Abstract
This study was to investigate and define what is considered as a current clinical practice in hemodynamic monitoring and vasoactive medication use after cardiac surgery in Italy. A 33-item questionnaire was sent to all intensive care units (ICUs) admitting patients after cardiac surgery. 71 out of 92 identified centers (77.2 %) returned a completed questionnaire. Electrocardiogram, invasive blood pressure, central venous pressure, pulse oximetry, diuresis, body temperature and blood gas analysis were identified as routinely used hemodynamic monitoring, whereas advanced monitoring was performed with pulmonary artery catheter or echocardiography. Crystalloids were the fluids of choice for volume replacement (86.8 % of Centers). To guide volume management, central venous pressure (26.7 %) and invasive blood pressure (19.7 %) were the most frequently used parameters. Dobutamine was the first choice for treatment of left heart dysfunction (40 %) and epinephrine was the first choice for right heart dysfunction (26.8 %). Half of the Centers had an internal protocol for vasoactive drugs administration. Intra-aortic balloon pump and extra-corporeal membrane oxygenation were widely available among Cardiothoracic ICUs. Angiotensin-converting enzyme inhibitors were suspended in 28 % of the Centers. The survey shows what is considered as standard monitoring in Italian Cardiac ICUs. Standard, routinely used monitoring consists of ECG, SpO2, etCO2, invasive BP, CVP, diuresis, body temperature, and BGA. It also shows that there is large variability among the various Centers regarding hemodynamic monitoring of fluid therapy and inotropes administration. Further research is required to better standardize and define the indicators to improve the standards of intensive care after cardiac surgery among Italian cardiac ICUs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The first hours of intensive care unit (ICU) care after cardiac surgery are a very dynamic period, during which a wide range of hemodynamic alterations can occur. Therefore, hemodynamic monitoring and the administration of inotropic drugs and vasopressors are critical issues in an adequate management of the patient’s perioperative cardiovascular function [1]. Owing to technological improvements, physicians can now choose between several different devices for assessing hemodynamic function, including the pulmonary artery catheter (PAC), transesophageal echocardiography (TEE) and the new, minimally invasive devices for monitoring cardiac output [2]. Which one is the best tool to assess circulatory function and which parameter clinicians should follow to guide fluid therapy is still a matter of debate [2, 3]. Inotropes and vasopressors are the cornerstone of hemodynamic therapy [1] and are used on 30–50 % of patients to improve cardiac performance after open-heart surgery and cardiopulmonary bypass [4, 5]. Several options are available, each one with its own pharmacological properties and circulatory effects [6]. Currently, no clear advantages of one agent over any other have been established since most of the numerous studies on inotropes have focused on hemodynamic effects, while and large, well-conducted, randomized clinical trials on clinically relevant end-points are lacking [7]. Moreover, some studies have pointed out a possible harmful effect of inotropes, which are drugs with consistent side effects (e.g. increased myocardial oxygen consumption and arrhythmias) [6, 8]. Despite controversies regarding these topics, a few data with respect to clinical practice in monitoring and the use of inotropes are available today. A 2003 survey in France showed a lack of standardization in catecholamine use [9], while another survey performed in Germany in 2005 highlighted consistent variability in hemodynamic monitoring and in the use of inotropes among the different cardiothoracic ICUs [10]. Recently, following the publication of specific guidelines for post-operative intensive care of cardiac surgery patients [11], an updated survey was performed among 81 German cardiac surgery departments. In this last survey, the choice of hemodynamic monitoring was homogenous throughout different centers, while the catecholamine choice for low cardiac output syndrome still varies considerably [12]. Therefore, the aim of our survey is to investigate the current clinical practice in Italy in the hemodynamic monitoring and in the use of inotropic drugs after cardiac surgery.
2 Materials and methods
The survey was endorsed by the Italian Society of Anesthesia and Intensive Care Medicine (SIAARTI). An invitation to participate in the survey was e-mailed to all members of the SIAARTI Study Group on Cardiothoracic and Vascular Anesthesia and all participating anesthesiologists were informed about the study aims. In addition, centers performing cardiac surgery in Italy were identified through the Italian Society of Cardiac Surgery (SICCH) website (http://www.sicch.it/) and further contact details of the Cardiothoracic ICUs were obtained from hospital websites and personal contacts. The chief of ICUs of non-responding centers were then personally contacted by e-mail and invited to participate.
Respondents were asked to indicate one or more (when necessary) options in response to each question and to return the completed questionnaire by fax or e-mail. Questionnaires were collected from June 2013 to December 2013.
The questionnaire was a modified version of the one used in the 2005 German survey [10] and consisted of 33 questions covering both intra- and postoperative issues; it was delivered in Italian and the English translation of its entire text is reported in “Appendix 1”.
We decided to exclude from the analysis all questionnaires received from Centers performing exclusively pediatric cardiac surgery.
