Abstract
A large body of evidence shows that plants release volatile chemicals upon attack by herbivores. These volatiles influence the performance of natural enemies. Nearly all the evidence on the effect of plant volatiles on natural enemies of herbivores concerns predators, parasitoids, and entomophagous nematodes. However, other entomopathogens, such as fungi, have not been studied yet for the way they exploit the chemical information that the plant conveys on the presence of herbivores. We tested the hypothesis that volatiles emanating from cassava plants infested by green mites (Mononychellus tanajoa) trigger sporulation in three isolates of the acaropathogenic fungus Neozygites tanajoae. Tests were conducted under climatic conditions optimal to fungal conidiation, such that the influence of the plant volatiles could only alter the quantity of conidia produced. For two isolates (Altal.brz and Colal.brz), it was found that, compared with clean air, the presence of volatiles from clean, excised leaf discs suppressed conidia production. This suppressive effect disappeared in the presence of herbivore-damaged leaves for the isolate Colal.brz. For the third isolate, no significant effects were observed. Another experiment differing mainly in the amount of volatiles showed that two isolates produced more conidia when exposed to herbivore-damaged leaves compared with clean air. Taken together, the results show that volatiles from clean plants suppress conidiation, whereas herbivore-induced plant volatiles promote conidiation of N. tanajoae. These opposing effects suggest that the entomopathogenic fungus tunes the release of spores to herbivore-induced plant signals indicating the presence of hosts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants can influence the effectiveness of the third trophic level (Price et al., 1980). For example, it is well established that natural enemies are attracted by volatile chemicals released by plants that are attacked by herbivores (Dicke and Sabelis, 1988; Dicke et al., 1990; Turlings et al., 1990). These so-called herbivore-induced plant volatiles (HIPV) help the natural enemies in locating their victims and thereby also help the plant in reducing the impact of herbivory. Whereas much work has been devoted to the effect of HIPV on predators and parasitoids, little is known about the impact on entomopathogens (Elliot et al., 2000). Recently, HIPV from the roots of Thuja plants were shown to attract entomopathogenic nematodes (Boff et al., 2001; van Tol et al., 2001), but other classes of entomopathogens, such as fungi, have not yet been investigated for their response to HIPV. One exception is the study by Brown et al. (1995), which suggests that tobacco plants under aphid attack produced volatiles that delay conidial germination by the entomopathogen Pandora neoaphidis (Remaudière and Hennebert) until after the conidia come into contact with the aphid integument. The same effect was obtained by exposing conidia to volatiles from mechanically damaged (macerated) leaves and to two alcohol-type components that are part of the blend of green leaf volatiles (GLV) (Brown et al., 1995). Hence it is not yet clear whether there is a separate role for HIPV. In this study, we addressed the question whether the fungal pathogen Neozygites tanajoae Delalibera, Hajek, & Humber uses HIPV to tune the production of spores to the presence of its host, the herbivorous mite Mononychellus tanajoa (Bondar).
The mite M. tanajoa is an important pest of cassava, a staple food crop widely cultivated in Africa. It lives on the underside of cassava leaves which it damages by feeding on leaf parenchyma cells, leaving discolored spots on the leaf, easily recognizable with the naked eye. To control M. tanajoa, biological control based on the use of phytoseiid predators and the fungal pathogen N. tanajoae has been developed. N. tanajoae is specific to M. tanajoa and has been found both in the Neotropics (Agudelo-Silva, 1986; Delalibera et al., 1992; Alvarez-Afanador et al., 1993) and in Africa (Bartkowski et al., 1988; Yaninek et al., 1996; Dara et al., 2001a). However, infection rates rarely exceeded 1% in Africa, whereas frequent epizootics were reported in the Neotropics (Delalibera et al., 1992; Elliot et al., 2002). Brazilian isolates of N. tanajoae were, therefore, imported into Benin, West Africa, and released in experimental fields in 1999. Postrelease monitoring showed significantly higher infection rates in fields where Brazilian isolates were released (Hountondji et al., 2002). Annual epizootics have been observed in Benin since then (Hountondji et al., 2003). However, laboratory virulence tests conducted on leaf discs under optimal conditions failed to show significant differences between the indigenous and the imported isolates (Dara et al., 2001b). Given the differential epizootic potential of the indigenous and imported isolates of N. tanajoae in the field, we suspect genetic variation among isolates of N. tanajoae to play a role, e.g., with respect to climatic conditions or response to HIPV.
Previous studies demonstrated that the phytoseiid predators Typhlodromalus aripo DeLeon and T. manihoti Moraes respond to volatiles emanating from cassava leaves infested by M. tanajoa, as opposed to volatiles from uninfested cassava leaves, mechanically damaged leaves, and M. tanajoa females alone (Janssen et al., 1990; Gnanvossou et al., 2001, 2003). These results suggest the release of volatile chemicals upon herbivore attack (HIPV), but definitive proof of their presence is yet to be given. Therefore, we first carried out a GC-MS analysis to identify the compounds in the blends of infested and clean cassava plants.
To assess the sporulation response of the mite pathogenic fungus (N. tanajoae) to HIPV, it is important to distinguish among the various kinds of spores produced. Two types are produced during the asexual phase: spherical spores called conidia and almond-shaped spores called capilliconidia (Oduor et al., 1996a). There is also a third type, so-called resting spores, which supposedly arise in the sexual phase. Resting spores are rarely found in the field, even during epizootics (Elliot et al., 2002; Hountondji et al., 2002), but the first two types are commonly observed. Conidia are discharged from infected herbivorous mites that are mummified. Most of these immobile spores end up on the leaf in a halo around the sporulating, mummified mite. Conidia germinate to give rise to capilliconidia, which represent the infective stage of N. tanajoae. Production of conidia and their germination into capilliconidia require specific climatic conditions, which are only met during nighttime in certain periods of the year in the tropics: (1) cool temperature, and (2) water-saturated air (Oduor et al., 1996a,b; Elliot et al., 2002). We chose to focus on conidiation because survival—and hence timing—is more critical here than in the capilliconidial phase. Capilliconidia can survive a few days until contacting and infecting a host, but conidia loose their viability within an hour below saturation conditions (Oduor, 1995). If the climatic conditions are not met, they fail to germinate and produce capilliconidia. Therefore, we measured the relation between the production of conidia and HIPV as a potential signal of host presence.
