Abstract
Hyalesthes obsoletus Signoret (Homoptera: Cixiidae) is a polyphagous planthopper that transmits stolbur phytoplasma (a causative agent of “yellows” disease) to various weeds, members of the Solanaceae, and wine grapes (Vitis vinifera L.) in Europe and the Middle East. Planthoppers were collected by hand vacuuming eight native plant species. Vitex agnus-castus L., a shrub in the Verbenaceae, hosted the largest number of H. obsoletus, although Olea europaea L. also served as a host for adults. Using a Y-olfactometer, we compared the planthoppers relative preference for V. agnus-castus, Convolvulus arvensis, and V. vinifera. V. agnus-castus was more attractive to both male and female H. obsoletus than the other plants. H. obsoletus antennal response was stronger to volatiles collected from V. agnus-castus than from Cabernet Sauvignon variety of V. vinifera. To determine if V. agnus-castus would serve as a reservoir for the pathogen, H. obsoletus were collected from leaf and stem samples of native V. agnus-castus, and were tested by polymerase chain reaction (PCR) for the presence of phytoplasma DNA. While 14% and 25% (2003 and 2004, respectively) of the insects tested positive for phytoplasma DNA, none of the plant samples tested positive. To determine if V. agnus-castus could serve as a host plant for the development of the planthopper, we placed emergence cages beneath field shrubs and enclosed wild-caught H. obsoletus in a cage with a potted young shrub. We found adult H. obsoletus in the emergence cases and planthopper nymphs in the soil of the potted plant. We concluded that V. agnus-castus is attractive to H. obsoletus, which seems to be refractory to phytoplasma infections and warrants further testing as a trap plant near vineyards.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The level of infection of a crop by phytoplasma is dependent on the number and abundance of insect vectors and of alternative/reservoir plants harboring the pathogen, provided that the planting material is not the source of infection. In some cases, such as the aster yellows of carrots, lettuce, and celery in the midwestern U.S., the relationship between the vector and native phytoplasma-infected host plants has been elucidated (Hoy et al., 1992). In other cases, such as the stolbur phytoplasma infection in various crop plants in Europe, the role of alternative host plants in disease epidemiology is not completely understood.
Hyalesthes obsoletus Signoret (Homoptera: Cixiidae), is a polyphagous vector of stolbur phytoplasma to some weeds, plants in the Solanaceae (Brcak, 1979), and wine grapes (Vitis vinifera L.) (Maixner, 1994; Sforza et al., 1998) in Europe, and is a suspected vector to wine grapes in Israel (Klein et al., 2001). Grape vines are probably not among the preferred plants of H. obsoletus, as it survives poorly on them (Maixner, 1994; Sforza et al., 1998). Brcak (1979) reported that H. obsoletus does not leave its primary central and east European host, Convolvulus arvensis L., as long as the plant remains suitable for feeding. Furthermore, he reported that as long as the planthopper remained on C. arvensis, a natural source of the phytoplasma, there were no observed incidents of stolbur infection in crops. H. obsoletus has been found on other weeds in addition to C. arvensis, some of which are also potential phytoplasma reservoirs. In vineyards in Germany, Maixner et al. (1995) demonstrated that C. arvensis and Solanum nigrum L. were infected with stolbur phytoplasma and could serve as an inoculum reservoir. In contrast, Weber et al. (1997) showed that in vineyards where Ranunculus bulbosus L. was the dominant weed, there were fewer infected H. obsoletus, and R. bulbosus was never found to harbor the phytoplasma. In France, H. obsoletus was found to complete its life cycle on Lavendula hybrida Reverchon (Moreau and Leclant, 1973), C. arvensis, Lavendula angustifolia Mill., and Lepidium draba L. (Sforza et al., 1999), and was shown to be infected by stolbur phytoplasma (Cousin and Moreau, 1991; Sforza et al., 1998).
Yellows disease was first observed in wine grapes in Israel in the 1980s, and has had a devastating effect. To date, over 50 ha of vineyards have been uprooted due to this disease. Vineyards in affected areas have been surveyed for the presence of phloem feeding insects, such as leafhoppers and planthoppers (Klein et al., 2001; Orenstein et al., 2003), and several candidate species, including H. obsoletus, have been identified.
