Abstract
Induced volatile terpenes have been commonly reported among diverse agricultural plant species, but less commonly investigated in odorous plant species. Odorous plants synthesize and constitutively store relatively large amounts of volatiles, and these may play a role in defense against herbivores. We examined the effect of herbivory and methyl jasmonate (MeJA) exposure on the release of volatile organic compounds (VOCs) in the marsh elder, Iva frutescens, which contains numerous constitutive VOCs, mainly mono- and sesquiterpenes. Our specific goal was to test for the presence of inducible VOCs in a naturally occurring plant already armed with VOCs. The abundant, native specialist leaf beetle Paria aterrima was used in herbivore induction trials. VOCs were sampled from herbivore wounded and unwounded, and from MeJA treated and untreated I. frutescens. Total VOC emissions were significantly greater in response to herbivory and MeJA treatment compared to unwounded controls. Herbivore wounding caused a substantial shift in the emission profile (42 VOCs from wounded, compared to 8 VOCs from unwounded I. frutescens), and MeJA had a similar yet less substantial influence on the emission pattern (28 VOCs from MeJA treated compared to 8 VOCs from untreated I. frutescens). Constitutive VOC emissions predominated, but some VOCs were detected only in response to herbivory and MeJA treatment, suggesting de novo synthesis. Several VOCs exhibited a delayed emission profile in contrast to the rapid release of constitutive VOCs, and principal components analysis revealed they were not associated with constitutive emissions. While I. frutescens contains many constitutive VOCs that are released immediately in response to herbivory, it also produces novel VOCs in response to feeding by the specialist P. aterrima and MeJA treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many plants from diverse lineages synthesize secondary metabolites that accumulate in specialized secretory cells, or whose production is induced by herbivory (Karban and Baldwin, 1997). Terpenoids are a diverse group of secondary compounds that serve a variety of functions in plants, ranging from heat stress resistance (Sharkey and Singsaas, 1995) to serving as cell membrane components, plant hormones, membrane-bound sugar carriers, and photoprotective pigments (Gershenzon and Croteau, 1993). Terpenoids may also play a direct defensive role in plant species that produce these compounds, as well as an indirect defensive role, by attracting natural enemies of herbivores (Paré and Tumlinson, 1999; Theis and Lerdau, 2003). Stored volatile organic compounds (VOCs) may be volatilized into the atmosphere by a healthy unwounded plant depending on their concentration and physiochemical properties (Niinemets et al., 2004). These stored VOCs are volatilized in greater quantity into the atmosphere upon tissue breakage during herbivore attack (Karban and Baldwin, 1997). While many plants contain large amounts of stored VOCs, others do not synthesize and emit them until a stimulus (such as herbivory) is perceived. Here, we use the term “constitutive” to refer to VOCs that are stored in the leaf and can be immediately released by mechanical wounding, “induced” to refer to VOCs that are increased in response to herbivory (which may include up-regulated constitutive VOCs), and “novel” to refer to unique induced VOCs that are not normally stored by the plant, but whose synthesis is up-regulated in response to herbivory.
Induced VOCs may be emitted hours or days after an attack, both from the site of wounding as well as systemically from undamaged plant leaves (Paré and Tumlinson, 1997a; 1999; Mattiacci et al., 2001). The synthesis of novel compounds not normally produced and accumulated may also be induced by herbivore oral secretions (Alborn et al., 1997; Paré et al., 2005). 13CO2 pulse-labeling experiments have demonstrated the de novo biosynthesis of novel VOCs emitted from herbivore-wounded plants (Paré and Tumlinson, 1997b).
Numerous studies have shown that third-trophic-level members use novel VOCs as indicators of prey location, thus facilitating a tritrophic interaction and an indirect defense to the wounded plant (Dicke et al., 1990; Drukker et al., 2000; De Boer and Dicke, 2004). For instance, the C11 homoterpene, (E)-4,8-dimethyl-1,3,7-nonatriene (herein referred to as nonatriene), and the C16 homoterpene, (E,E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene (herein referred to as TMTT), among other terpenes, are induced by a broad spectrum of herbivores feeding on their host plants, and these induced VOCs are attractive to foraging predators and parasitoids (Dicke et al., 1990; Turlings et al., 1991; Vanpoecke et al., 2001).
Novel VOCs induction has been a focal point for research in tritrophic interactions and indirect defense, while constitutive VOCs have garnered relatively less attention. Constitutive VOCs have been mainly studied for their roles in direct defense as toxins and herbivore deterrents (Wittstock and Gershenzon, 2002). However, herbivory may up-regulate the production of constitutive VOCs, and due to their volatility they may also play a role in indirect defense. In many coniferous trees, constitutive terpene synthase enzymes are up-regulated in response to herbivory (Litvak and Monson, 1998), and specialist bark beetle predators are attracted to both constitutive and induced VOCs from conifers (Raffa and Smalley, 1995). In angiosperms, constitutive VOCs from Zea maize, in addition to novel VOCs, enhance the ability of predators and parasites to find herbivore hosts (Fukushima et al., 2002). These findings suggest that constitutive VOCs may play a similar ecological role to induced VOCs.