Methods and Materials
Fungal Isolates
We used two Brazilian isolates that were successfully introduced in Benin (Altal.brz and Colal.brz) and one isolate found in Benin (Coton.ben). The isolates were preserved as mummified, infected M. tanajoa referred to as mummies. Mummies of Altal.brz and Colal.brz were collected from Alto Alegre and Colas das Almas in the state of Bahia, Brazil in 1995, respectively, whereas those of Coton.ben were collected in Cotonou, Benin, in 1997. Mummies of the three isolates were conserved inside tightly sealed photographic film canisters on dry cotton wool over another layer of cotton wool soaked in glycerol (serving as humidity trap) and maintained at 4°C. The stored isolates were cultured in vivo at approximately 6-mo intervals to minimize loss of viability. For each experiment, a new batch of mummies was produced for each isolate to be used within a maximum of 2 wk.
Analysis of Volatile Blends
We analyzed the volatile blends from infested and clean young cassava plants (ca. 6 wk old). Plants had the same number (nine) and the same size of leaves, and they were maintained in separate cages at 25°C in climate houses. Two clean plants and four plants infested with M. tanajoa were tested for emission of volatiles. Infested plants carried 450–600 adult female M. tanajoa. The collection was done following the procedure described by Agrawal et al. (2002). Per plant, volatiles were collected from all nine detached leaves and the plant apex and were put together into a 5-l glass desiccator. Volatiles were trapped on Tenax adsorbent (90 mg) packed in a 160× 4-mm-ID glass tube (Chrompack) by blowing purified air (ca. 100 ml/min) through the desiccator for 90 min. Incoming air was purified by drawing it through silica gel, activated charcoal, and Tenax. The Tenax tubes were stored atroom temperature in the dark until before retrieving the adsorbents through thermodesorption at 250°C for 10 min in a helium flow (10 ml/min). Desorbed products were cold-trapped at −90°C (M-16200, Chrompack) and analyzed withGC-MS. Compounds were identified by comparing the mass spectra obtained with those in the Wiley-Library and our own specialized library of natural products and by comparison of retention times (see Agrawal et al., 2002).
Exposure to HIPV
Two types of experiments were conducted to assess the effect of HIPV on conidiation of N. tanajoae. One was conducted in closed Petri dishes to compare conidiation of the three N. tanajoae isolates when exposed to volatiles emanating from small, excised leaf discs that were treated in various ways. The second experiment was conducted in a system with a regulated airflow containing either clean air or HIPV emanating from cassava leaves detached from the plant.
Diffusion in Closed Dish Environment
This experiment consisted of four treatments, differing in the sources of volatiles production and clean air as control (Figure 1). Volatiles emanated from leaf discs (2 cm diam) that were excised from the first fully expanded leaves of intact cassava plants (cv Agric) and that were subjected to the following treatments prior to the experiment:
-
Herbivore-damaged leaf discs from which all M. tanajoa and byproducts (web and feces) were removed (leaf damage scale rated 4 following the damage evaluation system proposed by Yaninek et al., 1989).
-
Leaf discs on which six young adult females of M. tanajoa, introduced 2 hr before the experiment (leaf damage scale 2), were feeding.
-
Clean leaf discs with six adult females of M. tanajoa mummified due to infection by N. tanajoae (mummies do not feed; hence no herbivore damage).
-
Leaf discs excised from herbivore-free plants (“clean” leaf discs).
Arrangement of coverslips to collect conidia (squares) and treated leaf discs (small circles) in a study of the effect of HIPV on conidiation for each isolate of the fungal pathogen N. tanajoae in closed dish experiment (large circle = Petri dish; see inset for a three-dimensional view of a Petri dish with mummies of the lid with, illustrating the “descending conidia” setup). The volatiles to which the mummies are exposed emanate from the leaf discs, which were treated as follows: clean (open circles), damaged by the herbivore M. tanajoa, which were removed just before the experiment (crossed circles), infested with M. tanajoa females that were not infected by the fungus (black circles), and clean leaf discs with M. tanajoa infected and mummified by N. tanajoae (semi-black circles). Control dishes had no leaf discs.
The method of “descending conidia” described by Papierok and Hajek (1997) was used to carry out conidiation. Three mummified mites (infected by N. tanajoae) were glued upside down using double-sided sticky tape, inside of the lid of a Petri dish (9 cm diam), with the distance between mummies being 4 cm. The conidia ejected from these mummies were shed over 2 × 2-cm coverslips on moist cotton at the bottom of the dish (Figure 1). Cotton inside the Petri dish was soaked with water until it reached the upper two thirds of the dish height, ensuring a maximum distance of 0.5 cm between coverslips and mummies. In between the coverslips, on the moist cotton, each dish had three leaf discs that received the same treatment (one of the four treatments mentioned earlier). Leaf discs were not provided in the control dishes. Each experiment involved five dishes per fungal isolate: four dishes for the different treatments and one for the control (Figure 1). The experiment was repeated three times. Petri dishes were closed with a lid that was fixed with two strips of tape at opposite sides of the lid to keep the mummies above the center of the coverslips. Petri dishes were covered with black cloth to avoid exposure to light (sporulation occurs at night) and incubated at 20 ± 1°C for 6 hr because previous experiments conducted with the three isolates showed that most conidia were produced within 6 hr at these conditions.
