and isoprenoid biosynthesis in higher plants. Physiol. Plant. 101:643–652.
Louda, S. M. and Potvin, M. A. 1995. Effect of inflorescence feeding insects on the demography and lifetime fitness of a native plant. Ecology 76:229–245.
Mahoro, S. 2002. Individual flowering schedule, fruit set, and flower and seed predation in Vaccinium hirtum Thunb. (Ericaceae). Can. J. Bot.-Rev. Can. Bot. 80:82–92.
Masson, C. and Mustaparta, H. 1990. Chemical information-processing in the olfactory system of insects. Physiol. Rev. 70:199–245.
McEvoy, P. B. and Coombs, E. M. 1999. Biological control of plant invaders: regional patterns, field experiments, and structured population models. Ecol. Appl. 9:387–401.
Metcalf, R. L. 1987. Plant volatiles as insect attractants. CRC Crit. Rev. Plant Sci. 5:251–301.
Metcalf, R. L., Lampman, R. L., and Lewis, P. A. 1998. Comparative kairomonal chemical ecology of diabroticite beetles (Coleoptera: Chrysomelidae: Galerucinae: Luperini: Diabroticina) in a reconstituted tallgrass prairie ecosystem. J. Econ. Entomol. 91:881–890.
Moore, R. 1975. The biology of Canadian weeds. 13: Cirsium arvense (L) Scop. Can. J. Plant Sci. 55:1033–1048.
Mothershead, K. and Marquis, R. J. 2000. Fitness impacts of herbivory through indirect effects on plant–pollinator interactions in Oenothera macrocarpa. Ecology 81:30–40.
Nuzzo, V. 1997. Element Stewardship Abstract for Cirsium arvense. The Nature Conservancy Arlington, VA.
Ohashi, K. and Yahara, T. 2000. Effects of flower production and predispersal seed predation on reproduction in Cirsium purpuratum. Can. J. Bot.-Rev. Can. Bot. 78:230–236.
Pellmyr, O. and Thien, L. B. 1986. Insect reproduction and floral fragrances—keys to the evolution of the angiosperms. Taxon 35:76–85.
Pilson, D. 2000. Herbivory and natural selection on flowering phenology in wild sunflower, Helianthus annuus. Oecologia 122:72–82.
Plepys, D., Ibarra, F., and Lofstedt, C. 2002. Volatiles from flowers of Platanthera bifolia (Orchidaceae) attractive to the silver Y moth, Autographa gamma (Lepidoptera: Noctuidae). Oikos 99:69–74.
Proctor, M., Yeo, P., and Lack, A. 1996. The Natural History of Pollination. Timber Press, Portland.
Raguso, R. A. 2001. Floral scent, olfaction and scent-driven foraging behavior, pp. 83–105, in L. Chittka, J.D. Thomson, (eds.). Cognitive Ecology of Pollination. Cambridge University Press.
Raguso, R. A. and Pichersky, E. 1999. A day in the life of a linalool molecule: chemical communication in a plant–pollinator system. Part 1: Linalool biosynthesis in flowering plants. Plant Species Biol. 14:95–120.
Roseland, C. R., Bates, M. B., Carlson, R. B., and Oseto, C. Y. 1992. Discrimination of sunflower volatiles by the red sunflower seed weevil. Entomol. Exp. Appl. 62:99–106.
Smart, L. E. and Blight, M. M. 1997. Field discrimination of oilseed rape, Brassica napus volatiles by cabbage seed weevil, Ceutorhynchus assimilis. J. Chem. Ecol. 23:2555–2567.
Smart, L. E. and Blight, M. M. 2000. Response of the pollen beetle, Meligethes aeneus, to traps baited with volatiles from oilseed rape, Brassica napus. J. Chem. Ecol. 26:1051–1064.
Sokal, R. S. and Rohlf, F. J. 1995. Biometry. Freeman and Co., New York.
Strauss, S. Y. 1997. Floral characters link herbivores, pollinators, and plant fitness. Ecology 78:1640–1645.
Theis, N. 2003. Targeting pollinators and evading herbivores: Floral scent emission in two species of Cirsium. PhD dissertation. State University of New York at Stony Brook, Stony Brook.
Theis, N. and Raguso, R. A. 2005. The effect of pollination on floral fragrance in thistles. J. Chem. Ecol. 31:2581–2600.
Tingle, F. C. and Mitchell, E. R. 1992. Attraction of Heliothis virescens (F) (Lepidoptera, Noctuidae) to volatiles from extracts of cotton flowers. J. Chem. Ecol. 18:907–914.
Wiesenborn, W. D. and Baker, T. C. 1990. Upwind flight to cotton flowers by Pectinophora gossypiella (Lepidoptera, Gelechiidae). Environ. Entomol. 19:490–493.
Acknowledgments
I thank Robert Raguso and Manuel Lerdau for discussion and comments; Laurel Reid, Eileen Rios, and Sarah Brice for help with data collection; Karen Goodell for help with bee identification; Robin Clery from Quest International for samples of dimethyl salicylate; and two anonymous reviewers for helpful comments. This work was supported in part by Sigma Xi Grants-in-Aid of Research, American Museum of Natural History Theodore Roosevelt Memorial Fund, U.S. Department of Education GAANN Fellowship, Sokal Travel Award, The Explorers Club Exploration Fund, and a Doctoral Dissertation Improvement Grant from the National Science Foundation (DEB# 0206300).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Theis, N. Fragrance of Canada Thistle (Cirsium arvense) Attracts Both Floral Herbivores and Pollinators. J Chem Ecol 32, 917–927 (2006). https://doi.org/10.1007/s10886-006-9051-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-006-9051-x