Emission of VOCs in response to herbivory, as well as attraction of third-trophic-level members, has been documented in many systems, most of which are crop species lacking large amounts of constitutive VOCs. While much progress has been made in understanding the function of VOCs and the mechanisms regulating VOC emissions, less emphasis has been placed on understanding herbivore stimulated VOCs in natural plant systems that can store relatively large amounts of constitutive VOCs compared to agricultural species. Baldwin and coworkers have pioneered efforts to elucidate the function of VOCs in nature by using native populations of tobacco (Nicotiana attenuata) and the odorous sagebrush (Artemisia tridentata) (Kessler and Baldwin, 2001). Southeastern salt marsh plant communities have a high occurrence of many largely constitutive VOC producing species such as the marsh elder, Iva frutescens (Asteraceae), and (southern) wax myrtle, Myrica cerifera (Myricaceae). The role of herbivore-induced VOCs in plant assemblages containing large amounts of constitutive VOCs is not known.
The objective of this research was to examine herbivore-induced VOC emissions in a native coastal plant, I. frutescens, which is armed with an array of constitutive VOCs (mainly mono- and sesquiterpenes). Our specific goal was to determine the potential for induced VOC emission in response to herbivory by a native herbivorous beetle, Paria aterrima (Chrysomelidae) (Wilcox, 1957). Plants containing a high amount of constitutive VOCs may immediately release stored VOCs, up-regulate constitutive VOCs, and may synthesize and emit novel VOCs in response to herbivory. We also tested for potential VOC induction by treating unwounded I. frutescens with the volatile methyl jasmonate (MeJA). VOC emissions (and other defense responses) are induced by the plant hormone jasmonic acid (JA), and its volatile methyl ester, MeJA (Dicke et al., 1999; Koch et al., 1999). Jasmonates are products of the octadecanoid pathway, which has been identified as one of the major signaling pathways in plant defense (Farmer and Ryan, 1990). Jasmonates may play a role in the up-regulation of VOCs, since treatment of plants with this compound can enhance both constitutive and novel VOC emissions (Martin et al., 2003). If I. frutescens contains herbivore-inducible VOC emissions, application of MeJA should stimulate the production and subsequent emission of novel VOCs, and potentially up-regulate constitutive VOC emissions.
Methods and Materials
Plants and Insects
Iva frutescens occurs naturally along the forest edge of salt marshes in southeastern North America. The salt marsh plant community shows a distinct zonation pattern with the grass, Spartina alterniflora, dominating the lower elevations, and rushes (Juncus sp.) occurring on the landward border. At higher elevations, the marsh transitions to a shrub zone that is dominated by Borrichia frutescens. I. frutescens occurs as scattered individuals throughout the shrub zone but dominates the landward edge. A M. cerifera zone occurs on the landward side of I. frutescens, and borders an inland oak–pine forest. A combination of physical stress and biotic interactions are thought to limit the distribution of salt marsh plants, with physical stress playing a primary role at low elevations and competition playing a larger role on the landward edge (Pennings and Callaway, 1992; Bertness and Yeh, 1994; Pennings and Moore, 2001).
I. frutescens plants approximately 2 m in height were collected in June 2000 from the marsh edge of the forest transition zone on Goat Island, at the North Inlet Marsh, Belle W. Baruch Marine Laboratory in Georgetown, SC, USA. Plants were pruned to approximately 1 m in height, and transplanted into 3-gal pots in the field using a 3:1 soil/sand mixture (Baccto lite™ potting soil: play sand), transported back to the University of South Carolina (USC) (Columbia, SC, USA), where they were fertilized using Peter's® 20/20/20 (5 ml of fertilizer per 3.78 l water) and maintained in a greenhouse. Plants were pruned to a height of approximately 1 m, and fertilized on an annual basis. Plants were moved in early May 2002 to a growth chamber where environmental conditions could be controlled. All herbivore wounding and MeJA treatment experiments took place in June 2002.
Paria aterrima beetles were collected in June 2002 from I. frutescens plants at Goat Island and transported back to USC. Beetles were maintained in plastic storage containers fitted with a mesh screen on the lid in the same growth chamber as experimental I. frutescens plants. Beetles were offered cotton balls saturated with water and branches of I. frutescens leaves as a food source. Branches were collected from the coastal site and stored in a cold room at 4°C in sealed plastic bags with moist paper towels to maintain freshness. New branches were placed in the beetle containers daily.
Growth Chamber Conditions
Growth conditions were maintained on a 14-hr photoperiod from 7:00 a.m. to 9:00 p.m., with a daytime temperature of 22°C and a nighttime temperature of 16°C. These conditions were maintained throughout the course of the experiments. The light regime was chosen to allow all volatile measurements to be made under daylight conditions when volatile emissions are greatest (Loughrin et al., 1994). I. frutescens approximately 1 m in height were used for experiments. These plants were in the original pots in which they were initially transplanted in the field, and appeared to be root-bound to some extent. Plants were watered every 2 d to saturation.
Herbivore Wounding Trials
For the herbivore wounding trials, the volatile collection system was placed over a selected branch and loosely fastened around the stem with a cable tie. Twenty beetles were inserted into the collection system through a small hole in the top of each bag, which was subsequently sealed with a cable tie. Insects were randomly chosen for each replicate, and no insects were reused. Beetles designated for a wounding trial were placed in a separate container with water, but without food for 24 hr prior to each trial. Once beetles were placed inside the collection bag, VOC emissions were collected in 1-hr intervals for 4 hr, with the traps changed every hour. After the first four collections, beetles were removed and VOC emissions were collected in 4-hr sampling periods at 4–8, 20–24, 32–36, 44–48, 56–60, and 68–72 hr. This sampling scheme was chosen to characterize any rapid changes in VOC emission during herbivory, as well as changes following herbivory. New traps were used for each sampling period. An unwounded plant was simultaneously sampled with a wounded plant. All wounding experiments were replicated with seven different plants, and initiated at the same time of day (11:00 a.m.).