After the incubation period, dishes were left open for about 15 min to allow coverslips, covered with water droplets, to surface-dry. Conidia on the coverslips were mounted by turning the coverslips upside down on glass slides with a drop of 0.1% lactophenol blue for staining the conidia. Counts were made under a dissecting microscope (Zeiss, STEMI 2000, 45× magnifications) with inverted light. A piece of cross-ruled transparency was attached under the slide to facilitate counting.
Airflow System
Conidiation experiments were also performed in an airflow box. This was a plastic, cylinder-shaped (12 cm diam, 8 cm high) box with a lid tightly sealed with Teflon and Parafilm. There was an air inlet and an outlet to expose the mummies to an airflow permeated or not with volatiles, according to treatment or control (Figure 2). Moistened cotton was placed on the bottom of this airflow box. On top of the cotton layer, there were five sporulation dishes (2.5 cm diam), with a coverslip on a thin layer of cotton on the bottom (inset in Figure 2). A single mummy was glued to the lid of the sporulation dish with double-sided sticky tape, right above the coverslip so that the conidia ejected from the mummy would end up on the coverslip. The conidia on this coverslip were counted following the same procedure as described above. To provide optimal conditions for conidiation, the experiment was carried out in the dark, in a climate room at 20°C, and at high humidity (due to moistened cotton in sporulation dish and airflow box). To facilitate air exchange with the airflow box, each sporulation dish had four equidistant holes (0.4 cm diam) along its lateral side.
Diagrammatic view of the airflow system. Air from the compressor was filtered by a bottle containing charcoal before going through airflow meters, the odor bottles (one of which contained herbivore-infested leaves; the other one was empty), the airflow boxes containing sporulation dishes (see inset), and exiting through another airflow meter. Airflow meters served to regulate the flow speed; the exit airflow meter ensures a uniform flow between the two arms of the system. Teflon tubing connected the different parts of the system.
In this experiment, HIPV (treatment) was tested against clean air (control). HIPV emanated from six young cassava leaves (third leaves from the apex) infested for 3 hr prior to the experiment 100 females of M. tanajoa (that were feeding on the leaves during the experiment) inside one of the two glass odor bottles (Figure 2). Leaves were kept fresh by inserting their petiole through a Parafilm cover into a small vial (1 cm diam, 5 cm high) with water. Airflow in the system was generated by means of a compressor (SERBATOI® AUTOCLAVI, Type ELTO, volume 50 l). The air moved through tubes from the compressor to a bottle containing a charcoal filter to make it clean, then consecutively to the airflow meters, the odor bottles, the airflow boxes, and finally to an airflow meter again (Figure 2). The airflow meters at the beginning and end of the two arms of the system are necessary to maintain a uniform airflow of 1 l/min. in both arms.
Using this setup, we assessed the effect of HIPV from M. tanajoa-infested cassava leaves on the conidiation of the two fungal isolates Colal.brz and Coton.ben. The experiment was replicated 10 times for each isolate. Sporulation was allowed to take place for 24 hr. Before each experiment, airflow boxes and sporulation dishes were washed, whereas cotton wool and coverslips were replaced. Prior to putting the herbivore-infested leaves in the odor bottle and placing the mummies in the sporulation dishes, clean air was allowed to flow through both arms of the system for 2 hr. For successive experiments, allocation of clean air or HIPV was alternated between the two arms in the split tube circuit shown in Figure 2.
Data Analysis
To estimate the original number of conidia produced, it was necessary to calculate the sum of the number of nongerminated conidia, germinating conidia, and capilliconidia that had developed from conidia while ignoring the shriveled remnants of the germinated conidia.
For the closed dish experiment, data for original numbers of conidia were square-root transformed before analysis. A generalized linear model (GLM) procedure (SAS Institute Inc., 1999) was applied to test differences in conidia production between the main factors and their interactions. Isolate and treatments were treated as fixed factors, whereas experiment was treated as a random factor. Tukey’s Studentized range test served to separate means at the 5% level.
With respect to the airflow experiment, data were analyzed using replicated goodness-of-fit tests against a 1:1 null hypothesis (Sokal and Rohlf, 1997, p. 716). In this way, we assessed the effect of HIPV on conidiation in each replicated experiments, and across the different experiments, to account for heterogeneity among the trials. If heterogeneity was significant, groups of homogeneous replicates were identified using an unplanned comparisons procedure, and a G-test on pooled results within homogeneous groups of replicates was subsequently carried out to scrutinize the extent to which heterogeneity affected the validity of the total G-statistic based on all the experiments (Sokal and Rohlf, 1997, p. 722).
Results
Composition of Volatile Blends
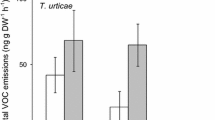
Figure 3 shows histograms of mean chromatogram peak areas for all volatiles that contributer >5% to the total chromatogram peak area. In the blend emanating from clean leaves, five volatiles were produced in relatively large amounts, namely, 2-butanon (2), 3-pentanon (3), (trans)-(E)-2-hexanal (8), (Z)-3-hexen-1-ol (11), and (E)-2-hexen-1-ol (12). In the blend emanating from infested leaves, these volatiles were produced in higher or lower quantities, particularly the production of (trans)-(E)-2-hexanal (eight) was somewhat decreased, and that of (Z)-3-hexen-1-ol (11) was increased. Moreover, these blends emanating from infested leaves contained six volatiles that were only present in trace quantities in the blends from clean leaves. In decreasing concentrations, these were (3E)-4,8-dimethyl-1,3,7-nonatriene (10), (E)-(trans)-beta ocimene (nine), 4,8,12-trimethyl-1,3(E),7(E), 11-tridecatetraene (15), methyl salicylate (14), and linalool (13) and, to a lesser extent, O-methyloxime-2-methylpropanal (one). Thus we found strong support for the release of volatiles by cassava plant upon herbivory by M. tanajoa, thereby confirming earlier inferences from behavioral experiments (Janssen et al., 1990; Gnanvossou et al., 2001). These volatiles are most likely to be herbivore-induced plant volatiles (HIPV) and not byproducts of the feces and the web (Sabelis et al., 1984; Dicke et al., 1990; Gnanvossou et al., 2001).
