Abstract
The demand for intraoperative monitoring (IOM) of lumbar spine surgeries has escalated to accommodate more challenging surgical approaches to prevent perioperative neurologic deficits. Identifying impending injury of individual lumbar roots can be done by assessing free-running EMG and by monitoring the integrity of sensory and motor fibers within the roots by eliciting somatosensory (SEP), and motor evoked potentials. However, the common nerves for eliciting lower limb SEP do not monitor the entire lumbar plexus, excluding fibers from L1 to L4 roots. We aimed to technically optimize the methodology for saphenous nerve SEP (Sap-SEP) proposed for monitoring upper lumbar roots in the operating room. In the first group, the saphenous nerve was consecutively stimulated in two different locations: proximal in the thigh and distal close to the tibia. In the second group, three different recording derivations (10–20 International system) to distal saphenous stimulation were tested. Distal stimulation yielded a higher Sap-SEP amplitude (mean ± SD) than proximal: 1.36 ± 0.9 µV versus 0.62 ± 0.6 µV, (p < 0.0001). Distal stimulation evoked either higher (73%) or similar (12%) Sap-SEP amplitude compared to proximal in most of the nerves. The recording derivation CPz–cCP showed the highest amplitude in 65% of the nerves, followed by CPz–Fz (24%). Distal stimulation for Sap-SEP has advantages over proximal stimulation, including simplicity, lack of movement and higher amplitude responses. The use of two derivations (CPz–cCP, CPz–Fz) optimizes Sap-SEP recording.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Elective lumbar spine fusion has exponentially increased, totaling more than 2 million surgeries over the past 15 years in the United States [12]. For many indications, including disc herniation, lumbar stenosis, and spondylolisthesis, using intraoperative neurophysiologic monitoring (IOM) techniques remains controversial. Nevertheless, IOM demand for spine surgery has had a massive increase in recent years [7], probably reflecting the escalation of perioperative neurologic deficits, co-morbidity, and subsequent health care and legal cost after elective spine surgery [20].
Conflicting data regarding the utility of IOM during lumbar spine surgery arises from the difficulty of identifying the injury of an individual lumbar root compared to central neurological pathways. For the latter, the benefit of monitoring corticospinal tract with motor evoked potentials (MEP) and dorsal column with somatosensory evoked potentials (SEP) has been validated for major spine surgeries [2, 6, 21]. For identifying impending injury of individual lumbar roots, IOM techniques comprise (I) recording spontaneous discharges of individual muscles to root irritation by free-running electromyography (EMG) which most surgeons prefer despite its low sensitivity [16] and (II) monitoring the integrity of sensory and motor fibers within the roots by eliciting SEP and MEP.
Common nerves used for lower limb SEP include such mixed-nerves as tibial (TN) and fibular nerves. These distal branches of the sciatic nerve, formed by conveying fibers from L4 to S3 lumbosacral roots, offer an excellent choice for monitoring lower lumbar and sacral roots during spine surgeries. However, SEP of these nerves alone do not assess the entire lumbar plexus and roots, notably lacking upper lumbar roots representation [14].
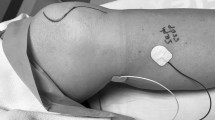
Alternatively, SEPs of the saphenous nerve (Sap-SEP) have been proposed for monitoring upper lumbar plexus and roots and femoral nerve during spine surgeries where these structures are at risk of injury [15, 18]. Fibers from L1, L2, and L3 roots, and partially but prominently from L4, convey forming the femoral nerve. The saphenous nerve, the longest sensory branch of the femoral nerve, supplies cutaneous innervation of the medial aspect of the leg and foot (Fig. 1).
Panel A illustration of the saphenous nerve and the two different techniques used for nerve stimulation: Proximal saphenous stimulation in the thigh, between the vastus medialis and the sartorius (cut) muscles, using a pair of subdermal needles and distal stimulation in the leg, between the tibia and the medial gastrocnemius muscle, using a pair of stick pad surface electrodes. Note that in both techniques the cathode electrode (black) is placed proximally to the anode (red). Panel B cutaneous territory or dermatome innervated by the saphenous nerve
The previously proposed Sap-SEP methodology for intraoperative monitoring purposes is invasive [18], induces leg movement that may interfere with the surgery, and presents a relatively high rate of failure. In this study, we aimed to technically optimize the use of Sap-SEP in the operating room by revisiting a more distal saphenous nerve stimulation and selecting the best cortical derivation for recording, which will, in turn, promote its implementation as IOM for lumbar spine surgeries.