Plant Volatile Collection
A push–pull volatile collection system was designed using Reynolds® oven bags. One top corner of a bag was fitted with a straight connector to serve as the inlet, while another straight connector was fitted to the opposite side of the bag at the base near the opening to serve as the exhaust. Connectors were fastened to the bags with cable ties. Compressed medical grade air was used as the inlet air supply. A gas regulator was connected to the gas cylinder to control the flow rate into the collection bag at 1.1 l min−1. An SKC, Inc. Aircheck sampler (model # 224-44XR) was used to pull headspace air through the bag and onto a 250-mg Tenax TA (Alltech, Inc.) trap fitted to the exhaust line on the bag. Headspace VOCs were sampled at 1 l min−1. Therefore, 90.9% of the air was sampled during each sampling period. A frame was constructed out of chicken wire and placed inside the bag to maintain a rigid structure around the branch being sampled to minimize VOC emissions due to artificial damage. For each wounding trial, the frame and bag were baked prior to use at 160°C for 30 min to eliminate any potential volatile contaminants. Control collections (bag and frame only) showed two artificial peaks from the bag and frame system (data not shown). These peaks were not included in I. frutescens VOC data analysis. For the herbivore wounding experiments, one branch from a plant was selected for wounding as described below. The frame and bag were placed over the branch, and volatiles were collected at intervals described above. VOC collections from collection bags containing only beetles revealed no detectable beetle-associated VOCs (data not shown).
Chemical Analysis
Each volatile trap was eluted with 2 ml of gas chromatography/mass spectroscopy (GC/MS) analytic grade pentane and stored in 2-ml auto sampler vials. Pentane was added in 1-ml aliquots and allowed to elute by gravity. A light stream of air was used to push the remaining pentane through the trap. The eluate was concentrated to 300 μl under a stream of compressed nitrogen. The concentrated sample was fractioned into two 150-μl samples. All samples were analyzed by using a Hewlett Packard 5890 series II gas chromatograph equipped with an HP-5 methyl-silicone capillary column (30 m × 0.25 mm) with helium as a carrier gas at a rate of 1.22 ml min−1. The injector temperature was set at 275°C. All injection volumes were 2 μl. The temperature program consisted of an initial hold at 50°C for 3 min, followed by an increase to a final temperature of 220°C at a rate of 5°C/min, followed by a hold at maximum temperature for 20 min. Samples were subsequently analyzed by mass spectroscopy with a Hewlett Packard 5971 series Mass Selective Detector. Volatile compounds collected from headspace emissions were identified by comparison of retention time and mass spectra to those contained in the National Bureau of Standards essential oil database, as well as the database of essential oil components identified by GC/MS (Adams, 1995). All VOCs were quantified by an external standard method based on two major constitutive VOCs from I. frutescens, the monoterpene limonene, and the sesquiterpene β-caryophyllene. Six-carbon volatiles, termed “green leaf volatiles” are produced from the catalytic activity of hydroperoxide lyase when cell membranes are damaged. They are among the most ubiquitous and earliest volatiles released in response to herbivory, and may have VOC-inducing capabilities (Farag and Paré, 2002). Green leaf volatiles, while presumably emitted in I. frutescens in response to herbivory, were not detected due to contamination from an unknown source in the portion of the chromatogram where these volatiles would have been detected.
Identification of Constitutive Leaf VOCs
Five g of fresh leaf material were collected from undamaged plants, and lipophilic components were extracted from the leaves in 20 ml GC/MS grade pentane by using a mortar and pestle to crush the leaves. Ten ml of the leaf extract were collected into a 50-ml polypropylene tubes and centrifuged for 10 min at 3600 rpm. One hundred and fifty μl aliquots were collected and analyzed by the GC/MS protocol described above. The volatiles obtained from the leaf extraction were used as a reference for preformed VOCs contained within I. frutescens, and also to generate reliable mass spectra for the identification of VOCs emitted in smaller quantities. Tentative identification of the compounds was carried out as described above.
Methyl Jasmonate Treatment
Five I. frutescens plants were exposed to exogenous MeJA based on the protocol described by Rodriguez-Saona et al. (2001). Briefly, 50 μl of a 9:1 (ethanol/MeJA) mixture were applied to two cotton-tipped applicators (100 μl total) and placed at the base of a single I. frutescens branch without physically touching any leaf material. Branches to be exposed to MeJA were enclosed in collection bags. Control plants were maintained in a separate growth chamber, and exposed to 100 μl of ethanol. Following an overnight 18-hr exposure period, cotton applicators were removed, new collection bags were placed over the treatment branch, and VOCs were immediately sampled for 1 hr.