Quantity of volatiles (expressed in mean peak areas ± SE) produced by leaves and apices from single clean cassava plants (open bars; N = 2) or from single cassava plants infested by M. tanajoa (closed bars; N = 4). The volatiles were identified by GC-MS. Only those that contributed >5% to the total peak area in the chromatograms are shown: (1) O-methyloxime-2-methylpropanal; (2) 2-butanon; (3) 3-pentanon; (4) 1-penten-3-on; (5) 1-butanol; (6) 1-penten-3-ol; (7) heptanal; (8) (trans)-(E)-2-hexenal; (9) (E)-(trans)-beta ocimene; (10) (3E)-4,8,-dimethyl-1,3,7,-nonatriene; (11) (Z)-3-hexen-1-ol; (12) (E)-2-hexen-1-ol; (13) linalool; (14) methyl salicylate; (15) 4,8,12-trimethyl-1,3(E),7(E),11-tridecatetraene.
Closed Dish Experiment
The collective response of all three isolates of N. tanajoa to the treatments was close significance (Table 1). No significant difference was observed between the conidia production of the isolates when all treatments were considered together (Table 1). However, a significant interaction was found between the treatments and the isolates (Table 1) indicating differences in responses to treatments among isolates. To detect trends among the different responses of the isolates, we plotted conidia production under all five treatments for each isolate (Figure 4).
Conidia production of three isolates (white bars: Coton.ben; black bars: Altal.brz; hatched bars: Colal.brz) of the fungal pathogen N. tanajoae, under five different treatments (see Figure 1), 6 hr after introducing mummies of the herbivorous mite M. tanajoa into the closed dish experiment.
Averaged overall treatments, the isolate Coton.ben produced 292.3 conidia per mummy and showed relatively little variation in response to the different treatments (coefficient of variation V* = 18.9%; Sokal and Rohlf, 1997). The isolate Altal.brz produced a mean number of 347.1 conidia per mummy and showed somewhat more variation in response to the different treatments (V* = 25.6%). Much of this variation can be attributed to the high conidia production under clean air. The isolate Colal.brz produced a mean number of 368.6 conidia per mummy, but the variation in response to the different treatments was much higher than in the other two isolates (V* = 44.9%). Much of this variation here can be attributed to the strikingly high conidia production under clean air and when exposed to volatiles from leaf discs infested by M. tanajoa.
The variation expressed by the isolate Colal.brz in the bar plot points to a trend in responses to clean air as well as to volatiles from leaf discs fed upon by M. tanajoa. This trend was also indicated by the nearly significant difference between the treatments (Table 1). To identify potential causes for this trend, a Tukey test was applied following the GLM analysis to separate means between the treatments. This showed a significant difference (P < 0.05) between clean air and volatiles from clean leaf discs: 428.8 ± 41.46 conidia per mummy vs. 275.1 ± 47.48 conidia per mummy (mean ± SE), respectively. Furthermore, given the high variability and large differences in the responses to the treatments by the isolate Colal.brz, an ANOVA was conducted for this isolate alone, and significant differences in conidia production were found between the treatments (P = 0.003, F = 4.01, df = 4, N = 45). Subsequent Tukey tests to compare the means showed a significant difference between conidia production (P < 0.05) under volatiles from leaf discs fed upon by M. tanajoa and volatiles from clean leaf discs, but no significant difference between leaf discs fed upon by M. tanajoa and clean air (Figure 4).
Airflow System
Given that Colal.brz gave the most striking differences between clean air and volatiles from clean leaf discs and virtually no difference between clean air and volatiles from leaves fed upon by M. tanajoa, we decided to challenge the latter result by repeating the experiment with the following modifications: (1) using intact leaves as volatiles sources (instead of leaf discs), (2) starting with a higher infestation of herbivorous mites on cassava leaves (30× more mites than in closed dish experiment), and (3) allowing the herbivorous mites to feed longer on cassava leaves (3 d instead of 2 hr). Using the replicated G-test for total G-statistics, we found that conidia production was significantly higher in an air stream with volatiles from cassava leaves infested by M. tanajoa than under clean air conditions (404.7 conidia per mummy vs. 354.0 conidia per mummy; hence 14% more conidia; total G = 967.52, df = 10, P < 0.001). In total, there were five significant replicates in favor of the control with a difference in conidia production of only 46% (±20.6 SE) of the treatment (Figure 5). There were four significant replicates in favor of the treatment (volatiles from infested leaves), and here the difference was 187% (±71.9 SE) of the control (Figure 5). Indeed, heterogeneity among replicates was significant (heterogeneity G = 933.52, df = 9, P < 0.001). Unplanned comparisons showed groups of one, two, and more often three replicates that were homogeneous (Figure 5, right-hand panel). Taken together, the comparisons indicated a large variability among replicates (i.e., small homogeneous groups). It should be noted that whenever homogeneous groups showed a significantly higher effect of HIPV on conidia production in the treatment, the numerical difference was more pronounced than when the higher effect was found in the control.
Conidia production of a Brazilian isolate of N. tanajoae (Colal.brz) in the presence (black bars) and absence (open bars) of volatiles from intact cassava leaves infested by the herbivorous mite M. tanajoa in the airflow system experiment. Replicates of each treatment were carried out simultaneously with a replicate of the other treatment, and the results are therefore shown pairwise for 10 replicates. Significant differences among simultaneous replicates of the two treatments are indicated by an asterisk. Since replicated G-test indicated significant heterogeneity among the replicates, unplanned comparisons were carried out to test for homogeneity among replicates. The right-hand panel shows the groups of homogeneous replicates; significant differences between the two treatments using the pooled G-statistic within homogenous replicates are indicated by an asterisk.