Alternative plant hosts for the phytoplasmas have not been identified in Israel. During the middle of the growing season (June–August), there are few living plants, and virtually no leafhoppers or planthoppers are captured in vineyards (Klein et al., 2001; Orenstein et al., 2003). Because the last precipitation in wine-grape production areas is in mid-April (Orenstein et al., 2003), by May all nonirrigated areas are dry and only deep-rooted plants remain green throughout summer. Two generations of H. obsoletus are found annually in Israeli vineyards; there is a small peak population of adults in May and early June, and another larger peak in September and October (Klein et al., 2001; Orenstein et al., 2003). The goal of this research was to determine the native host plant(s) of H. obsoletus and their ability to serve as a reservoir for phytoplasmas.
Methods and Materials
Native Plant Screening
This study took place in the Golan Heights at an elevation of 350 m in the south to 800 m in the north. We collected insects from native plants that remained green throughout the summer; Amaranthus retroflexus L., C. arvensis, Inula viscose L., Myrtus communis L., Salix alba L., Tamarix sp., and Vitex agnus-castus L. We used a modified leaf blower (Echo model No. PB 1000), in which air intake and exhaust ports were switched, and intake was fitted with a fine mesh nylon bag. Vacuum sampling collects all insects indiscriminately and does not rely on their attractiveness to a trap. In the first year (2003), a minimum of three plants of each species were sampled two to four times in May–June and once in September in the central-southern Golan. During the second year (2004), a minimum of three plants of each species were sampled two to four times in May–June and eight times from September to November; V. agnus-castus was sampled in central and northern Golan. H. obsoletus were found during spring in an olive (Olea europaea L.) orchard, and therefore sampled twice in spring and eight times in autumn.
Captured H. obsoletus were counted and recorded by sex when collected in large numbers. Live specimens were maintained at room temperature in screen-covered (50 mesh) boxes containing putative feeding plants until they were used in further trials.
Plant Choice Assay
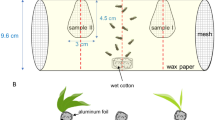
The host plant preference of H. obsoletus was determined by olfactometry. The olfactometer consisted of a Y-shaped glass tube, 2 cm diam. The base was 9 cm long, and the two arms of the olfactometer were each 16 cm in length. Each arm was attached to a flow meter and an odor source container (glass sphere, 12 cm diam) into which test plant branches were placed. In each case, charcoal-filtered air (1 l min−1) was forced through each arm of the apparatus. There were six replicates of each plant pairing: V. agnus-castus vs. C. arvensis and V. agnus-castus vs. V. vinifera. V. agnus-castus was used in each pair comparison because of the larger number of planthoppers captured in this species. In Israel, the majority of V. vinifera are grafted on Richter 110, but three other rootstocks are also used. We tested Richter 110 and Castel 216. In each replicate (six replicates with females and six with males), a group of five to seven planthoppers was placed in the base tube of the Y-olfactometer and allowed to move upwind towards either of the two arms of the olfactometer. Trial periods were 5 min in length, and the location of each test planthopper was recorded at the conclusion of each trial. To compensate for any positional bias, the olfactometer was rotated 180° after every three replicates. In order to avoid contamination by remaining plant volatiles, the olfactometer was washed with acetone and dried after each experiment.
Volatile Collection
Volatiles were collected from blooming V. agnus-castus and Cabernet Sauvignon variety of V. vinifera. As noted above, samples of V. vinifera from both rootstocks were used. Charcoal purified air was forced through a 500-ml glass sampling jar containing a plant branch, then through a 200-mg SuperQ trap at a rate of 0.2 l min−1 to collect organic chemicals released from test plants. Traps were washed with pentane to elute the captured volatiles. The resulting extract was concentrated to a volume of 10 μl and stored at −20°C.
Electroantennograms
In order to examine antennal sensitivity to volatiles of various host plants, electroantennograms (EAG) were recorded from detached antennae of male and female H. obsoletus exposed to test volatiles. The base of each antenna was inserted into a glass capillary tube, with a silver electrode lining serving as a grounding probe, while the tip of the antennae was inserted into a second capillary tube, with a silver electrode lining serving as a recording probe. Both glass capillaries were filled with 0.1 N KCl. Antennae were positioned by using a micromanipulator (INR-05, Syntech, Hilversum, The Netherlands). Two microliters of concentrated plant volatiles were applied to an approximately 1 cm2 piece of filter paper (Whatman No. 1). One microliter of pentane was applied to filter paper to serve as a blank. The filter paper was allowed to air dry for 20 sec to allow the solvent to evaporate, and was then placed inside a glass Pasteur pipette (15 cm long). The pipette tip was inserted through a side hole in a glass tube (0.6 cm diam and 15 cm long) through which charcoal-filtered, humidified air flowed at 0.5 ml min−1. Within each stimulus pipette that contained the filter paper with plant volatiles, 3 ml of air were forced into the constant air stream by a mechanical puffing that delivered puffs of 0.4-sec duration. At least 2-min intervals were maintained between each release of plant volatiles. The presentation order of stimuli for one antenna was randomized within the set (two extracts and one blank). EAG recordings were made by using a serial data acquisition controller (Syntech IDAC-232, The Netherlands).