Statistical Analyses
The experimental design consisted of a series of repeated measures on individual plants over time. Therefore, we used multivariate analysis of variance (MANOVA) and repeated-measures analysis of variance (ANOVA) to test for main effects on emissions of multiple or single VOCs, respectively. Individual VOCs with significant main effects (P < 0.05) were compared across time using Tukey tests to correct for multiple comparisons. VOC emissions in response to MeJA treatment were analyzed using a t test. All statistical tests were carried out using SAS V8.2 (SAS Institute, Cary, NC, USA). We also compared VOC emissions across time and treatment by using principal components analysis of VOC emissions. Twenty-two individual VOCs, comprising >0.5% at any sampling time, were used in the analysis. Principal components analysis reduces multivariate data to a smaller set of orthogonal, uncorrelated components that account for the maximum amount of variability. The first principal component accounts for the greatest amount of variation in the data, while additional components account for successively smaller amounts of variation.
Results
Constitutive VOCs
Iva frutescens leaves contain a complex mixture of secondary compounds dominated by monoterpenes and sesquiterpenes (Table 1). Sixty-one compounds comprising 63.2 (±5.5) μg g fr wt−1 of material were recovered by pentane extraction. Fifty-three compounds were identified either to a specific compound or, at the least, a class of terpene based on molecular ions in mass spectra, while eight compounds could not be reliably identified to any class. Sesquiterpenes and oxygenated sesquiterpenes were the most diverse class recovered, followed by the monoterpenes. The major constituents (each >5%) of the total mixture in order of decreasing concentration were limonene, germacrene D, unknown sesquiterpene 3, β-caryophyllene, and α-pinene (Table 1).
Herbivore-Induced VOC Emissions
Unwounded I. frutescens plants constitutively emitted a mixture of eight detectable VOCs consisting of the monoterpenes α-pinene, sabinene, β-pinene, limonene, and the sesquiterpenes β-caryophyllene, α-humulene, germacrene D, and cis-β-guaiene, all of which occur in high concentration within leaf material (Table 1). The production of these VOCs in unwounded plants did not differ over the 72-hr sampling period. Table 1 also contains the mean for all compounds recovered in response to herbivory across the sampling periods, which on average were higher than unwounded plants.
Statistical analysis of the dataset by MANOVA and repeated-measures ANOVA yielded similar results (Table 2). Herbivore wounding has a significant effect on the emission of VOCs over time (P < 0.001) (Table 2, Fig. 1). Significant interactions between treatment and sampling period were detected in repeated-measures ANOVA, indicating that the treatment effect varied significantly with time (Table 2). No significant nested effects of plant within treatment were observed (Table 2). Beetles feeding on I. frutescens caused total VOC emissions to increase in the first 4 hr during feeding compared to unwounded controls (P < 0.05) (Fig. 1). Herbivory caused a 22-, 14-, 16-, and 14-fold increase in total VOC emissions during hours 1, 2, 3, and 4, respectively. Following the 4hr feeding period, total VOC emissions declined dramatically, but remained elevated up to 48 hr after initial wounding (P < 0.05) (Fig. 1).
Total emissions collected from herbivore wounded and unwounded Iva frutescens over 72 hr in response to 4 hr of herbivory by P. aterrima. Sample periods 1–4 collected during wounding. Sample periods 5–10 collected after wounding. Data points represent mean ± SE of seven plants. Asterisk (*) indicates significantly increased emissions in the herbivore wounded treatment (Tukey, P < 0.05).
The influence of herbivory on VOC emissions over time is also displayed in principal component space (Fig. 2). Mean principal component scores at each sampling period are plotted for wounded and unwounded I. frutescens, and describes the influence of time and wounding on the variation in VOC emissions. Principal component 1 accounts for 61% of the total variation in the data, while component 2 accounts for 10%. Wounded plants show a steady relaxation toward unwounded plants, and four distinct clusters are seen over time (Fig. 2). The clusters indicate sampling periods with similar variation in VOC emissions. Active herbivore wounding sampling periods 1–4 hr, postwounding sampling periods 8, 24, and 36 hr, postwounding samplings periods 48, 60, 72 hr, and all unwounded principal component scores show distinct associations over time in principal component space (Fig. 2).
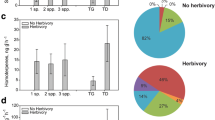
Herbivory caused a distinct compositional change in the total blend by stimulating the emission of 42 compounds including most constitutive VOCs. VOCs comprising greater than 0.5% of the total blend are displayed in Fig. 3 (22 total compounds). With the exception of the monoterpenes α-pinene, sabinene, β-pinene, and limonene and the sesquiterpenes β-caryophyllene and Germacrene D, all other VOCs were present in extremely small quantities (all less than 1% of the total blend). However, most of these VOCs remained significantly elevated 2 d after wounding, and persisted for the 72hr duration of the experiment. Two novel compounds were detected in the herbivore treatment, p-cymene, and an unidentified oxygenated sesquiterpene 3 (Figs. 3 and Fig. 4). These two compounds were unique in that they were not detected in I. frutescens leaf extracts, nor in MeJA-induced emissions (discussed below). Significant emissions of the unique oxygenated sesquiterpene 3 were immediately detected in response to herbivory (Tukey, P < 0.05), and these emissions remained significantly elevated 24 hr after herbivory (Fig. 4).