2 Methods and technical considerations
We reviewed the data from two different groups of patients undergoing elective spine surgeries. All data were collected before the surgery started during general anesthesia maintained using total intravenous anesthesia with propofol and remifentanil or with propofol and inhalational anesthetic agents at < 0.5 mean alveolar concentration (MAC).
The first group included 104 Sap-SEPs from 52 patients to test the best methodology for stimulating the saphenous nerve based upon nerve accessibility, the amplitude of the evoked response, and leg movement. In each patient of this group, the saphenous nerve was consecutively stimulated using two different locations (Fig. 1 panel A):
-
(A)
Proximal saphenous stimulation in the thigh, as recently proposed by Silverstein et al. [18], using a pair of subdermal needles of 13 mm length (Technomed USA; White Bear Lake, MN, USA) placed in the groove between the vastus medialis and the sartorius muscles, using anatomical landmarks, about 10 cm above the knee [18].
-
(B)
Distal saphenous stimulation in the leg using a pair of round stick pad electrodes of 19 mm diameter (Natus Neurology, Inc., Middleton, WI, USA) placed on the skin surface between the tibia and the medial gastrocnemius muscle, about 15 cm above the ankle, analogous to the method used for saphenous nerve conduction studies [3, 5].
For both stimulating techniques, proximal and distal electrodes were assigned as cathode and anode, respectively. Stimuli of 0.5 ms duration at the intensity of 40 to 50 mA and 0.7 Hz repetition rate were applied. Electrical stimulation within the above intensity range provided a theoretical supramaximal stimulus to activate myelinated sensory axons [17]. At least two reproducible averaged responses of 70 to 130 sweeps were obtained for each stimulating condition. For Sap-SEP recording in this first group of patients, cork-screw electrodes (Natus Neurology, Inc., Middleton, WI, USA) paced at the scalp in a CPz–Fz derivation (10–20 International system) was used. In each patient, saphenous nerve distal stimulation followed immediately after proximal stimulation to elicit both Sap-SEP in similar anesthetic conditions. A potential was identified when a positive peak (P37) followed by a negative peak (N42) became visibly distinct from noise and was consistently reproducible among trials. Traces without a visible potential or not reproducible were considered absent potentials. The potential amplitude was measured by peak-trough P37–N42 [1, 9]. Leg movement to saphenous stimulation was visually assessed and compared between both stimulating methodologies. The movement was categorized on a qualitative scale as follows: (0) none, (1) mild movement that did not disturb the surgical field, and (2) strong movement that did disturb the surgical field.
The second group included 100 Sap-SEP from 50 patients to test the scalp derivation for the highest Sap-SEP amplitude to distal saphenous stimulation delivered at the same intensity and rate as previously described. In each patient of this group, three different derivations of the 10–20 International system were tested (Fig. 2):
-
(I)
CPz–Fz CPz active electrode, referenced to Fz.
-
(II)
CPz–cCP CPz active electrode, referenced to CP3 for right or CP4 for left-sided saphenous nerve stimulation.
-
(III)
iCP–cCP CP4 or CP3 active electrode, referenced to CP3 or CP4 for right or left-sided saphenous nerve stimulation, respectively. iCP indicates the CP electrode ipsilateral to the side stimulated (i.e. for right stimulation iCP will be CP4). cCP indicates the CP electrode contralateral to the side stimulated (i.e. for right stimulation cCP will be CP3).
Representation of the scalp recording electrodes according to the 10 to 20 International Electrode System (Axial view). Three different derivations were used for recording optimization. Panel A CPz–Fz: being CPz the active electrode (A), referenced to Fz (R). Panel B CPz–CP3 or CPz–CP4: being CPz the active electrode, referenced to CP3 or CP4 for the right and left saphenous stimulation, respectively. Panel C CP4–CP3 for right saphenous stimulation, being CP4 the active electrode referenced to CP3; CP3–CP4 for left saphenous stimulation, being CP3 the active electrode referenced to CP4. Of note, iCP and cCP indicates the CP electrode ipsi- and contralateral to the side stimulated (i.e. for right stimulation iCP will be CP4, and cCP will be CP3)
We used a Nim-Eclipse® E4 NS (Medtronic Xomed, Inc., Jacksonville, FL, USA) setting the filter bandwidth to 30–300 Hz and storing the data for later offline analysis. All studies were performed by the same team of neurophysiologists.