H. obsoletus Development on V. agnus-castus
To determine if V. agnus-castus serves as a host plant upon which H. obsoletus can complete its development, two evaluation methods were used: evaluation of H. obsoletus emerging from wild V. agnus-castus and development of caged H. obsoletus on potted V. agnus-castus. Emergence boxes were made of a 50 × 50 × 40 cm wooden frame covered on five sides with 50 mesh screening. One box was placed, open side down, under the canopy of each of three V. agnus-castus plants in the center-south of the Golan at the beginning of May 2003 and again at the beginning of September. One yellow sticky trap was placed in each box to catch any emerging adult insects, and traps were replaced once every 2 wk. In May 2004, one box was placed under the canopy of each of 15 V. agnus-castus plants. One yellow sticky trap was placed in each box to catch any emerging adult insects, and traps were replaced once every 2 wk until emergence ceased; this was repeated in autumn starting at the beginning of September and continuing until emergence ceased. Additionally, in spring, 50 mesh screening was draped over the bottom branches of two different shrubs and secured to the ground, encompassing a semicircular area 1.5 m from the trunk. One yellow sticky trap was suspended on a branch from each of these shrubs and changed asdescribed.
In September 2003, 40 adult H. obsoletus were vacuum-collected and placed in a 1 × 1 × 1 m cage covered with 50 mesh screening and containing one V. agnus-castus plant. The shrub was planted in a 10-l pail: the bottom 2/3 of the potting mix was a mixture of 70:30 peat moss:volcanic gravel, and the top 1/3 was a mixture of 1:1 coconut fiber:perlite. The plant was watered with an automatic irrigation system. Since adults are difficult to discern on the plant through the net, in early May of the next year, when the first adults were observed in the field, the pot was removed from the cage and the soil around the roots was carefully examined for presence of H. obsoletus nymphs.
Since C. arvensis is a known host in Europe, in May 2003 and 2004, we dug up >50 plants in areas where V. agnus-castus were also found, and searched the roots for H. obsoletus nymphs.
Phytoplasma Infection in V. agnus-castus
Samples of H. obsoletus adult males and females were analyzed individually by polymerase chain reaction (PCR) to determine if they were positive for phytoplasma. Plants on which were they were captured were observed for typical signs and symptoms of phytoplasma infection (proliferation of small leaves, “witches’ broom,” or yellow leaves) through the duration of the study, and they were analyzed by PCR for presence of phytoplasma at the end of the season (October) in 2003 and2004.
DNA Extraction and Polymerase Chain Reaction
DNA extracted from phytoplasma-infected Vinca minor L. (periwinkle) was used as a positive control, and DNA extracted from asymptomatic V. minor served as a negative control. Insects were homogenized individually in 150 μl extraction buffer, and 1 g of plant tissue was homogenized in a total of 3 ml of extraction buffer (2% cetyl trimethyl ammonium bromide (CTAB), 100 mM Tris–HCl pH 8.0, 1.4 M NaCl, 40 mM EDTA, 0.2% β-mercaptoethanol), then put in a 65°C water bath for 30 min with periodic vortexing. Both insect and plant samples were centrifuged (1500 × g for 5 min), the supernatant was mixed with an equal volume of chloroform:isoamyl alcohol (24:1 v/v), and recentrifuged (20,000 × g for 5 min). The supernatant was mixed with 0.6 volume of isopropanol for 15 min then centrifuged (20,000 × g for 20 min) to pellet the DNA. The extracted DNA was washed twice with 70% ethanol, then resuspended in 60 μl distilled H2O, and stored at −20°C.