Volatile organic compound (VOC) blend emitted from wounded (closed bars) and unwounded (open bars) I. frutescens during the first hour of herbivory (A), 24 hr (B), 48 hr (C), and 72 hr (D) after initial wounding by P. aterrima beetles. Twenty-two VOCs greater than 5% of the total blend are plotted. Values are mean ± SE of seven plants. Identities of compounds are listed numerically in Table 1. Asterisk (*) indicates significant difference from unwounded controls (Tukey, P < 0.05).
Delayed volatile organic compound (VOC) emissions [p-cymene, oxygenated sesquiterpene 3, and (E)-4,8-dimethyl-1,3,7-nonatriene], and unique VOC emissions (p-cymene, and oxygenated sesquiterpene 3) from herbivore wounded I. frutescens. Asterisk (*) indicates significant difference from untreated controls (Tukey, P < 0.05). Values are mean ± SE of seven plants.
p-Cymene, nonatriene, and unidentified sesquiterpene 3 exhibited a delayed emission pattern that was distinct from VOCs released immediately upon wounding (Fig. 4). These compounds were not detected in wounded I. frutescens plants during the initial 2 hr of herbivory. Beginning at the third hour, they were detected in only one I. frutescens plant, which accounts for the large amount of variability in emission at that collection period. Significant emissions of unidentified sesquiterpene 3, p-cymene, and nonatriene were not detected until 4, 24, and 36 hr, respectively (Tukey, P < 0.05) (Fig. 4). Nonatriene and p-cymene emissions rapidly waned 48 hr after herbivory, but remained detectable throughout the experiment (Fig. 4). Unidentified sesquiterpene 3 emissions were not detected in I. frutescens after 24 hr (Fig. 4). The relationship between the constitutive and novel VOCs in I. frutescens is also shown in the plot of VOC loading factors in principal component space 1 and 2 (Fig. 5). Loading factors indicate the similarity between individual VOCs and the principal components on which they are plotted. All 22 VOCs used in the analysis have positive loadings on principal component 1, and most of the major constitutive mono- and sesquiterpenoids are closely associated in principal component space (Fig. 5). The herbivore novel compounds [p-cymene (10) and oxygenated sesquiterpene 3 (61)] and the delayed VOC, nonatriene (12), do not cluster with any of the major constitutive terpenoids (Fig. 5).
Relationship of 22 major individual VOC loadings (≥0.5% of total blend) in principal component space. Numbers correspond to compounds listed in Table 1. The intersection of the dotted lines represents the origin of the VOC loading factors. VOC loadings were obtained from the factor pattern matrix of the principal components analysis, and represent the correlations between VOCs and the principal components on which the values are plotted. Thus, the distance between any two VOCs is an indication of the relationship between them. Similar shapes represent a class of terpenoid. Squares = monoterpenoids, triangles = sesquiterpenoids, diamonds = homoterpenes. Closed shapes = constitutive VOCs. Open shapes = delayed/herbivore specific VOCs.
MeJA-Induced VOC Emissions
Exposure of I. frutescens to MeJA caused a 10-fold increase in total VOC emissions compared to untreated controls (P < 0.01) (Fig. 6). MeJA-elicited VOC emissions were similar to, yet distinct from, VOC blends emitted from herbivore-treated I. frutescens. The blend of VOCs emitted in response to MeJA was less complex compared to the blend released in response to herbivory. MeJA-treated plants emitted a total of 28 compounds compared to 42 released in response to herbivory (Table 1). Some compounds were unique to the MeJA-treated plants including (E)-β-ocimene, hexenyl butyrate, methyl butyrate, cis-jasmone, and methyl salicylate (Table 1). While MeJA elicited the emission of novel VOCs, it also stimulated significant emissions of constitutive VOCs (Fig. 7). MeJA stimulated the release of nonatriene, and caused a 14-fold increase in α-pinene emissions, a 5-fold increase in sabinene, and an 8-fold increase in limonene emissions (Fig. 7). The herbivore wounding and MeJA treatment results can not be statistically compared because they were conducted as separate experiments; however, it is interesting to observe the similarities in some of the VOCs emitted from I. frutescens 24 hr after wounding, and after 18 hr of MeJA exposure (Figs. 6 and 7).
Total volatile organic compound (VOC) emissions from methyl jasmonate treated and untreated; herbivore wounded and unwounded (24 hr after initial wounding) I. frutescens. Herbivore and methyl jasmonate experiments were not simultaneously conducted, preventing statistical comparison of the two treatments. Methyl jasmonate treated and untreated bars represent mean ± SE of five plants. Asterisk (*) indicates significant difference from untreated controls (t test, P < 0.05). Herbivore wounded and unwounded bars represent mean ± SE of seven plants.
Individual constitutive and nonconstitutive volatile organic compounds (VOCs) emitted from methyl jasmonate treated and untreated I. frutescens, and herbivore wounded and unwounded (24 hr after initial herbivory) I. frutescens. Methyl jasmonate treated and untreated bars represent the mean ± SE of five plants. Herbivore wounded and unwounded bars represent the mean ± SE of seven plants. Asterisk (*) indicates methyl jasmonate treatment is significantly different from methyl jasmonate untreated plants (t test, ***P < 0.05).