To test whether the absence of a response in the closed dish environment implies the same response under conditions where volatiles come from intact leaves infested by a larger population of herbivorous mites feeding for a longer period, we also assessed conidiation of another isolate, i.e., Coton.ben. We found that conidia production of this isolate was significantly higher in an air stream with volatiles from cassava leaves infested by M. tanajoa than under clean air (251.0 conidia per mummy vs. 183.8 conidia per mummy; hence 37% more conidia). This can be inferred from the replicated G-test for total G-statistic (total G = 693.11, df = 10, P < 0.001). In total, there were two replicates with significantly more conidia under clean air stream, whereas there were eight replicates with significantly more conidia under an air stream with volatiles from infested leaves (Figure 6). Significant heterogeneity was observed among replicates (heterogeneity G = 588.93, df = 9, P < 0.001). Unplanned comparisons showed that apart from one group of two replicates there were four groups of four replicates that were homogeneous (Figure 6, right-hand panel). Replicated G-tests within the latter four homogeneous groups were all significant and all in favor of a higher production of conidia when exposed to HIPV. Note that the homogeneous group size for Coton.ben is somewhat larger than for the other isolate (Colal.brz), indicating a relatively lower level of heterogeneity.
Conidia production of a Benin isolate of N. tanajoae (Coton.ben) in the presence (black bars) and absence (open bars) of volatiles from intact cassava leaves infested by the herbivorous mite M. tanajoa in the airflow system experiment. Replicates of each treatment were carried out simultaneously with a replicate of the other treatment and the results are, therefore, shown pairwise for 10 replicates. Significant differences among simultaneous replicates of the two treatments are indicated by an asterisk. Since replicated G-test indicated significant heterogeneity among replicates, unplanned comparisons were carried out to test for homogeneity among replicates. The right-hand panel shows the groups of homogeneous replicates; significant differences between the two treatments using the pooled G-statistic within homogenous replicates are indicated by an asterisk.
Discussion
Based on the results of our closed dish experiments, we conclude that volatiles from clean leaves (green leaf volatiles; GLV) suppressed conidiation in the two Brazilian isolates (Colal.brz and Altal.brz), whereas volatiles from leaves infested by M. tanajoa stimulated conidiation in the Brazilian isolate Colal.brz (Figure 4). Evidence for inhibition by GLV comes from the significantly lower number of conidia produced in the presence of volatiles emanating from excised clean leaf discs than produced with clean air. Evidence for stimulation by HIPV was only found for the Brazilian isolate Colal.brz. It is based on the higher number of conidia produced in the presence of volatiles from M. tanajoa-infested leaf discs than produced under exposure to volatiles from clean leaf discs.
It is puzzling that conidia production did not differ between the treatment with clean air and that with volatiles from leaf discs fed upon by M. tanajoa. To further analyze this, we carried out a similar experiment, but now following another setup (i.e., the airflow system) in which the amount of volatiles from infested leaves was increased (six intact leaves instead of three excised leaf discs; 30 times more M. tanajoa feeding for 3 d instead of 2 hr). This experiment showed that for the Benin isolate (Coton.ben), conidia production is significantly higher when exposed to volatiles from infested leaves than when exposed to clean air (Figure 6). For the Brazilian isolate (Colal.brz), we found much heterogeneity among replicates and apart from a bias in favor of higher conidia production under exposure to HIPV we did not obtain statistical evidence for an effect of HIPV (Figure 5). Possibly, each isolate has a unique optimum in its response to different concentrations of HIPV. The significant effects for the Brazilian isolate (Colal.brz) using the closed dish experiment and that for the Benin isolate (Coton.ben) are consistent with the hypothesis that HIPV stimulates conidiation.
Inhibition of conidiation in response to volatiles from herbivore-free plants has been reported earlier for the aphid pathogen P. neoaphidis when exposed to volatiles from macerated tobacco leaves (Brown et al., 1995). Inhibition of growth was also reported in response to isothiocyanate in other insect fungi (e.g., Klingen et al., 2002) and in response to phytoalexins in plant pathogens (Baily, 1970; Fraser, 1970). Some fungi were also found to produce volatiles with a self-inhibiting effect (Chitarra et al., 2004). Whereas evidence for inhibition by volatile chemicals exists, stimulation of conidiation by herbivore-induced plant volatiles is a novel finding. It should be noted that our experiments were done under climatic conditions that are optimal for sporulation, even in the absence of chemical signals (Oduor et al., 1996a). Thus the effects of HIPV and GLV were inevitably of a relative nature. Therefore, it would be worthwhile to repeat them under conditions that are marginal for sporulation.
From a functional point of view, it is in the interest of the entomopathogenic fungus to delay conidiation until HIPV signals that herbivores are nearby. However, this leaves unexplained why conidiation readily takes place in clean air, even more so because inside a mummy the fungus would be able to survive under dry conditions for more than 8 mo (Oduor et al., 1995). We hypothesize that the fungus does not gain by delaying sporulation in an environment without cues from plants, and it may only successfully infect in the event that there is an unlucky herbivore passing by.