In those cases in which more than one questionnaire was returned by the same hospital (i.e. more than one physician from the same hospital answered the first email), divergences between answers given in the questionnaires were resolved by contacting the Head of Department.
No specific data regarding individual patients were collected. Research was carried out in compliance with the Helsinki Declaration.
Due to the descriptive nature of the survey, no specific statistical analysis was performed. Data are presented as percentages calculated on the number of responding Centers, without any adjustment for the center’s activity (i.e. a certain monitoring device or drug or fluid is used in “xx” % of “Centers”, not of “patients”). To estimate the fraction of responding hospitals among the total potential respondents, Centers performing adult cardiac surgery in Italy were identified through the Italian Society of Cardiac Surgery (SICCH) website (http://www.sicch.it/).
3 Results
A total of 92 Centers performing adult cardiac surgery were identified. We received 84 completed questionnaires from a total of 73 hospitals; only three questionnaires from two hospitals performing pediatric cardiac surgery were excluded, leaving for further analysis 81 questionnaires from 71 Centers, or 77.2 % of the overall potential respondents. The full list of responding Centers is available in the “Appendix 2”, and the full list of answers is available upon request.
3.1 General data
69 % of the institutions were public hospitals and 31 % were private; 33.8 % were university hospitals. The total number of procedures reported in the questionnaires was of 43,858 cardiac surgical procedures were performed in the responding Centers within the last year, with an average of 617.7 procedures/center/year (minimum, 200; maximum, 1600). Coronary artery bypass graft (CABG) was the most frequently performed operation (14306 operations/year), followed by aortic valve surgery (8363), mitral valve surgery (6634), combined CABG and valvular surgery (6330), aortic surgery (4282) and tricuspid valve surgery (1299); other procedures account for 2644 operations/year. Post-operative ICU was managed by anesthesiologists in 81.7 % of Centers, by cardiac surgeons in 4.2 %, by both sub-specialties in 11.3 %, by cardiac surgeons and cardiologists in 1.4 % and by all the three sub-specialties in 1.4 %. ICU was dedicated in 76 % of Centers and mixed in 24 %.
3.2 Hemodynamic monitoring
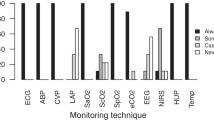
Electrocardiogram (ECG), pulse oximetry (SpO2), and blood gas analysis (BGA) were used routinely in all the Centers. Other procedures for monitoring included invasive blood pressure (BP—98.6 %), central venous pressure (CVP—98.6 %), diuresis (98.6 %), body temperature (98.6 %). End-tidal CO2 (etCO2) monitoring was less frequently used (74.6 %). PAC was used routinely in only 12 Centers (18.3 %). Central venous oxygen saturation (ScvO2) and mixed venous oxygen saturation (SvO2) were part of standard monitoring in 28.6 % and 18.3 % of Centers, respectively (Fig. 1).
ECG, BGA (blood gas analysis) have been used routinely in all the centers. Other monitoring procedures included invasive blood pressure (99 %), central venous pressure (99 %), diuresis (99 %), body temperature (99 %) and pulse oximetry (95 %); end-tidal CO2 (etCO2) monitoring was less frequently used (75 %). PAC has been used routinely in 17 %. Central Venous Oxygen Saturation (ScvO2) and Mixed Venous Oxygen Saturation (SvO2) were part of standard basic monitoring in 29 and 18 % of centers, respectively
With respect to advanced hemodynamic monitoring devices availability, Continuous Cardiac Output Pulmonary Artery Catheter Thermo Dilution, CCO PAC TD (Vigilance™-Edward LifeSciences, Irvine, California, USA) was available in 88.7 % of ICUs, a Thermo Dilution transpulmonary Thermal Indicator (TD tp ThI, PiCCO™) in 42.2 %, an uncalibrated Arterial Pressure waveform Cardiac Output measuring device (CO AP, Vigileo™ (Edwards LifeSciences, Irvine, California, USA) in 46.8 %, Thermo Dilution transpulmonary lithium Indicator (TD tp lI, LiDCO™, Calibrated devices) in 4.2 %, TEE in 100 % and transthoracic echocardiography (TTE) in 92.9 %.
When advanced hemodynamic monitoring was needed, 16.9 % of Centers reported the routine use of CCO PAC, whereas 57.7 % used TEE and 53.5 % used TTE. CO AP, TD tpThI and TD tp lI were very rarely chosen as routine devices (2.8, 1.4 and 0 %, respectively) (Fig. 2).