Discussion
Many plants respond to insect attack by releasing VOCs that are known to attract natural enemies of herbivores (Paré and Tumlinson, 1999). A short 4-hr feeding period by P. aterrima on I. frutescens caused a dramatic quantitative and qualitative change in VOC emissions over time. Herbivory induced the emissions of both constitutive and novel VOCs, and dramatically changed the composition of the total blend released. Unwounded I. frutescens emitted a mixture of eight components that were detected by gas chromatography. Wounded I. frutescens, however, emitted a mixture of 42 compounds most of which are stored constitutively in the plant and only released in significant quantities in response to herbivory. Principal components analysis by treatment and time also shows the effect of wounding on VOC emissions. Sampling periods grouped into four main clusters, indicating the similarity in variation of VOC emissions at those time periods. The aggregation of postwounding sampling periods 8, 24, and 36 hr, separate from active wounding sampling periods, and the remaining postwounding sampling periods (Fig. 2), may be the result of the release of novel and delayed VOC emissions that persisted mainly during these sampling periods.
VOCs are produced by two main biochemical pathways. The cytosolic mevalonic acid pathway produces the sesquiterpenes, and the plastidal methyl erythritol phosphate pathway produces the monoterpenes (Lichtenthaler et al., 1997). Both pathways result in the formation of precursors that react with specific terpene synthases to produce a wide variety of VOCs. Upon formation, VOCs are typically localized to specialized secretory cells where they may be stored. In this system, the quantitative and qualitative increase in VOC emissions in response to herbivory may be the result of several mechanisms. (1) The release of novel induced VOCs may be the result of de novo synthesis. (2) The increased emissions of constitutive VOCs may be the result of increased synthesis due to herbivore/MeJA up-regulation of constitutive terpene synthases. (3) Increased emission of constitutive VOCs may be the result of passive release from stored pools upon tissue breakage. The high emission during active feeding suggests mechanical release can be an important means for herbivore-stimulated VOC emissions. Casual observation of I. frutescens leaves indicates an abundance of nonglandular stellate trichomes. No obvious specialized storage structures were detected in cross sections of leaf material; however, it was observed that simple abrasion of the leaves results in no release of odorous compounds. Mechanical disruption of the lamina (breaking leaves), however, produces an immediate burst of odorous material, suggesting internal storage of compounds. Therefore, when cells are disrupted, an immediate bust of odor indicates that mechanism (3) may be in operation. While all three mechanisms may be operating to some degree during herbivory, the results of the MeJA-treated plants suggest that mechanisms (1) and (2) are responsible for the sustained increase in VOC emissions. Constitutive as well as novel VOC emissions were significantly elevated in undamaged leaf tissue exposed to MeJA, suggesting that the elevated emissions are not simply due to passive volatilization from ruptured storage structures.
Jasmonates are potent regulators of several defense pathways in plants, and both JA and its methyl conjugate, MeJA, have been directly implicated in the regulation of VOCs in plants (Reymond and Farmer, 1998; Walling, 2000; Farmer et al., 2003). Our results indicate that I. frutescens responds to MeJA treatment by emitting several VOCs, some of which were detected from the herbivore-wounded I. frutescens, and some that were unique to the MeJA treatment. MeJA-induced multiple biosynthetic pathways in I. frutescens including the shikimic acid pathway (producing methyl salicylate), the octadecanoid pathway (producing cis-jasmone), as well as the mevalonate-dependent and -independent terpenoid pathways (producing mono- and sesquiterpenes). MeJA stimulates these same pathways in agricultural species such as tomato, lima bean, and corn (Thaler et al., 1996; Dicke et al., 1999; Schmelz et al., 2003). Martin et al. (2003) recently demonstrated that MeJA application induced the activities of both constitutive and novel terpene synthases in Norway spruce. The up-regulation of constitutive and novel VOCs suggests that I. frutescens invests in the production of both constitutive and novel VOCs following herbivore attack.
The function of VOCs has been debated ever since their discovery. Terpenes may function as direct defenses by deterring herbivores (Karban and Baldwin, 1997). They may also protect vital photosynthetic material from oxidative stress, or provide thermal tolerance and UV-B protection to leaf material (Niinemets et al., 2004). VOCs can influence the behavior of herbivores as well as predators and parasitoids (Paré and Tumlinson, 1999; Heil, 2004). A large quantitative and qualitative increase in VOC emissions was detected in response to a short 4-hr feeding period. In nature, P. aterrima occur in high abundances. Larvae emerge in early spring (April), develop into adults by late spring (May), and continue feeding through midsummer (July). Because of these extended feeding periods by P. aterrima, field emissions of constitutive and induced VOCs may be even more dramatic than those detected in this laboratory assay. We speculate that while the production of large amounts of constitutive VOCs may provide some direct defensive and/or physiological functions, the large variation in VOC emissions in response to herbivory provides a basis for trophic interactions in this system. How effective VOCs may be at serving as infochemicals to higher trophic levels has yet to be determined in this setting. However, it has been shown that constitutive VOC emissions play an important role in increasing the effectiveness of foraging parasitoid wasps (Fukushima et al., 2002). Many studies aimed at understanding the ecological function of VOCs in nature have focused on agricultural species planted in monoculture field plots. In these settings, the chemical landscape is relatively homogenous compared to a native plant assemblage. I. frutescens coexists with several odorous plant species that contain their own unique VOC profiles (Wang, 2001), and the background levels of VOCs in the atmosphere may be considerably more heterogeneous relative to an agricultural field. Whether herbivores and predators/parasitoids can exploit VOC emissions in this chemically complex landscape remains to be determined. P. aterrima specializes on I. frutescens (Wang, 2001), and may exploit components of I. frutescens VOC mixture to locate host-plant material.