Whereas predators and parasitoids are well known to exploit HIPV as a source of information on the location of their victims, entomopathogens have received little attention in this respect. Infochemicals in general have been shown to play a role in this class of natural enemies (baculovirus: Felton and Duffey, 1990; Hoover et al., 1998; entomopathogenic fungi: Brown et al., 1995; entomopathogenic nematodes: Choo et al., 1989; Lei et al., 1992; Lewis et al., 1992, 1993, 1996; Grewal et al., 1993a,b, 1994; Kanagy and Kaya, 1996; Wang and Gaugler, 1998; Boff et al., 2001; Boff and Smits, 2001; van Tol et al., 2001; Cutler and Webster, 2003), but most of these studies concern entomopathogenic nematodes. Among them, recent work by van Tol et al. (2001) and Boff et al. (2001) showed that HIPV from roots of weevil-infested Thuja plants attracts the entomopathogenic nematode Heterorhabditis megidis. However, fungi are essentially motionless, and attraction cannot, therefore, play a role. Here we showed that HIPV may help to tune conidia production to the presence of herbivores on plants. This paves the way for further tests on the benefits to the plant of releasing volatiles upon herbivore attack.
Another major question for future research is “which components of the volatile blends emanating from herbivore-free and herbivore-infested cassava plants are responsible for inhibition or stimulation?”. It is interesting to note that (trans)-(E)-2-hexanal, a major component of the blend of volatiles from herbivore-free cassava plants (Figure 3), has been shown to have an inhibiting effect on conidiation of the aphid pathogen P. neoaphidis (Brown et al., 1995). As shown by our GC-MS analysis (Figure 3), there are other components of GLV [e.g., (Z)-3-hexen-1-ol; (E)-2-hexen-1-ol; 3-pentanon; 2-butanon], and HIPV [e.g., (3E)-4,8,-dimethyl-1,3,7,-nonatriene; (E)-(trans)-beta ocimene; 4,8,12-trimethyl-1,3(E),7(E),11-tridecatetraene; methyl salicylate; linalool], and these are yet to be tested for inhibition or stimulation of conidiation in N. tanajoae.
The conidiation responses of the entomopathogenic fungus to HIPV showed much more heterogeneity than the behavioral responses of predatory mites to HIPV (Janssen et al., 1990; Margolies et al., 1997; Gnanvossou et al., 2001; Aratchige et al., 2004). For example, our experiments resulted in mummies yielding 1000 conidia and mummies yielding none, whereas the average number of conidia was about 250. If our hypothesis on the function of HIPV for the plant and the entomopathogen holds, then why is there so much variability? One explanation may be that we did not sufficiently standardize the conditions of the host and/or the entomopathogen. Whereas we chose adult females as the only hosts under test, we did not select a uniform female size because this is a hard task to carry out with arthropods as small as mites. Variation in host size may cause variation in conidia yield and thereby create noise in our experimental data. Pathogen quality may also have varied in our experiments, for example, because within-host density of hyphal bodies (preceding spore formation) may differ between hosts due to variation in the number of capilliconidia infecting the host or due to variation in host quality. Our current method to propagate the entomopathogen in vivo is such that these variations inevitably arise. Development of in vitro culturing methods would be a breakthrough in helping to standardize pathogen quality. If further standardization would not substantially reduce variability in the response to HIPV, then there may be a functional reason for its existence. Entomopathogens have a risky life because they are immobile and the infective stages are especially short-lived and sensitive to abiotic conditions. This is why entomopathogens may not use HIPV as the only signal for sporulation, but possibly several other cues related to the presence of potential hosts (e.g., feces, silk). If there are many cues affecting sporulation, and the presence of those cues varies in time and space, then one may either expect entomopathogens to show the full repertoire of responses or a subset if the ability to perceive the cues entails significant costs. In the latter case, variability in responsiveness to the different cues is expected, even among the descendants of a single spore if there is a selective advantage to spreading the risk.
References
A. A. Agrawal A. Janssen J. Bruin M. A. Posthumus M. W. Sabelis (2002) ArticleTitleAn ecological cost of plant defence: Attractiveness of bitter cucumber plants to natural enemies of herbivores Ecol. Lett. 5 377–385
P. Agudelo-Silva (1986) ArticleTitleA species of Triplosporium (Zygomycetes: Entomophthoraceae) infecting Mononychellus progressivus (Acari: Tetranychidae) in Venezuela Fla. Entomol. 69 444–446
J. M. Alvarez-Afanador A. Acosta C. A. Belloti A. R. Braun (1993) ArticleTitleEstudios de patogenicidad de un hongo asociado a Mononychellus tanajoa (Bondar) acaro plaga de la yuca (Manihot esculenta Crantz) Rev. Colomb. Entomol. 19 3–5
N. S. Aratchige I. Lesna M. W. Sabelis (2004) ArticleTitleBelow-ground plant parts emit herbivore-induced plant volatiles: Olfactory responses of a predatory mite to tulip bulbs infested by rust mites Exp. Appl. Acarol. 33 21–30
J. A. Baily (1970) Phytoalexins and the ability of leaf tissues to inhibit fungal growth T. Preece (Eds) Ecology of Leaf Surface Micro-organisms Academic Press London 519–528
J. Bartkowski M. O. Odindo W. A. Otieno (1988) ArticleTitleSome fungal pathogens of the cassava green spider mites Mononychellus spp. (Tetranychidae) in Kenya Insect Sci. Appl. 9 457–459
M. I. C. Boff P. H. Smits (2001) ArticleTitleEffects of density, age and host cues on the dispersal of Heterorhabditis megidis Biocontrol Sci. Technol. 11 505–514
M. I. C. Boff F. C. Zoon P. H. Smits (2001) ArticleTitleOrientation of Heterorhabditis megidis to insect hosts and plant roots in a Y-tube sand olfactometer Entomol. Exp. Appl. 98 329–337
G. C. Brown G. L. Prochaska D. F. Hildebrand G. L. Nordin D. M. Jackson (1995) ArticleTitleGreen leaf volatiles inhibit conidial germination of the entomopathogen Pandora neoaphidis (Entomopthorales: Entomophthoraceae) Environ. Entomol. 24 1637–1643
G. S. Chitarra T. Abee F. M. Rombouts M. A. Posthumus J. Dijksterhuis (2004) ArticleTitleGermination of Penicillium paneum conidia is regulated by 1-Octen-3-ol, a volatile self-inhibitor Appl. Environ. Microbiol. 70 2823–2829
H. Y. Choo H. K. Kaya T. M. Burlando R. Gaugler (1989) ArticleTitleEntomopathogenic nematodes: Host finding ability in the presence of plant roots Environ. Entomol. 18 1136–1140
G. C. Cutler J. M. Webster (2003) ArticleTitleHost-finding ability of three entomopathogenic nematode isolates in the presence of plant roots Nematology 5 601–608
Dara, S. K., Lomer, C. J., Hountondji, F. C. C., and Yaninek, S. J. 2001a. Seasonal incidence of two fungal pathogens Neozygites floridana (Zygomycotina: Zygomycetes) and Hirsutella thompsonii (Deuteromycotina: Hyphomycetes), in mite populations on cassava in Benin, pp. 503–507, in M. O. Akoroda and J. M. Ngeve (eds.). Proceedings of 7th Triennial Symposium of the International Society for Tropical Root Crops—Africa Branch (ISTRC-AB), Cotonou, Benin, 11–17 October 1998.
