Abstract
Significant evidence outlines that the management of the high-risk surgical patient with perioperative hemodynamic optimization leads to significant benefits. This study aimed at studying the current practice of hemodynamic monitoring and management of Italian anesthesiologists. An invitation to participate in a web-based survey was published on the web site of the Società Italiana di Anestesia Analgesia Rianimazione Terapia Intensiva. Overall, 478 questionnaires were completed. The most frequently used monitoring techniques was invasive blood pressure (94.1 %). Cardiac output was used in 41.3 % of the cases mainly throughout less-invasive methods. When cardiac output was not monitored, the main reason given was that other surrogate techniques, mainly central venous oxygen saturation (40.5 %). Written protocols concerning hemodynamic management in high-risk surgical patients were used by the 29.1 % of the respondents. 6.3 % of the respondents reported not to be aware if such document was available at their institution. 86.3 % of the respondents reported that they usually optimize high risk patients but to use blood flow assessment rarely (39.7 %). The most used parameter in clinical practice to assess the effects of volume loading were an increase in urine output and arterial blood pressure together with a decrease in heart rate and blood lactates. The 45.1 % or the respondents outlined that hemodynamic optimization in the high risk patients is of major clinical value. Our study outlines an important gap between available evidence and clinical practice emphasizing the need for a better awareness, more information and knowledge on the specific topic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In order to provide safe and effective anesthesia, the anesthesiologist has to be able to monitor and interpret physiologic variables to choose therapeutic interventions that can benefit patients’ outcome [1]. In fact, in the perioperative setting, hemodynamic monitoring provides information relating to cardiac output (CO), volume status and ultimately tissue perfusion. However, despite the anesthesiologist have an array of devices to choose from, no single device or technique provides a complete assessment of hemodynamic status and the use of all devices in every situation is neither practical nor appropriate [2]. Furthermore, hemodynamic monitoring can be used to react when a problem has been recognized or when preemptive hemodynamic interventions can be used to reduce mortality and prevent postoperative complications [3]. These preemptive interventions are referred to as goal directed therapy (GDT). From this point of view, the high risk surgical patient represents an ideal scenario for the application GDT. This can be achieved using different hemodynamic monitoring systems and GDT protocols. It has been demonstrated that their use can reduce significantly the rate of postoperative complications and, for the highest risk groups, it can reduce postoperative mortality [3–6]. Morbidity and mortality are particularly important nowadays due to the advancing age of the surgical population, which has led to an increased prevalence of comorbidities. Together with the expansion of surgical techniques, this means that the assessment of perioperative risk is complex; this is an increasing problem and perioperative clinicians are in need of more robust risk stratification tools that can allow a better stratification and decision making process [3, 7]. As a result, a large debate is ongoing about which of the available monitoring techniques and devices, parameters or therapeutic goals can be considered the “best choice” in this setting [3, 7–9]. Despite some quality and design heterogeneity, significant evidence outlines that the management of the high-risk surgical patient with GDT leads to significant benefits [10–13]. Recent data from Europe and America suggests that, despite this level of evidence, clinicians are not routinely using this approach [14]. The aim of this study was to study the current practice of hemodynamic monitoring and management of Italian anesthesiologists.
2 Materials and methods
A survey of 20 questions was conceived to assess the current trends in hemodynamic monitoring and management in high-risk surgical patients (“Appendix”). It was previously approved and endorsed by Società Italiana di Anestesia Analgesia Rianimazione e Terapia Intensiva (SIAARTI), it was anonymous and non-attributable. An invitation to participate was published on the society’s web-site; therefore no ethical approval or consent from participants was needed. The survey was performed by means of a secure web database (Survey Monkey, Palo Alto, CA, USA). The system was set-up so that each responder could participate only once. It was opened for 3 consecutive months. Survey questions and definition of surgical high risk patient are presented in the additional file.
2.1 Statistical analysis
Results were analyzed according to the number of responses for each given question.
Data were presented as mean SD and proportions.
3 Results
3.1 Respondents
Overall, 478 questionnaires were completed with an overall response rate of 14.9 %. The majority of the responders were young: in fact the 55.9 % completed the post-graduate training after year 2000, 18.1 % between 1999 and 1990, 21.4 % between 1989 and 1980 and 4.6 % before 1980. The 48.2 % of the responders declared to practice at regional, the 35.4 % at teaching and the 14.7 % at provincial hospitals whereas the remaining 6.6 % reported to work at private institutions. Major abdominal surgery (51.8 %) was the most frequent area of practice with lower numbers for critical care (19.3 %), thoracic/cardiac surgery (11 %), and neuro-surgery (4.1 %). In the 13.8 % of the cases, other types of surgical specialties reported as the main area of practice. The most part of the respondents (67.9 %) reported to care for 1–5 high-risk patients per week whereas 6–10 patients per week was the answer selected by the 13.5 %, more than 11 by the 2.7 %. Finally, the 15.8 % reported not to be directly involved in the management of this kind of patient.