While most compounds were immediately detected upon herbivory, some constitutive compounds (nonatriene) as well as the novel VOCs (p-cymene) exhibited a delayed emission pattern. Our results showed that nonatriene and p-cymene were not detected in significant quantities until 24 hr after herbivory, and peak emissions occurred 36 hr after initial herbivory. While we did not assay enzyme activity, the results suggest that novel compounds, and those showing delayed emissions may be the result of a herbivore-specific response, whereas the immediate release and increased emission of constitutive VOCs may indicate a general wound response. In some systems, herbivore oral secretions are required to stimulate emissions of novel VOCs, and these emissions are typically induced hours or days after herbivory (Alborn et al., 1997; Boumeester et al., 1999; Degenhardt and Gershenzon, 2000). Furthermore, nonatriene, p-cymene, and oxygenated sesquiterpene 3 were not associated with the major constitutive VOC emissions in principal component space (Fig. 3). The placement of these compounds away from the major constitutive mono- and sesquiterpenes may be the result of a difference in the dynamics or regulation of their emissions. While many constitutive VOCs were released immediately in response to herbivory and rapidly waned thereafter, some constitutive and novel VOCs had a different dynamic, with prolonged sustained emissions following herbivory.
References
Adams, R. P. 1995. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy. Allured Publishing, Carol Stream, IL.
Alborn, H. T., Turlings, T. C. J., Jones, T. H., Stenhagen, G., Loughrin, J. H., and Tumlinson, J. H. 1997. An elicitor of plant volatiles from beet armyworm oral secretion. Science 276:945–949.
Bertness, M. D. and Yeh, S. M. 1994. Cooperative and competitive interactions in the recruitment of marsh elders. Ecology 75:2416–2429.
Boumeester, H. J., Verstappen, F. W. A., Posthumus, M. A., and Dicke, M. 1999. Spider mite-induced (3S)-(E)-nerolidol synthase activity in cucumber and lima bean. The first dedicated step in acyclic C11-homoterpene biosynthesis. Plant Physiol. 121:173–180.
De Boer, J. G. and Dicke, M. 2004. The role of methyl salicylate in prey searching behavior of the predatory mite Phytoseiulus persimilis. J. Chem. Ecol. 30:255–271.
Degenhardt, J. and Gershenzon, J. 2000. Demonstration and characterization of (E)-nerolidol synthase from maize: A herbivore-inducible terpene synthase participating in (3E)-4,8-dimethyl-1,3,7-nonatriene biosynthesis. Planta 210:815–822.
Dicke, M., Gols, R., Ludeking, D., and Posthumus, M. A. 1999. Jasmonic acid and herbivory differentially induce carniviore-attracting plant volatiles in lima bean plants. J. Chem. Ecol. 25:1907–1922.
Dicke, M., Vanbeek T. A., Pesthumus M. A., Bendom, N., Vanbokhoven, H., and Degroot, A. E. 1990. Isolation and identification of volatile kairomone that affects acarine predator–prey interactions—involvement of host plant in its production. J. Chem. Ecol. 16:381–396.
Drukker, B., Bruin, J., Jacobs, G., Kroon, A., and Sabelis, M.W. 2000. How predatory mites learn to cope with variability in volatile plant signals in the environment of their herbivorous prey. Exp. Appl. Acarol. 24:881–895.
Farag, M. A. and Paré , P. W. 2002. C6-green leaf volatiles trigger local and systemic VOC emissions in tomato. Phytochemistry 61:545–554.
Farmer, E. E. and Ryan, C. A. 1990. Interplant communication: Airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc. Natl. Acad. Sci. USA 87:7713–7716.
Farmer, E. E., Almeras, E., and Krishnawurthy, V. 2003. Jasmonates and related oxylipins in plant responses to pathogenesis and herbivory. Curr. Opin. Plant Biol. 6:372–378.
Fukushima, J., Kainoh, Y., Honda, H., and Takabayashi, J. 2002. Learning of herbivore-induced and nonspecific plant volatiles by a parasitoid Cotesia kariyai. J. Chem. Ecol. 28:579–586.
Gershenzon, J. and Croteau, R. 1993. Terpenoid biosynthesis: The basic pathway and formation of monoterpenes, sesquiterpenes, and diterpenes, pp. 333–388, in T. S. Moore Jr. (ed.). Lipid Metabolism in Plants. CRC Press, Boca Raton, FL.
Heil, M. 2004. Direct defense or ecological costs: Responses of herbivorous beetles to volatiles released by wild lima bean (Phaseolus lunatus). J. Chem. Ecol. 30:1289–1295.
Karban, R. and Baldwin, I. T. 1997. Induced Responses to Herbivory. Univ. Chicago Press, Chicago.
Kessler, A. and Baldwin, I. T. 2001. Defensive function of herbivore-induced plant volatile emissions in nature. Science 291:2141–2214.
Koch, T., Krumm, T., Jung, V., Engelberth, J., and Boland, W. 1999. Differential induction of plant volatile biosynthesis in the lima bean by early and late intermediates of the octadecanoid-signaling pathway. Plant Phsyiol. 121:153–162.