For advanced hemodynamic monitoring, CCO PAC TD Continuous Cardiac Output Pulmonary Artery Catheter Thermo Dilution, (Vigilance™-Edward LifeSciences, Irvine, California, USA) was available in 89 % of ICUs, a Thermo Dilution transpulmonary Thermal Indicator (TD tp ThI, PiCCO™) in 42 %, uncalibrated Arterial Pressure waveform Cardiac Output measuring device (CO AP, Vigileo™ (Edwards LifeSciences, Irvine, California, USA) in 47 %, Thermo Dilution transpulmonary lithium Indicator (TD tp lI, LiDCO™, Calibrated devices) 4 %, TEE 100 % and TTE in 93 %. When advanced hemodynamic monitoring was needed, 17 % of centers reported routine use of CCO PAC TD, whereas 57.7 % used TEE and 53.5 % used TTE. Minimally invasive devices for monitoring cardiac output have been very rarely chosen as routine devices (2.8, 1.4 and 0 %, respectively)
The most important indications for PAC placement were pulmonary arterial pressure (PAP) monitoring (85.9 %), hemodynamic instability (84.7 %), cardiac output (CO) monitoring (84.7 %) and monitoring of therapy with inotropes (79.3 %).
For PAP monitoring, a mean pre-operative pulmonary artery (PA) pressure value of 40.7 mmHg or higher was chosen as cut-off, and a mean pre-operative ejection fraction (EF) of 32.9 % or lower was a cut-off for CO monitoring (when PA pressure and EF were available pre-operatively, either estimated with echocardiography or measured through heart catheterization). If PAC was used, cardiac index (CI) and SvO2 were measured continuously in 50.7 % of Centers, intermittently in 19.7 %, and in both ways in 29.6 %.
The most important indications for a TEE evaluation were hemodynamic instability (92.9 %), valve function (74.6 %), suspected cardiac tamponade (73.2 %), and suspected regional wall motion abnormalities (66.1 %). Of these, first-line indications were hemodynamic instability and valve function (55.3 and 30.3 %, respectively). In all the centers a physician trained in TEE use was available in the hospital 24 h a day (anesthesiologists in 35.2 %, cardiologists in 12.7 % and both sub-specialties in 52.1 %).
3.3 Fluid therapy
The first choice for volume replacement were crystalloid solutions in 86.8 % of Centers, artificial colloids in 11.8 %, both indifferently in 1.5 % and both albumin and crystalloids in 1.5 %. Among crystalloids, the most frequently used were balanced electrolyte solutions. The second choice was artificial colloids in 66.7 % of Centers (Hydroxyethyl starch [HES] being the most frequently used), crystalloids in 27.3 % and albumin in 4.5 %. Blood products were used only as third choice in 20.9 % of Centers. Gelatins were available in 56.3 % of Centers, and in 70 % of them there was a daily dose limit. Starch products were available in 73.2 % of Centers, with a daily dose limit in 73 % of them. Albumin was available in 93 % of ICUs as 3.5, 5 or 20 % solution (1.4, 25.3 and 83.1 % of ICUs, respectively) (Fig. 3).
The first choice for volume replacement in Operating room (OR) and in ICU were crystalloid solutions in 86 % of centers, artificial colloids in 12 %, albumin and crystalloids in 1 %. The second choice were artificial colloids in 67 % of centers (Hydroxyethyl starch [HES] being the most frequently used), crystalloids in 27 % and albumin in 4 %. Blood products were used only as third choice, in 21 % of centers. Gelatins were available in 56 % of centers, and in 70 % of them there was a daily dose limit. Starch products were available in 73.2 % of centers, with a daily dose limit in 73 % of them. Albumin was available in 93 % of ICUs as 3.5, 5 or 20 % solution (1.4, 25.3 and 83.1 % of ICUs, respectively)
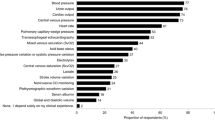
For monitoring fluid therapy and volume status, CVP was used most frequently (26.7 %), followed by arterial BP (19.7 %) and echocardiography (5.6 %). A wide range of other tools were used in the remaining Centers [e.g. wedge pressure (PCWP), stroke volume variation (SVV), inferior vena cava diameter, combinations of indices…], where each one reported to be first choice in only one or two hospitals; overall, so-called “dynamic” indices were used in 36.6 % of hospitals (Fig. 4).
For monitoring fluid therapy and volume status, CVP was used most frequently (27 %), followed by invasive arterial pressure (20 %) and SVV/PPV (10 %). “Other” include other indices or combination of indices that could not be aggregated under other indices. CI cardiac index, CO cardiac output, combined dyn + stat: combined dynamic and static indices, CVP central venous pressure, HR heart rate, iAP invasive arterial pressure, PAP pulmonary arterial pressure, PCWP pulmonary capillary wedge pressure, PPV pulse pressure variation, RAP right atrial pressure, ScvO 2 central venous oxygen saturation, SvO 2 mixed venous oxygen saturation, SVV stroke volume variation, TEE transesophageal echocardiography, TTE transthoracic echocardiography, V. cava vena cava
To reach the target value of the monitored index, crystalloids boluses were used in 18.3 % of Centers, colloids boluses in 22.5 %, and both in 57.7 % of centers.