Dara, S. K., Lomer, C. J., and Hountondji, F. C. C. 2001b. Release of the entomopathogenic fungus, Neozygites floridana (Zygomycetes: Entomophthorales) for control of the cassava green mite Mononychellus tanajoa (Acari: Tetranychidae): An in vivo approach, pp. 559–562, in M. O. Akoroda and J. M. Ngeve (eds.). Proceedings of the 7th Triennial Symposium of the International Society for Tropical Root Crops—Africa Branch (ISTRC-AB), Cotonou, Benin, 11–17 October 1998.
I. Delalibera D. R. Sosa-Gomes SuffixJr G. J. De Moraes J. A. De Alencar W. Farias-Araujo (1992) ArticleTitleInfection of Mononychellus tanajoa (Acari: Tetranychidae) by the fungus Neozygites sp. (Entomophthorales) in Northeastern Brazil Fla. Entomol. 75 145–147
M. Dicke M. W. Sabelis (1988) ArticleTitleHow plants obtain predatory mites as bodyguards Neth. J. Zool. 38 148–165
M. Dicke M. W. Sabelis J. Takabayashi J. Bruin M. A. Posthumus (1990) ArticleTitlePlant strategies of manipulating predator–prey interactions through allelochemicals: Prospects for application in pest control J. Chem. Ecol. 16 3091–3118
S. L. Elliot M. W. Sabelis A. Janssen L. P. S. Van der Geest E. A. M. Beerling J. Fransen (2000) ArticleTitleCan plants use entomopathogens as bodyguards? Ecol. Lett. 3 228–235
S. L. Elliot G. J. De Moraes J. Mumford (2002) ArticleTitleImportance of ambient saturation deficits in an epizootic of the fungus Neozygites floridana in cassava green mites (Mononychellus tanajoa) Exp. Appl. Acarol. 27 11–25
G. W. Felton S. S. Duffey (1990) ArticleTitleInactivation of baculovirus by quinones formed in insect-damaged plant-tissues J. Chem. Ecol. 16 1221–1236
A. K. Fraser (1970) Growth restriction of pathogenic fungi on the leaf surface T. Preece (Eds) Ecology of Leaf Surface Micro-organisms Academic Press London 529–536
D. Gnanvossou R. Hanna M. Dicke J. S. Yaninek (2001) ArticleTitleAttraction of the predatory mites Typhlodromalus manihoti and Typhlodromalus aripo to cassava plants infested by cassava green mite Entomol. Exp. Appl. 101 291–298
D. Gnanvossou R. Hanna M. Dicke (2003) ArticleTitleInfochemical-mediated niche use by the predatory mites Typhlodromalus manihoti and T. aripo (Acari: Tetranychidae) J. Insect Behav. 16 523–535
P. S. Grewal R. Gaugler S. Selvan (1993a) ArticleTitleHost recognition of entomopathogenic nematodes: Behavioral response to contact with host feces J. Chem. Ecol. 19 1219–1231
P. S. Grewal R. Gaugler E. E. Lewis (1993b) ArticleTitleHost recognition behavior by entomopathogenic nematodes during contact with insect gut contents J. Parasitol. 79 495–503
P. S. Grewal E. E. Lewis R. Gaugler J. F. Campbell (1994) ArticleTitleHost finding behavior as a predictor of foraging strategy in entomopathogenic nematodes Parasitology 108 207–215
K. Hoover M. J. Stout S. A. Alaniz B. D. Hammock S. S. Duffey (1998) ArticleTitleInfluence of induced plant defenses in cotton and tomato on the efficacy of baculoviruses on noctuid larvae J. Chem. Ecol. 24 253–271
F. C. C. Hountondji C. J. Lomer R. Hanna A. J. Cherry S. K. Dara (2002) ArticleTitleField evaluation of Brazilian isolates of Neozygites floridana (Entomophthorales: Neozygitaceae) for the microbial control of the cassava green mite in Benin, West Africa Biocontrol Sci. Technol. 12 361–370
Hountondji, F. C. C., Hanna, R., and Cherry, A. 2003. Microbial control of cassava green mite by the fungus Neozygites tanajoae in Benin, pp. 122–125, in R. Hanna and M. Toko (eds.). Proceedings of the 3rd Inter-regional Meeting of the Africa-wide Cassava Green Mite Biocontrol Project. International Institute of Tropical Agriculture, Biological Control Centre for Africa, 20–22 February 2002, Cotonou, Republic of Benin.