PCR amplification of DNA was performed by using a Tgradient thermocycler (Tamar Laboratory Supplies, Israel). The purified DNA from plant and insect samples was passed through one 30-cycle PCR reaction in the presence of universal primers defined as rU3 and fU5 (Lorenz et al., 1995). Amplification was carried out in a total of 20 μl; 0.1 μl (2.5 units μl−1) Taq polymerase (Promega), 16.5 μl amplification buffer (supplied with Taq polymerase, and dNTPS), and 5–10 ng test DNA. The PCR reaction began a 5-min 94°C heat shock step followed by a 2-min step at 50°C, a 2-min step at 72°C, 35 cycles of 92°C (30 sec), 55°C (30 sec), 72°C (45 sec). In the last cycle, the 72°C step was extended for 7 min as an elongation step. The amplified PCR products were analyzed by electrophoresis of 12 μl of reaction mixture in 1.5% agarose gel (40mM Tris–HCl, pH 7.5; 20 mM acetic acid; 1 mM EDTA), stained with ethidium bromide, and visualized with a UV transilluminator. The size standard 100-bp DNA ladder used in gels was obtained from MBI Fermentas.
Statistical Analysis
Comparisons between the numbers of planthoppers caught from the different plant species were analyzed by one-way analysis of variance (ANOVA), and means were separated by the Tukey HSD multiple range test using JMP 5.1 software (SAS software). Results of the olfactometer tests were analyzed by the log-likelihood ratio (G-statistic). Kruskal–Wallis test was used to compare the EAG responses to the headspace preparations and the control. Medians were separated by the Median-Notch method. All tests were conducted at α = 0.05 level.
Results
Native Host Plants
In the first year, more specimens of H. obsoletus were present on V. agnus-castus than or any other plant species (df 7, 118, F =153.86, P < 0.001) (Table 1). H. obsoletus were found on M. communis only in autumn. Repeated sampling of four wild V. agnus-castus shrubs in June yielded 218 H. obsoletus individuals with a male:female ratio of 58:42. Vacuuming the same four shrubs in October yielded 564 specimens, with a male:female ratio of 46:54. In spring of the second year, more specimens of H. obsoletus were present on V. agnus-castus, followed by olives, than for any other plant species (df = 9, 51, F = 87.47, P < 0.001) (Table 2). In autumn, significantly more specimens of H. obsoletus were present on V. agnus-castus than on all other plants (df = 10, 210, F = 34.04, P < 0.001) (Table 2). H. obsoletus were found on olive trees in spring, and only until mid-October in autumn.
Since H. obsoletus was present in large numbers on V. agnus-castus, further analysis was possible. Significantly fewer H. obsoletus were found on V. agnus-castus in the northern Golan (El Rom), and only until the end of October, whereas in the south-center of the Golan, they were abundant until the end of November. In October 2004, the monthly average low temperature in the north was 14.0°C, whereas in the center-south it was 16.5°C. By November, theaverage low temperature was 7.5°C in the north and 11.3°C in the center-south.
Olfactometer and Electroantennogram Trials
In an olfactometer assay, both sexes of H. obsoletus significantly preferred V. agnus-castus over grape vines (V. vinifera) and C. arvensis (Table 3), although the number of females choosing V. agnus-castus over grape vines was higher than that of males. Conversely, more males chose V. agnus-castus over C. arvensis. There was no rootstock effect.
Antennal response of adults (Table 4) was significantly higher to V. agnus-castus volatiles than to grape vines (Cabernet Sauvignon) or the blank control (df = 3, 20, F = 9.58, P < 0.001). The antennal response to the grape vine was not different from the response to the blank control. There was no rootstock effect.
H. obsoletus Development on V. agnus-castus
H. obsoletus were caught on sticky traps in emergence boxes placed under V. agnus-castus in the field. In the first year (2003) a total of eight adults were caught in May; in autumn, 10 adults were caught from the beginning of September until the middle of October. In the second year (2004), more cages were set up and emergence was monitored in spring and autumn (Table 5). In spring, adults emerged for a maximum of 6 wk, whereas in autumn they emerged over a period of almost 3 mo. In the spring, approximately half the area under each of two shrubs was covered with net; because the entire emergence area was not covered, few planthoppers were caught. Assuming a nonclustered distribution for this species, an estimated mean number of 37 (2 × number captured from 1/2 of each shrub) adult H. obsoletus emerged from each shrub. In May, we found 25 third to fifth instar H. obsoletus nymphs on the caged V. agnus-castus that were exposed to mated females in September. No H. obsoletus nymphs were found on any of the 50 + C. arvensis plants that were uprooted in spring of 2003 and 2004.