3.4 Inotropes and vasoactive drugs
For treatment of low cardiac output syndrome (LCOS), the first choice was dobutamine (40 % of Centers), followed by dopamine (27.1 %), epinephrine (20 %) and levosimendan (18.6 %). The second choice was epinephrine (43.9 %), then levosimendan (19.7 %) and dobutamine (13.6 %). The third choice was levosimendan (43.5 % of Centers), followed by epinephrine (20.9 %) and norepinephrine (15.2 %) (Figs. 5, 6a).
For treatment of Low Cardiac Output Syndrome (LCOS), the first choice was dobutamine (40 % of centers), followed by dopamine (27 %), epinephrine (20 %) and levosimendan (19 %). Second choice was epinephrine (44 %), then levosimendan (20 %) and dobutamine (14 %). Third choice was levosimendan (44 % of centers), followed by epinephrine (21 %) and norepinephrine (15 %). LCOS: Low Cardiac Output Syndrome
A) For treatment of Low Cardiac Output Syndrome (LCOS), the first choice was dobutamine (40 % of centers), followed by dopamine (27 %), epinephrine (20 %), levosimendan (19 %), enoximone (7 %), norepinephrine (3 %) and milrinone (1 %). B) To treat right heart dysfunction, first line drugs were epinephrine (27 % of centers) and dobutamine (25 %), levosimendan (6 %), iNO (6 %) and norepinephrine (6 %). iNO inhaled nitric oxide, LCOS Low Cardiac Output Syndrome, PDE3 phosphodiesterase-3
When combination therapy was needed, the first choice was dobutamine plus norepinephrine (16.2 %), followed by levosimendan plus norepinephrine (11.8 %). Second choices were epinephrine plus phosphodiesterase-3 (PDE3) inhibitors and levosimendan plus norepinephrine (17.7 and 12.9 %, respectively). A large variability was however observed among the Centers, with 24 different combinations reported. For treatment of severe inflammatory response syndrome (SIRS) or vasoplegic syndrome, norepinephrine was used in the vast majority of cases (98.6 %). Terlipressin and vasopressin were used in 15.5 and 12.7 % of ICUs, respectively. To treat right heart dysfunction, first line drugs were epinephrine (26.8 % of Centers) and dobutamine (25.3 %) (Fig. 6b). The second choice were PDE3 inhibitors (23.5 %), dobutamine (17.6 %) and epinephrine (14.7 %). The third choice was enoximone (24.1 %), followed by levosimendan (18.9 %) and epinephrine (12.1 %).
Nitroglycerine was considered as a first-line vasodilator (59.1 % of ICUs), followed by sodium nitroprusside (31 %). Inhaled vasodilators were available in 74.6 % of ICUs, with inhaled nitric oxide (iNO) being the most widely used (94.3 %). iNO was indeed first choice for treatment of severe pulmonary hypertension (51.4 % of Centers), followed by enoximone (12.8 %) and prostanoids (10 %). Second choices were sildenafil (29.7 %) and prostanoids (18.7 %). A written protocol for administration of inotropes and vasoactive drugs was present in 52.1 % of ICUs. Angiotensin-converting-enzyme (ACE) inhibitors were suspended before surgery in 28.1 % of Centers, with half of them withholding the drug 24 h before intervention.
3.5 Transfusion of blood products
A written transfusion protocol was present in 69.6 % of Centers. In 60.6 % of ICUs, there was a threshold for starting transfusion of red blood cells. Mean values of 7.79 g/dL hemoglobin and 24.2 % hematocrit were indicated as the lower limit for red blood cells transfusion. When a threshold was not contemplated, transfusions were mainly guided by SvO2 (64.3 %), hemorrhages (64.3 %), patient age (53.6 %) or lactate levels (46.4 %). Moreover, 15 % of Centers reported the use of “physiological” indices together with fixed hemoglobin/hematocrit values.
3.6 Mechanical circulatory support
Devices for mechanical support were available in all Centers. IABP was available in 97.1 % of Centers, ECMO in 95.5 %. In 57.7 % of ICUs it was possible to use a left ventricle assisting device (LVAD), in 46.5 % a right VAD, and in 42.3 % a biventricular VAD. Veno-venous ECMO was used to treat severe respiratory insufficiency in 76.1 % of Centers; this service was managed mainly by anesthesiologists (42.6 %) or anesthesiologists and cardiac surgeons (35.2 %).
4 Discussion
To the best of our knowledge, this is the first study describing routine clinical practice in Italian cardiothoracic ICUs. A total of 71 Centers contributed to our survey, accounting for 78.8 % of the hospitals performing cardiac surgery; our study can therefore be considered representative of postoperative management of cardiac surgery patients in Italy.