Statistic analysis was performed to determine differences in amplitude of Sap-SEP between the two different stimulating methodologies in the first group and among the three different scalp derivations in the second group of patients. Two-tailed p < 0.05 was considered significant. Subgroups were stratified by gender, obesity (defined as BMI > 30 kg/m2), medical history of diabetes, and the use of inhalational anesthetic agents (< 0.5 MAC).
This prospective analysis of retrospectively collected data was approved by the IRB at Mount Sinai System (IRB # 18-01277).
3 Results
The first group comprised 52 patients (32 males), aged between 23 and 90 years (55 ± 17) for analysis of 104 Sap-SEPs combining both sides were analyzed. This group included 11 patients with a medical history of diabetes and 20 patients with obesity showing BMI ≥ 30. Distal stimulation elicited a significantly higher Sap-SEP amplitude (mean ± SD) than proximal stimulation: 1.36 ± 0.9 µV versus 0.62 ± 0.6 µV (p < 0.0001). This difference persisted statistically significant in the subgroup analyses of patients with a history of diabetes or obesity. In 97% of the nerves tested, distal stimulation elicited either higher (73%) or nearly equal amplitude of not less than 20% difference (12%) compared to proximal stimulation, as shown in Fig. 3. Either stimulating methodology showed no significant differences in Sap-SEP amplitude for gender, age, side stimulated, or use of inhalational anesthetic agents. Proximal stimulation elicited no response in 12 saphenous nerves despite the presence of reproducible Sap-SEPs evoked by distal stimulation. Only two patients presented with a higher Sap-SEP amplitude to proximal compared to distal stimulation. One of these patients had diabetes, which may explain this uncommon finding as might be expected from a distally prominent neuropathic process.
Saphenous nerve somatosensory evoked potential (Sap-SEP) amplitude differences between proximal and distal stimulation for the same saphenous nerve. In most of the nerves tested, distal stimulation elicited either higher (73%) or nearly equal amplitude (12%) compared to proximal stimulation. Remarkably, proximal stimulation elicited no response in 11.5% of the nerves tested despite the presence of reproducible Sap-SEPs evoked by distal stimulation. Only two patients (3%) presented with a higher Sap-SEP amplitude to proximal compared to distal stimulation
Distal stimulation caused no leg movements as compared to proximal stimulation, which induced slight (51%) or strong (49%) movement in all the cases. Pearson correlation coefficients for Sap-SEP amplitude and site of stimulation indicated no correlation for BMI and proximal stimulation and weakly positive correlation for diabetes and distal stimulation (r = 0.07, p = 0.48).
The second group comprised 50 patients (27 males), aged between 17 and 88 years (56 ± 16) for analysis of 100 Sap-SEPs combining both sides were analyzed. The derivation CPz–cCP showed the highest initial amplitude in 65% of the nerves, followed by CPz–Fz (24%) and iCP–cCP (12%), as shown in Fig. 4. The mean amplitude (mean ± SD) for CPz–cCP was 1.5 ± 0.8 µV, for iCP–cCP was 1.3 ± 0.7 µV and for CPz–Fz was 1 ± 0.6 µV.
4 Discussion
Distal stimulation technique elicits a higher amplitude Sap-SEP than proximal stimulation. In our group of patients, the mean amplitude of Sap-SEP elicited by distal stimulation approximately doubled the corresponding value evoked by proximal stimulation (p < 0.0001, Fig. 3) and was up to 10 times higher in individual cases. Moreover, in 97% of the cases, the distally elicited responses either exceeded or equaled the proximally evoked counterpart (Fig. 3). Distal stimulation also elicited Sap-SEP universally even though proximal stimulation evoked no reliable potential in 12% of the cases. These differences probably reflect the variable depth of the saphenous nerve in the thigh based on the patient’s habitus, which, in some patients, makes it difficult to identify the anatomical landmarks to place the stimulating needles optimally. The use of a longer Teflon-isolated needle available in the market may circumvent this technical limitation partially at the risk of a substantial increase in cost. Yet, our percentage of Sap-SEP elicitability by proximal stimulation was similar or higher than previously published data [15, 18]. As an additional drawback, proximal stimulation often induces unwanted limb movement (75%) associated with muscle activation that may interfere with surgery. Summarizing, advantages of distal over proximal saphenous stimulation include higher Sap-SEP amplitude, non-invasiveness, less dependence on variable anatomical landmarks for localizing the nerve for optimal stimulation, no induction of leg movement, 100% elicitability and consistency among different patients.