V. agnus-castus Infection with Phytoplasma
V. agnus-castus plants in the field were examined for signs and symptoms of phytoplasma infection. Although no symptoms were observed throughout the entire season, leaf and stem samples were collected from each plant in October and were analyzed by PCR for presence of phytoplasma DNA. Results of these analyses were negative for all 40 V. agnus-castus samples and healthy periwinkle, and were positive for phytoplasma-infected periwinkle (Figure 1). Of the H. obsoletus collected from these plants, 12.24% of the females (6 of 49 individuals) and 1.92% of the males (2 of 52 individuals) tested positive in 2003, and 17.78% of the females (8 of 45 individuals) and 7.5% of the males (3 of 40 individuals) tested positive in 2004.
Photograph of 1.5% agarose gel stained with ethidium bromide, showing amplified products from universal primers rU3 and fU5 for carrots and leafhoppers. Lane: M. molecular markers (in addition to those indicated, 8000, 5000, 4000, 3500, 2500 bp are also present), 1–3 Hyalesthes obsoletus, 4–5 Vitex agnus-castus, 6 healthy Vinca minor, 7 Stolbur-infected Vinca minor.
Discussion
This is the first study identifying V. agnus-castus as the preferred developmental host plant of adult H. obsoletus as observed, by vacuum sampling, on A. retroflexus, C. arvensis, M. communis, O. europaea, and V. agnus-castus. Other than C. arvensis, these are native plants that remain green throughout the year. This is consistent with findings of other researchers in Europe (Hoch and Remane, 1986; Maixner et al., 1995; Sforza et al., 1998). In central and eastern Europe, C. arvensis is the primary host for the planthopper (Brcak, 1979); however, in Israel aerial portions of this plant are completely dried by May when the first generation of H. obsoletus appears, except in irrigated areas where it is usually controlled along with other weeds.
C. arvensis does not appear in the field until after the first rains, usually in November–December, when H. obsoletus are usually no longer found (Orenstein et al., 2003). Since the weed and the planthopper are temporally different, it was not surprising that examination of the roots of more than 100 C. arvensis yielded no H. obsoletus nymphs. Furthermore, based on olfactometer and electroantennagram bioassays with C. arvensis and V. agnus-castus, the planthopper responded differently to host plant volatiles; response was higher to V. agnus-castus. Our results indicate that V. agnus-castus is a viable developmental host for the planthopper. When adult H. obsoletus were contained in a cage with young potted V. agnus-castus, nymphs developed in the same length of time as planthopper development in the field. Differences in the numbers of H. obsoletus captured on the shrub in the northern (fewer individuals) vs. central-southern Golan are consistent with previous findings on the distribution of the planthopper in vineyards (Orenstein et al., 2003) and with the distribution of phytoplasma (less incidence in the north) (Orenstein et al., 2001).
Having determined that V. agnus-castus is a preferred host plant and supports the complete development of H. obsoletus, it was necessary to determine if the shrub would serve as a reservoir for the pathogen. To date, no signs or symptoms of yellows disease have been observed in V. agnus-castus or other wild plants examined in this study. Phytoplasmas can be detected in plants that do not exhibit symptoms of infection, and the part of the plant assayed can becritical to detection (Constable et al., 2003). Asymptomatic plants may beresistant or tolerant to phytoplasma infection. Fourteen percent to 25% of the H. obsoletus adults tested by PCR were positive for phytoplasma. Sforza etal. (1998) found between ∼28% and 39% of H. obsoletus in France were infected with phytoplasma. Since there is a latent period between insect feeding on an infected plant and its capability to transmit the phytoplasma, a positive PCR result does not necessarily mean that the specific insect is capable of transmitting the phytoplasma. However, since these insects were collected in October, at the end of the second peak of H. obsoletus activity (Klein et al., 2001; Orenstein et al., 2003), it is reasonable to assume that some were capable of transmitting phytoplasma.
The attraction of H. obsoletus to V. agnus-castus, as well as the apparent lack of phytoplasma infection in this plant species, suggests that there is potential for using V. agnus-castus as a trap plant near vineyards to direct them away from grapevines. Identifying the volatile plant attractants and incorporating them in traps may be effective in protecting crops from inoculation by H. obsoletus. We are currently working on identifying the dominant components of the attractant compounds.