Our survey revealed that ECG, IBP, CVP, SpO2, diuresis, body temperature and etCO2 are considered as standard monitoring for cardiac surgery patients. Our results are similar to those obtained by the 2005 and the following German surveys [10, 12, 13]. PAC, which was considered part of standard monitoring in 2005 German survey, is reported to be routinely used only in a minority of Italian centers. This result is consistent with the most recent German survey [12]. Once considered essential for monitoring the cardiovascular status of the critically ill, the use of PAC has largely decreased in recent years [14]. Although there is general agreement that PAC should be reserved only for high-risk patients, such as those with severe pre-operative ventricular dysfunction or undergoing complex cardiac operations [15, 16], a recent study questioned the benefits of PAC even in high-risk patients [17]. Nevertheless, a recent survey on the use of PAC in cardiac surgery showed that more than two-third of practitioners use PAC in over 75 % of patients [18]. TEE allows a quick and reliable examination of heart anatomy and function, thus permitting rapid diagnosis of life-threatening conditions that could complicate cardiac surgery [19]. In a 2002 survey among American and Canadian cardiovascular anesthesiologists, TEE was available only in 56 % of the surveyed Centers [20]. In the 2005 German survey, TTE and TEE were used for advanced hemodynamic monitoring only in 38 and 32 % of Centers, respectively [10]. These percentages increased in our study to 92.8 and 100 % of Centers, with almost 76.1 % of them reporting to “always” use echocardiography (either TT or TE) for advanced hemodynamic monitoring. Our data are similar to those of the last German survey, which reported a 95 % median use of TEE [12]. Moreover, in all Italian surveyed ICUs a TEE expert was available around the clock, compared with 65 % of the 2005 German survey and 72 % of another German survey [13] performed in 2008 (following the release of the S3 guidelines for post-cardiac surgery intensive care [11] ). Thus, our study confirms that echocardiography is currently considered as a fundamental tool for the management of cardiac surgery patients and is routinely used in all the cardiothoracic ICUs.
In our study, crystalloids (Ringer’s solution in particular) were the favorite fluids for volume replacement (87 % of Centers), whereas in the 2005 German survey, colloids were the first choice [10]. Recent large randomized trials found that HES use was associated with increased mortality and incidence of acute kidney injury (AKI) in septic and general ICU patients [21, 22], whereas other studies found a neutral [23] or even beneficial effect of colloids [24]. Due to concerns regarding HES safety, the European Medicines Agency (EMA) suspended their use in June 2013; in October 2013, HES use was authorized again, albeit with some limitations. Of note, our survey was designed prior to the EMA suspension and publication of the CHEST [21] trial and 6S trial [22]. However, several answers were collected after CHEST and 6S trials publication and during the suspension period and the final analysis was made after HES re-introduction; therefore, our results regarding HES use should be interpreted cautiously [25].
It is interesting to note that, despite the amount of literature published on the topic and their recognized limitations [26, 27], CVP and blood pressure are still the preferred reference parameters to monitor fluid therapy, as they were in the German surveys [10, 12, 13]. These results could be due to limitations of the so-called “dynamic indices” in cardiac surgery patients: open-chest conditions, arrhythmias, right heart failure and the use of low tidal volumes have recently been found to reduce the efficacy of dynamic indices to predict fluid responsiveness [28–30]. Moreover, proper use of dynamic indices often requires placement of specific devices [31] which are not yet routinely used in all cardiac surgical patients, while a central venous catheter is almost always used.
According to our study, dobutamine is Italian physicians’ first choice when an inotrope is needed to treat LCOS. A similar result was obtained in a French survey on the use of inotropes following cardiac surgery [32]. In the 2005 German survey the first line LCOS treatment was epinephrine, while in the most recent German survey the first line treatment consisted in either dobutamine and epinephrine [10, 12]. While in Germany epinephrine is still considered as a first choice agent, in Italy it is generally the second choice, and in France the third.
Dopamine was reported to be among the first choices ad inotropic agent to treat LCOS, although the SOAP-II trial showed that, in patients with shock in general ICU, norepinephrine may be a better agent [33]. However, in the SOAP-II trial dopamine was used at a high-dosage associated with prominent α-adrenergic effect (thus more as a vasopressor agent rather than inotrope), while to treat LCOS after cardiac surgery generally a “pure” β-adrenergic inotropic effect is sought. Our hypothesis is that cardiac anesthesiologist use dopamine at β-adrenergic stimulating dose, and switch to another inotropic agent in case excessive dopamine doses are required. In a context of lack of clear indications from available studies, these results show that Italian physicians generally follow the recommendations of experts and guidelines [7, 11, 34] regarding inotropic therapy; still, the choice of first-line inotropes to treat LCOS is variable, with several Centers reporting preference for dopamine, epinephrine or levosimendan. Third choice in our survey was levosimendan, which was not even mentioned in the previous European surveys [10, 13, 32] and was considered only as a second line treatment in the 2013 German survey [12]. Levosimendan is a relatively new calcium-sensitizing agent with inodilator properties, nevertheless, our study shows that levosimendan has now entered routine clinical practice. On the contrary, few Centers reported to use PDE3 inhibitors, which were among the preferred drugs used to treat LCOS in the 2005 German survey [10] and are currently the second-line drug of choice [12]. When considering a combination of drugs, we found an even larger variability with around 20 combinations reported for first, second and third choice.