Using the optimal location for saphenous stimulation thus established, we directed our effort to optimize Sap-SEP recordings by testing three different scalp derivations (Fig. 2). Considering the considerable convergence in the cortical projections of the cutaneous, muscle spindle, and joint afferents [11] and similar dipole orientation for PTN-SEP and Sap-SEP [4, 13], we tested a few derivations routinely used for TN-SEP. Of these, the CPz–cCP montage showed the highest Sap-SEP amplitude, followed by the CPz–Fz (Fig. 4). During surgery, the Sap-SEP waveform probably changes, reflecting a shift in the dipole orientation secondary to the anesthesia effect. As previously advocated, the use of two derivations throughout the surgery helps avoid a wrong interpretation of SEP decrements [8, 10, 19]. We wish to stress that distal stimulation of the saphenous nerve to obtain Sap-SEP poses no additional technical difficulty compared to the TN-SEP, although Sap-SEP conveys pure cutaneous information. In response to a new emphasis of reproducibility in IOM [10], we have documented that Sap-SEPs evoked by distal stimulation show a low trial-to-trial amplitude variation and good trace superimposable consistency (Fig. 5). This low variation persisted even in the three patients with the smallest Sap-SEP amplitude (0.28 µV) (Fig. 3, the lower trace of the group labeled 3%). Furthermore, the degree of Sap-SEP amplitude changes during surgery, where the patients suffered no injury, was comparable to TN-SEP change (9.07% and 9.58% respectively), underlining its potential as an added IOM technique.
This optimized methodology offers a simple and non-invasive approach, adding no additional cost, to IOM of lumbar spine surgeries. With the advance of more challenging operative procedures in lumbar spine surgeries, like the trans-psoas lateral approach, recording of free-running EMG of individual muscles falls short of preventing neurological injury. A large group study [16] reported that free-running EMG had the highest rate of false negatives among all IOM methodologies, showing seven times higher miscounts than SEP. Implementing these new SEP modalities may help improve the sensitivity and specificity of IOM in general and monitoring of upper lumbar roots and lumbar plexus in particular. Sap-SEP wider applications should be studied in further studies.
5 Conclusions
This study technically advances a desired methodology for the Sap-SEP that includes distal stimulation of the saphenous nerve and the use of two scalp derivations, CPz–cCP and CPz–Fz, for an optimal recording. This approach, non-invasive and based on less variable anatomical landmarks, elicits a higher amplitude Sap-SEP without producing a movement that may interfere with surgery. A more reliable recording of Sap-SEP promotes its application in the operating room, particularly when surgery poses a risk of injury to femoral nerve, upper lumbar roots, and lumbar plexus.
References
Aminoff MJ, Eisen AA. AAEM minimonograph 19: somatosensory evoked potentials. Muscle Nerve. 1998;21:277–90. https://doi.org/10.1002/(sici)1097-4598(199803)21:3%3c277::aid-mus1%3e3.0.co;2-7.
Eccher M. Intraoperative neurophysiologic monitoring: are we really that bad? J Clin Neurophysiol. 2012;29:157–9. https://doi.org/10.1097/WNP.0b013e31824ff6d0.
Eisen A, Elleker G. Sensory nerve stimulation and evoked cerebral potentials. Neurology. 1980;30:1097–105.
Kaukoranta E, Hamalainen M, Sarvas J, Hari R. Mixed and sensory nerve stimulations activate different cytoarchitectonic areas in the human primary somatosensory cortex SI. Neuromagnetic recordings and statistical considerations. Exp Brain Res. 1986;63:60–6.
Kimura J. Electrodiagnosis in diseases of nerve and muscle: principles and practice. Oxford: OUP; 2013.
Krishnakumar R, Srivatsa N. Multimodal intraoperative neuromonitoring in scoliosis surgery: a two-year prospective analysis in a single centre. Neurol India. 2017;65:75–9. https://doi.org/10.4103/0028-3886.198189.
Laratta JL, Ha A, Shillingford JN, Makhni MC, Lombardi JM, Thuet E, Lehman RA, Lenke LG. Neuromonitoring in spinal deformity surgery: a multimodality approach. Glob Spine J. 2018;8:68–77. https://doi.org/10.1177/2192568217706970.