Lichtenthaler, H. K., Rohmer, M., and Schwender, J. 1997. Two independent biochemical pathways for isopentenyl diphosphate and isoprenoid biosynthesis in higher plants. Plant Physiol. 101: 643–652.
Litvak, M. E. and Monson, R. K. 1998. Patterns of induced and constitutive monterpene production in conifer needles in relation to insect herbivory. Oecologia 114:531–540.
Loughrin, J. H., Manukian, A., Heath, R. R., Turlings, T. C. J., and Tumlinson, J. H. 1994. Diurnal cycle of emission of induced volatile terpenoids by herbivore-injured cotton plants. Proc. Natl. Acad. Sci. USA 91:11836–11840.
Martin, D. M., Gershenzon, J., and Bohlmann, J. 2003. Induction of volatile terpene biosynthesis and diurnal emission by methyl jasmonate in foliage of Norway Spruce. Plant Phsyiol. 132:1586–1599.
Mattiacci, L., Rocca, B. A., Scascighini, N., D’Alessandro, M., Hern, A., and Dorn, S. 2001. Systemically induced plant volatiles emitted at the time of “danger”. J. Chem. Ecol. 27:2233–2252.
Niinemets, U., Loreto, F., and Reichstein, M. 2004. Physiological and physiochemical controls on foliar volatile organic compound emissions. Trends Plant Sci. 9:180–186.
Paré, P. W. and Tumlinson, J. H. 1997a. De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Phsyiol. 114:1161–1167.
Paré, P. W. and Tumlinson, J. H. 1997b. Induced synthesis of plant volatiles. Nature 385:30–31.
Paré, P. W. and Tumlinson, J. H. 1999. Plant volatiles as a defense against insect herbivores. Plant Phsyiol. 121:325–331.
Paré, P. W., Farag, M. A., Krishnamachari, V., Zhang, H., Ryu, C. M., and Kloepper, J. W. 2005. Elicitors and priming agents initiate plant defense responses. Photosynth. Res. 85:149–159.
Pennings, S. C. and Callaway, R. M. 1992. Salt-marsh plant zonation—The relative importance of competition and physical factors. Ecology 73:681–690.
Pennings, S. C. and Moore, D. J. 2001. Zonation of shrubs in western atlantic salt marshes. Oecologia 126:587–594.
Raffa, K. E. and Smalley, E. B. 1995. Interaction of pre-attack and induced monoterpene concentrations in host conifer defense against bark beetle–fungal complexes. Oecologia 102:285–295.
Reymond, P. and Farmer, E. E. 1998. Jasmonates and salicylate as global signals for defense gene expression. Curr. Opin. Plant Biol. 1:404 –411.
Rodriguez-Saona, C., Crafts-Brandner, S. J., Paré, P. W., and Henneberry, T. J. 2001. Exogenous methyl jasmonate induces volatile emissions in cotton plants. J. Chem. Ecol. 27:679–695.
Schmelz, E. A., Alborn, H. T., Banchio, E., and Tumlinson, J. H. 2003. Quantitative relationships between induced jasmonic acid levels and volatile emission in Zea mays during Spodoptera exigua herbivory. Planta 216:665–673.
Sharkey, T. D. and Singsaas, E. L. 1995. Why plants emit isoprene. Nature 374:769.
Thaler, J. S., Stout, M. J., Karban, R., and Duffey, S. S. 1996. Exogenous jasmonates simulate insect wounding in tomato plants (Lycopersicon esculentum) in the laboratory and field. J. Chem. Ecol. 22:1767–1781.
Theis, N. and Lerdau, M. 2003. The evolution of function in plant secondary metabolites. Int. J. Plant Sci. 164:S93–S102.
Turlings, T. C. J., Tumlinson, J. H., Heath, R. R., Proveaux, A. T., and Doolittle, R. E. 1991. Isolation and identification of allelochemicals that attract the larval parasitoid, Cotesia marginiventris (Cresson) to the microhabitat of one of its hosts. J. Chem. Ecol. 17:2235–2251.
Vanpoecke, R. M. P., Posthumus, M. A., and Dicke, M. 2001. Herbivore-induced volatile production by Arabidopsis thaliana leads to attraction of the parasitoid Cotesia rubecula: Chemical, behavioral, and gene-expression analysis. J. Chem. Ecol. 27:1911–1928.
565 Walling, L. L. 2000. The myriad of plant responses to herbivores. J. Plant Regul. 19:195–216.
Wang, M. 2001. Convergence of foliar monoterpenes in plant communities. PhD dissertation, University of South Carolina, Columbia.
Wilcox, J. A. 1957. A revision of the North American species of Paria Lec. (Coleoptera: Chrysomelidae). N.Y. State Mus. Sci. Serv. Bull. 365:1–45.
Wittstock, U. and Gershenzon, J. 2002. Constitutive plant toxins and their role in defense against herbivores and pathogens. Curr. Opin. Plant Biol. 5:1–8.
Acknowledgment
We thank the Belle W. Baruch Institute for access to the field site. Special thanks to Dr. Min Wang and Dr. Randi Hansen for their assistance with transplanting of I. frutescens.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Degenhardt, D.C., Lincoln, D.E. Volatile Emissions from an Odorous Plant in Response to Herbivory and Methyl Jasmonate Exposure. J Chem Ecol 32, 725–743 (2006). https://doi.org/10.1007/s10886-006-9030-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-006-9030-2