4.1 Limitations of the study
We have no data to confirm whether the survey reflects the demographic population of all Italian cardiac ICUs. This is a general limitation of all our surveys. We do not have any outcome data to confirm whether a certain intervention is actually beneficial or not. In order to simplify the questionnaire, no difference was made between LCOS with high or low systemic vascular resistances, between primary or secondary right ventricular failure, or between the different types of patients.
Another possible bias is the difference in clinical habits between anesthesiologists in the same center. However the questionnaires were addressed to the head of every center. Every physician may have a different background, experience and different ideas about the same patient, but the main procedures monitoring and clinical decision in the same centers usually follow a protocol.
4.2 Conclusions
This survey describes the current situation concerning Italian anesthetic management following cardiac surgery, and we can summarize our findings as follows:
-
Standard, routinely used monitoring consists of ECG, SpO2, etCO2, invasive BP, CVP, diuresis, body temperature, and BGA.
-
TEE is used in every cardiothoracic Center.
-
PAC is routinely used in 18.3 % of Centers.
-
Crystalloids are the fluid of choice for volume replacement.
-
Fluid therapy is guided mainly by CVP and blood pressure.
-
Dobutamine is the inotrope of choice for treatment of LCOS.
-
Epinephrine is the inotrope of choice for treatment of right ventricular failure.
-
Half of the surveyed Centers have an internal protocol for inotropes administration.
The use of inotropes, particularly when a combined therapy is needed, is variable among Centers. We found that, despite their limitations, CVP and arterial pressure are still the preferred indices to guide fluid therapy. Our survey shows important controversies concerning these fundamental topics.
Further research is required to better standardize and define the indicators to improve the standards of intensive care after cardiac surgery among Italian cardiac ICUs.
References
St. André AC, DelRossi A. Hemodynamic management of patients in the first 24 hours after cardiac surgery. Crit Care Med. 2005;33:2082–93. doi:10.1097/01.CCM.0000178355.96817.81.
Vincent JL, Rhodes A, Perel A, et al. Clinical review: update on hemodynamic monitoring—a consensus of 16. Crit Care. 2011;15:229–36. doi:10.1186/cc10291.
Marik PE, Monnet X, Teboul JL. Hemodynamic parameters to guide fluid therapy. Ann Intensive Care. 2011;1:1. doi:10.1186/2110-5820-1-1.
Müller M, Junger A, Bräu M, et al. Incidence and risk calculation of inotropic support in patients undergoing cardiac surgery with cardiopulmonary bypass using an automated anaesthesia record-keeping system. Br J Anaesth. 2002;89:398–404. doi:10.1093/bja/89.3.398.
Ahmed I, House CM, Nelson WB. Predictors of inotrope use in patients undergoing concomitant coronary artery bypass graft (CABG) and aortic valve replacement (AVR) surgeries at separation from cardiopulmonary bypass (CPB). J Cardiothorac Surg. 2009;4:24. doi:10.1186/1749-8090-4-24.
Overgaard CB, Dzavík V. Inotropes and vasopressors: review of physiology and clinical use in cardiovascular disease. Circulation. 2008;118:1047–56. doi:10.1161/CIRCULATIONAHA.107.728840.
Gillies M, Bellomo R, Doolan L, Buxton B. Bench-to-bedside review: inotropic drug therapy after adult cardiac surgery—a systematic literature review. Crit Care. 2005;9:266–79. doi:10.1186/cc3024.
Nielsen DV, Hansen MK, Johnsen SP, Hansen M, Hindsholm K, Jakobsen CJ. Health outcomes with and without use of inotropic therapy in cardiac surgery: results of a propensity score-matched analysis. Anesthesiology. 2014;120:1098–108. doi:10.1097/ALN.0000000000000224.
Leone M, Vallet B, Teboul JL, Mateo J, Bastien O, Martin C. Survey of the use of catecholamines by French physicians. Intensive Care Med. 2004;30:984–8. doi:10.1007/s00134-004-2172-1.
Kastrup M, Markewitz A, Spies C, et al. Current practice of hemodynamic monitoring and vasopressor and inotropic therapy in post-operative cardiac surgery patients in Germany: results from a postal survey. Acta Anaesthesiol Scand. 2007;51:347–58. doi:10.1111/j.1399-6576.2006.01190.x.
Carl M, Alms A, Braun J, et al. S3 guidelines for intensive care in cardiac surgery patients: hemodynamic monitoring and cardiocirculary system. Ger Med Sci. 2010;. doi:10.3205/000101.
Sponholz C, Schelenz C, Reinhart K, Schirmer U, Stehr SN. Catecholamine and volume therapy for cardiac surgery in Germany—results from a postal survey. PLoS One. 2014;9:e103996. doi:10.1371/journal.pone.0103996.
Kastrup M, Carl M, Spies C, Sander M, Markewitz A, Schirmer U. Clinical impact of the publication of S3 guidelines for intensive care in cardiac surgery patients in Germany: results from a postal survey. Acta Anaesthesiol Scand. 2013;57:206–13. doi:10.1111/aas.12009.