A. Janssen C. D. Hofker A. R. Braun N. Mesa M. W. Sabelis A. C. Bellotti (1990) ArticleTitlePreselecting predatory mites for biological control: The use of an olfactometer Bull. Entomol. Res. 80 177–181
J. M. N. Kanagy H. K. Kaya (1996) ArticleTitleThe possible role of marigold roots and α-terthienyl in mediating host finding behaviour by steinernematid nematodes Nematologica 42 220–231
I. Klingen A. Hajek R. Meadow J. A. A. Renwick (2002) ArticleTitleEffect of brassicaceous plants on the survival and infectivity of insect pathogenic fungi BioControl 47 411–425
Z. Lei T. A. Rutherford J. M. Webster (1992) ArticleTitleHeterorhabditid behavior in the presence of the cabbage maggot, Delia radicum, and its host plants J. Nematol. 24 9–15
E. E. Lewis R. Gaugler R. Harrison (1992) ArticleTitleEntomopathogenic nematode host finding: Response to host contact cues by cruise and ambush foragers Parasitology 105 309–315
E. E. Lewis R. Gaugler R. Harrison (1993) ArticleTitleResponse of cruiser and ambusher entomopathogenic nematodes (Steinernematidae) to host volatile cues Can. J. Zool. 71 765–769
E. E. Lewis M. Ricci R. Gaugler (1996) ArticleTitleHost recognition behavior predicts host suitability in the entomopathogenic nematode Steinernema carpocapsae (Rhabditidae: Steinernematidae) Parasitology 113 573–579
MARGOLIES, D. C., SABELIS, M. W., and BOYER, J. E. JR. 1997. Response of a phytoseiid predator to herbivore-induced plant volatiles: Selection on attraction and effect on prey exploitation. J. Insect Behav. 10:695–709.
Oduor, G. I. 1995. Abiotic factors and epizootiology of Neozygites cf. floridana, a fungus pathogenic to the cassava green mite. Ph.D. thesis, University of Amsterdam, 101 p.
G. I. Oduor J. S. Yaninek L. P. S. Van der Geest G. J. De Moraes (1995) ArticleTitleSurvival of Neozygites cf. floridana (Zygomycetes: Entomophthorales) in mummified cassava green mites and the viability of its primary conidia Exp. Appl. Acarol. 19 479–488
G. I. Oduor G. J. De Moraes L. P. S. Van der Geest J. S. Yaninek (1996a) ArticleTitleProduction and germination of primary conidia of Neozygites floridana (Zygomycetes: Entomophthorales) under constant temperature, humidity, and light conditions J. Invertebr. Pathol. 68 213–222
G. I. Oduor J. S. Yaninek L. P. S. Van der Geest G. J. De Moraes (1996b) ArticleTitleGermination and viability of capilliconidia of Neozygites floridana (Zygomycetes: Entomophthorales) under constant temperatures, humidities and light conditions J. Invertebr. Pathol. 67 267–278
B. Papierok A. E. Hajek (1997) Entomophthorales L. Lacey (Eds) Manual of Techniques in Insect Pathology Academic Press San Diego 187–212
P. W. Price C. E. Bouton P. Gross B. A. McPheron J. N. Thompson A. E. Weis (1980) ArticleTitleInteractions among three trophic levels: Influence of plant on interactions between insect herbivores and natural enemies Ann. Rev. Ecolog. Syst. 11 41–65
Sabelis, M. W., Afman, B. P., and Slim, P. J. 1984. Location of distant spider-mite colonies by Phytoseiulus persimilis: Localisation and extraction of a kairomone, pp. 431–440, in D. A. Griffiths & C. E. Bowman (eds.). Acarology VI, Vol. 1.
SAS Institute. (1999). SAS System for Windows, Statistics, Release 6.12, version 8. SAS Institute, Cary, NC.
R. R. Sokal F. J. Rohlf (1997) Biometry: The Principles and Practice of Statistics in Biological Research EditionNumber3rd edn. Freeman and Company New York
T. C. J. Turlings J. H. Tumlinson W. J. Lewis (1990) ArticleTitleExploitation of herbivore-induced plant odors by host-seeking parasitic wasps Science 250 1251–1253
R. W. H. M. Van Tol T. C. Van der Sommen M. I. C. Boff J. Van Bezooijen M. W. Sabelis P. H. Smits (2001) ArticleTitlePlants protect their roots by alerting the enemies of grubs Ecol. Lett. 4 292–294
Y. Wang R. Gaugler (1998) ArticleTitleHost and penetration site location by entomopathogenic nematodes against Japanese beetle larvae J. Invertebr. Pathol. 72 313–318
Yaninek, J. S., De Moraes, G. J., and Markham, R. H. 1989. Handbook on the cassava green mite (Mononychellus tanajoa): A guide to its biology and procedures for implementing classical biological control. International Institute of Tropical Agriculture, Ibadan, 140 p.
J. S. Yaninek S. Saizonou A. Onzo I. Zannou D. Gnanvossou (1996) ArticleTitleSeasonal and habitat variability in the fungal pathogens: Neozygites cf. floridana and Hirsutella thompsonii, associated with cassava mites in Benin Biocontrol Sci. Technol. 6 23–33
Acknowledgments
We are grateful to Andy Cherry for supporting the realization of this study and Honoré Dossounon, Roland Bocco, and Kevin Yenou for their technical assistance. We also thank Martijn Egas, Michiel van Wijk, Alexis Onzo, and Désiré Gnanvossou for constructive comments on the manuscript. This research was supported by the International Institute of Tropical Agriculture with funds from Danish International Development Assistance (Danida) and the International Fund for Agricultural Development (IFAD), and by the University of Amsterdam.
This is IITA Ms. number IITA/04/JA/15.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hountondji, F.C.C., Sabelis, M.W., Hanna, R. et al. Herbivore-induced Plant Volatiles Trigger Sporulation in Entomopathogenic Fungi: The Case of Neozygites tanajoae Infecting the Cassava Green Mite. J Chem Ecol 31, 1003–1021 (2005). https://doi.org/10.1007/s10886-005-4244-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-005-4244-2