MacDonald DB. Individually optimizing posterior tibial somatosensory evoked potential P37 scalp derivations for intraoperative monitoring. J Clin Neurophysiol. 2001;18:364–71.
MacDonald DB, Al Zayed Z, Stigsby B. Tibial somatosensory evoked potential intraoperative monitoring: recommendations based on signal to noise ratio analysis of popliteal fossa, optimized P37, standard P37, and P31 potentials. Clin Neurophysiol. 2005;116:1858–69. https://doi.org/10.1016/j.clinph.2005.04.018.
MacDonald DB, Dong C, Quatrale R, Sala F, Skinner S, Soto F, Szelenyi A. Recommendations of the International Society of Intraoperative Neurophysiology for intraoperative somatosensory evoked potentials. Clin Neurophysiol. 2019;130:161–79. https://doi.org/10.1016/j.clinph.2018.10.008.
Macefield G, Burke D, Gandevia SC. The cortical distribution of muscle and cutaneous afferent projections from the human foot. Electroencephalogr Clin Neurophysiol. 1989;72:518–28.
Martin BI, Mirza SK, Spina N, Spiker WR, Lawrence B, Brodke DS. Trends in lumbar fusion procedure rates and associated hospital costs for degenerative spinal diseases in the United States, 2004 to 2015. Spine (Phila Pa 1976). 2019;44:369–76. https://doi.org/10.1097/BRS.0000000000002822.
Nakanishi K, Inoue K, Hadoush H, Sunagawa T, Ochi M. Dipole orientation of receptive fields in the somatosensory cortex after stimulation of the posterior tibial nerve in humans. J Clin Neurophysiol. 2014;31:236–40. https://doi.org/10.1097/WNP.0000000000000044.
Nuwer MR. Intraoperative monitoring of neural function. Amsterdam: Elsevier; 2008.
Overzet K, Kazewych M, Jahangiri FR. Multimodality intraoperative neurophysiological monitoring (IONM) in anterior hip arthroscopic repair surgeries. Cureus. 2018;10: e3346. https://doi.org/10.7759/cureus.3346.
Raynor BL, Padberg AM, Lenke LG, Bridwell KH, Riew KD, Buchowski JM, Luhmann SJ. Failure of intraoperative monitoring to detect postoperative neurologic deficits: a 25-year experience in 12,375 spinal surgeries. Spine (Phila Pa 1976). 2016;41:1387–93. https://doi.org/10.1097/BRS.0000000000001531.
Rosenfalck A. Early recognition of nerve disorders by near-nerve recording of sensory action potentials. Muscle Nerve. 1978;1:360–7. https://doi.org/10.1002/mus.880010504.
Silverstein J, Mermelstein L, DeWal H, Basra S. Saphenous nerve somatosensory evoked potentials: a novel technique to monitor the femoral nerve during transpsoas lumbar lateral interbody fusion. Spine (Phila Pa 1976). 2014;39:1254–60. https://doi.org/10.1097/BRS.0000000000000357.
Taniguchi M, Nadstawek J, Pechstein U, Schramm J. Total intravenous anesthesia for improvement of intraoperative monitoring of somatosensory evoked potentials during aneurysm surgery. Neurosurgery. 1992;31:891–7.
Thirumala P, Zhou J, Natarajan P, Balzer J, Dixon E, Okonkwo D, Hamilton DK. Perioperative neurologic complications during spinal fusion surgery: incidence and trends. Spine J. 2017;17:1611–24. https://doi.org/10.1016/j.spinee.2017.05.020.
Thuet ED, Winscher JC, Padberg AM, Bridwell KH, Lenke LG, Dobbs MB, Schootman M, Luhmann SJ. Validity and reliability of intraoperative monitoring in pediatric spinal deformity surgery: a 23-year experience of 3436 surgical cases. Spine (Phila Pa 1976). 2010;35:1880–6. https://doi.org/10.1097/BRS.0b013e3181e53434.
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the Ethical Standards of the Institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study. This study was approved by the IRB at Mount Sinai System (IRB # 18-01277).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sánchez Roldán, M.Á., Mora Granizo, F., Oflidis, V. et al. Optimizing the methodology for saphenous nerve somatosensory evoked potentials for monitoring upper lumbar roots and femoral nerve during lumbar spine surgery: technical note. J Clin Monit Comput 36, 1079–1085 (2022). https://doi.org/10.1007/s10877-021-00737-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-021-00737-6