Gershengorn HB, Wunsch H. Understanding changes in established practice: pulmonary artery catheter use in critically ill patients. Crit Care Med. 2013;41:2667–76. doi:10.1097/CCM.0b013e318298a41e.
Vincent JL, Pinsky MR, Sprung CL, et al. The pulmonary artery catheter: in medio virtus. Crit Care Med. 2008;36:3093–6. doi:10.1097/CCM.0b013e31818c10c7.
Cowie B. Does the pulmonary artery catheter still have a role in the perioperative period? Anaesth Intensive Care. 2011;39:345–55.
Chiang Y, Hosseinian L, Rhee A, Itagaki S, Cavallaro P, Chikwe J. Questionable benefit of the pulmonary artery catheter after cardiac surgery in high-risk patients. J Cardiothorac Vasc Anesth. 2015;29:76–81. doi:10.1053/j.jvca.2014.07.017.
Judge O, Ji F, Fleming N, Liu H. Current use of the pulmonary artery catheter in cardiac surgery: a survey study. J Cardiothorac Vasc Anesth. 2015;29:69–75. doi:10.1053/j.jvca.2014.07.016.
Reeves ST, Finley AC, Skubas NJ, et al. Basic perioperative transesophageal echocardiography examination: a consensus statement of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. Anesth Analg. 2013;117:543–58. doi:10.1213/ANE.0b013e3182a00616.
Jacka MJ, Cohen MM, To T, Devitt JH, Byrick R. The use of and preferences for the transesophageal echocardiogram and pulmonary artery catheter among cardiovascular anesthesiologists. Anesth Analg. 2002;94:1065–71. doi:10.1097/00000539-200205000-00003.
Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367:1901–11. doi:10.1056/NEJMoa1209759.
Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.4 versus Ringer’s Acetate in severe sepsis. N Engl J Med. 2012;367:124–34. doi:10.1056/NEJMoa1204242.
Guidet B, Martinet O, Boulain T, et al. Assessment of hemodynamic efficacy and safety of 6 % hydroxyethylstarch 130/0.4 vs. 0.9 % NaCl fluid replacement in patients with severe sepsis: the CRYSTMAS study. Crit Care. 2012;16:R94. doi:10.1186/cc11358.
Annane D, Siami S, Jaber S, et al. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA. 2013;310:1809–17. doi:10.1001/jama.2013.280502.
Meybohm P, Van Aken H, De Gasperi A, et al. Re-evaluating currently available data and suggestions for planning randomised controlled studies regarding the use of hydroxyethyl starch in critically ill patients—a multidisciplinary statement. Crit Care. 2013;17:R166. doi:10.1186/cc12845.
Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med. 2013;41:1774–81. doi:10.1097/CCM.0b013e31828a25fd.
Cavallaro F, Sandroni C, Antonelli M. Functional hemodynamic monitoring and dynamic indices of fluid responsiveness. Minerva Anestesiol. 2008;74:123–35.
de Waal EE, Rex S, Kruitwagen CL, Kalkman CJ, Buhre WF. Dynamic preload indicators fail to predict fluid responsiveness in open-chest conditions. Crit Care Med. 2009;37:510–5. doi:10.1097/CCM.0b013e3181958bf7.
Lansdorp B, Lemson J, van Putten MJ, de Keijzer A, van der Hoeven JG, Pickkers P. Dynamic indices do not predict volume responsiveness in routine clinical practice. Br J Anaesth. 2012;108:395–401. doi:10.1093/bja/aer411.
Vincent JL, Pelosi P, Pearse R, et al. Perioperative cardiovascular monitoring of high-risk patients: a consensus of 12. Crit Care. 2015;19:224. doi:10.1186/s13054-015-0932-7.
Monnet X, Teboul JL. Passive leg raising: five rules, not a drop of fluid! Crit Care. 2015;19:18. doi:10.1186/s13054-014-0708-5.
Bastien O, Vallet B. French multicentre survey on the use of inotropes after cardiac surgery. Crit Care. 2005;9:241–2. doi:10.1186/cc3482.
De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362:779–89. doi:10.1056/NEJMoa0907118.
Mebazaa A, Pitsis AA, Rudiger A, et al. Clinical review: practical recommendations on the management of perioperative heart failure in cardiac surgery. Crit Care. 2010;14:201. doi:10.1186/cc8153.
Acknowledgments
We acknowledge all the Centers that participated in our survey. The full list of responding Centers is available in the “Appendix”.
Conflict of interest
The Authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the SIAARTI Study Group on Cardiothoracic and Vascular Anesthesia.
Appendices
Appendix 1









Appendix 2
Centers that contributed to this survey, listed in alphabetical order of town:
-
1.
Ospedali Riuniti Umberto I-Lancisi-Salesi, Ancona
-
2.