References
J. Brcak (1979) Leafhopper and planthopper vectors of plant disease agents in central and southern Europe K. Maramorosch K. F. Harris (Eds) Leafhopper Vectors and Plant Disease Agents Academic Press USA 97–154
F. E. Constable K. S. Gibb R. H. Symons (2003) ArticleTitleSeasonal distribution of phytoplasmas in Australian grapevines Plant Pathol. 52 267–276
M. T. Cousin J. P. Moreau (1991) Yellow decline of Lavandin (Hybrid L. Officinalis × L.Latifolia): A MLO (mycoplasma-like organism) disease. Symptoms, experimental transmission, ultrastuctural studies, role of leafhoppers, methods of control S. P. Raychaudhuri (Eds) Recent Advances in Medicinal, Aromatic and Spice Crops, Vol. 1 Today and Tomorrow’s Printers and Publishers New Delhi, India 59–62
H. Hoch R. Remane (1986) ArticleTitleEvolution und speziation der zikaden-gattung Hyalesthes Signoret, 1865 (Homoptera Auchenorrhyncha Fulgoroidea Cixiidae) Marbg. Entomol. Publ. 2 1–427
C. W. Hoy S. E. Heady T. A. Koch (1992) ArticleTitleSpecies composition, phenology and possible origins of leafhoppers (Cicadellidae) in Ohio vegetable crops J. Econ. Entomol. 85 2336–2343
M. Klein P. G. Weintraub M. Davidovich L. Kuznetsova T. Zahavi A. Ashanova S. Orenstein E. Tanne (2001) ArticleTitleMonitoring phytoplasma-bearing leafhoppers/planthoppers in vineyards in the Golan Heights, Israel J. Appl. Entomol. 125 19–23
K. H. Lorenz B. Schneider U. Ahrens E. Seemüller (1995) ArticleTitleDetection of the apple proliferation and pear decline phytoplasmas by PCR amplification of ribosomal and nonribosomal DNA Phytopathology 85 771–776
M. Maixner (1994) ArticleTitleTransmission of German grapevine yellows (Vergilbungskrankheit) by the planthopper Hyalesthes obsoletus (Auchenorrhyncha: Cixiidae) Vitis 33 103–104
M. Maixner U. Ahrens E. Seemuller (1995) ArticleTitleDetection of the German grapevine yellows (Vergilbungskrankheit) MLO in grapevine, alternative hosts and a vector by a specific PCR procedure Eur. J. Plant Pathol. 101 241–250
J. P. Moreau F. Leclant (1973) ArticleTitleContribution to the study of two lavandin insects Hyalesthes obsoletus Sign. and Cechenotettix martini Leth. (Homoptera Auchenorrhyncha) Ann. Zool. Ecol. Ani. 5 361–364
S. Orenstein T. Zahavi P. G. Weintraub (2001) ArticleTitleDistribution of phytoplasma in grapevines in the Golan Heights, Israel, and development of a new universal primer Vitis 40 219–223
S. Orenstein T. Zahavi D. Nestel R. Sharon M. Barkalifa P. G. Weintraub (2003) ArticleTitleSpatial dispersion patterns of potential leafhopper and planthopper (Homoptera) vectors of phytoplasma, and their associated phytoplasmas, in wine vineyards Ann. Appl. Biol. 142 341–348
R. Sforza D. Clair X. Daire J. Larrue E. Boudon-Padieu (1998) ArticleTitleThe role of Hyalesthes obsoletus (Hemiptera: Cixiidae) in the occurrence of Bois Noir of grapevines in France J. Phytopathol. 146 549–556
R. Sforza T. Bourgoin S. W. Wilson E. Boudon-Padiew (1999) ArticleTitleField observations, laboratory rearing and descriptions of immatures of the planthopper Hyalesthes obsoletus (Hemiptera: Cixiidae) Eur. J. Entomol. 96 409–418
Weber, A., Maixner, and M., Reinert, W. 1997. Monitoring of field populations of the vector Hyalesthes obsoletus for infestation with ‘Vergilbungskrankheit’. Proc. of the 12th ICVG Meeting, pp. 67–68.
Acknowledgments
The authors thank LeAnn Beanland for critical comments on this manuscript. This research was supported in part by a grant from the Chief Scientist, Ministry of Agriculture, State of Israel and the Wine-Grape Growers Board.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharon, R., Soroker, V., Wesley, S.D. et al. Vitex agnus-castus is a Preferred Host Plant for Hyalesthes obsoletus. J Chem Ecol 31, 1051–1063 (2005). https://doi.org/10.1007/s10886-005-4247-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-005-4247-z