A.O. S. G. Moscati, Avellino
-
3.
A.O.U. Policlinico Giovanni XXIII, Bari
-
4.
Anthea Hospital, Bari
-
5.
Casa di Cura S.Maria, Bari
-
6.
Ospedali Riuniti, Bergamo
-
7.
Clinica Humanitas Gavazzeni, Bergamo
-
8.
Policlinico S.Orsola-Malpighi, Bologna
-
9.
Villa Torri Hospital, Bologna
-
10.
Spedali Civili, Brescia
-
11.
Istituto Ospedaliero Fondazione Poliambulanza, Brescia
-
12.
Istituto Clinico San Rocco, Brescia
-
13.
Casa di Cura Pineta Grande, Castelvolturno
-
14.
A.O.G.Brotzu, Cagliari
-
15.
A.O.U. Vittorio Emanuele, Catania
-
16.
Policlinico Universitario Magna Graecia, Catanzaro
-
17.
A.O. S. Croce e Carle, Cuneo
-
18.
A.O.U. Careggi, Firenze
-
19.
A.O.U. S.Martino, Genova
-
20.
P. O. Vito Fazzi, Lecce
-
21.
Città di Lecce Hospital, Lecce
-
22.
P.O. Alessandro Manzoni, Lecco
-
23.
A.O. Ospedale Civile, Legnano
-
24.
A.O. Carlo Poma, Mantova
-
25.
Ospedale del Cuore G. Pasquinucci, Massa
-
26.
A.O. Ospedali RiunitiPapardo-Piemonte, Messina
-
27.
Istituto Clinico Sant’Ambrogio, Milano
-
28.
A.O. Niguarda Ca’ Granda, Milano
-
29.
Ospedale Luigi Sacco, Milano
-
30.
IRCCS Centro Cardiologico Monzino, Milano
-
31.
IRCCS Ospedale San Raffaele, Milano
-
32.
IRCCS Istituto Clinico Humanitas, Milano (Rozzano)
-
33.
IRCCS Policlinico S.Donato, Milano (San Donato)
-
34.
IRCCS MultiMedica, Milano (Sesto S.Giovanni)
-
35.
Casa di Cura Hesperia Hospital, Modena
-
36.
A.O. San Gerardo, Monza
-
37.
A.O. Specialistica dei Colli, Ospedale Monaldi, Napoli
-
38.
Clinica Mediterranea, Napoli
-
39.
A.O.U. Maggiore della Carità, Novara
-
40.
Policlinico Università di Padova, Padova
-
41.
ISMETT, Palermo
-
42.
Villa Maria Eleonora Hospital, Palermo
-
43.
A.O.U. Ospedale Maggiore, Parma
-
44.
A.O. Ospedale R. Silvestrini, Perugia
-
45.
A.O.U. Pisana – Cisanello, Pisa
-
46.
A.O. S. Carlo, Potenza
-
47.
ICLAS – Istituto Clinico di Alta Specialità, Rapallo
-
48.
Villa Maria Cecilia Hospital, Ravenna (Cotignola)
-
49.
A.O. S.Filippo Neri, Roma
-
50.
A.O. S.Camillo Forlanini, Roma
-
51.
Ospedale S.Andrea, Roma
-
52.
Casa di Cura European Hospital, Roma
-
53.
Policlinico Tor Vergata, Roma
-
54.
Policlinico Universitario A. Gemelli – Università Cattolica, Roma
-
55.
Policlinico Umberto I – Università Sapienza, Roma
-
56.
Policlinico Universitario Campus Bio-medico, Roma
-
57.
Ospedale Civile SS. Annunziata, Sassari
-
58.
A.O.U Senese, Ospedale S. Maria alle Scotte, Siena
-
59.
Casa di Cura Villa Verde, Taranto
-
60.
Ospedale Civile G. Mazzini, Teramo
-
61.
A.O. Santa Maria, Terni
-
62.
A.O. Ospedale Mauriziano Umberto I, Torino
-
63.
A.O.U Città della Salute e della Scienza – Molinette, Torino
-
64.
Ospedale Santa Chiara, Trento
-
65.
Ospedale S.Maria dei Battuti Ca’ Foncello, Treviso
-
66.
A.O.U. Ospedali Riuniti, Trieste
-
67.
A.O.U. S.Maria della Misericordia, Udine
-
68.
Ospedale di Circolo e Fondazione Macchi, Varese
-
69.
Ospedale dell’Angelo, Venezia (Mestre)
-
70.
A.O.U. Ospedale Civile Maggiore, Verona
-
71.
Ospedale San Bortolo, Vicenza
Rights and permissions
About this article
Cite this article
Bignami, E., Belletti, A., Moliterni, P. et al. Clinical practice in perioperative monitoring in adult cardiac surgery: is there a standard of care? Results from an national survey. J Clin Monit Comput 30, 347–365 (2016). https://doi.org/10.1007/s10877-015-9725-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-015-9725-4