3.2 Monitoring techniques
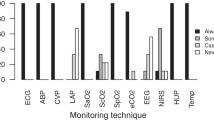
In theatres invasive pressure monitoring was available for 95.1 % of the respondents in whereas a CO monitor was available in only the 58.2 % of the cases. The most frequently used monitoring techniques was invasive blood pressure. CO was used in 41.3 % of the cases (fourth place) mainly throughout less-invasive methods (Tables 1, 2). When CO was not monitored, the main reason given was that other surrogate techniques, mainly central or mixed venous oxygen saturation (Table 3) were used, as answered by 58 % of respondents. When we asked pulse pressure variation, 64 % of the respondents using this parameter reported to use a subjective visual estimate of the monitor’s trace. Finally, 92 % of the participants to this survey outlined that the available hemodynamic monitoring system they could be improved.
3.3 Hemodynamic management habits, attitudes and beliefs
Written protocols concerning hemodynamic management in high-risk surgical patients were used by the 29.1 % of the respondents. 6.3 % of the respondents reported not to be aware if such document was available (or not) at their institution. Nearly everyone (97.4 %) agreed that oxygen delivery is of major importance for patients undergoing high-risk surgery. 86.3 % of the participants to the survey reported that they usually optimize high risk patients. Blood flow assessment was rarely used to optimize (Table 4). Nevertheless, the 45.1 % or the respondents outlined that, in their opinion, hemodynamic optimization in the high risk patients is of major clinical value particularly when started before (45.1 %) or immediately after (6.9 %) the induction of anesthesia whereas the remaining 38.2 and 9.8 % voted for the intra-operative and post-operative period respectively.
The preferred parameters to assess patients actual volume status are shown in Table 5. Clinical experience, blood pressure, central venous pressure (CVP), and urine output were the most frequently considered indicators. When we asked about which parameter was used in clinical practice to assess the effects of volume loading, an increase in urine output and arterial blood pressure together with a decrease in heart rate and blood lactates were the most frequently selected options (Table 6). Moreover, when we asked about the parameters with the best capability to predict an increase of CO after volume loading, the arterial blood pressure and the stroke volume variation gathered the major consensus (Table 7). Finally, we inquired about the fluid of choice for plasmatic expansion with the following results: crystalloids 54.8 %, 140/04 hydroxyethyl starch 36.2 %, gelatins 4.9 %, fresh frozen plasma 1.9 %, Albumin 1.1 %, Dextrans 1.1 %.
4 Discussion
To our knowledge this is the first time that a tentative national survey about this topic has ever been published. Our results are in line with the ones published by Cannesson et al. [14] in a survey among North American and European anesthetists. In their study nearly all respondents agreed that hemodynamic optimization was important but only 34 % did monitor CO in the context of high-risk surgery. Our results are similar. We found that while the majority of the respondents agree that optimization of high-risk surgical patient is important, in more than 80 % of the cases no flow related parameter is used. The most used parameters to optimize patients are blood pressure, heart rate and CVP. These parameters have significant limitations when used to target flow, with the majority of the studies showing none or poor correlation between them and flow. Pierrakos et al. [15] demonstrated that mean arterial pressure changes cannot be used to follow changes in CO. Several studies have investigated the performance of CVP as either a preload index or as a marker of fluid responsiveness. Most studies failed to show that it can be used to guide fluid management. The overall results have been pooled together in two meta-analysis by Marik in 2006 and 2013 [16, 17]. The results of both meta-analysis show that if one uses the CVP as target or to predict fluid responsiveness may well just toss a coin and would stand the same chances of being right of wrong. This is in line with a recent revision of the Surviving Sepsis Campaign too, that despite still recommending the use of CVP, suggests that when flow parameters are available, they should be preferred CVP. The limitations of pressures have been exposed in many GDT studies. In the majority of these studies there were no differences between heart rate, blood pressure and CVP between control groups and GDT groups [18–20]. In our survey we found that protocols are used by a minority of respondents. A flow monitor is not enough to perform GDT unless coupled with a protocol that changes therapy based on the flow information. While this may seem obvious, our survey proves that it isn’t and the majority of the respondents does not use the monitor with a protocol. If flow monitors are used without clearly defined protocols they don’t lead to better outcomes [21]. Not only that, if invasive monitors (i.e. the pulmonary artery catheter) are inserted and no protocol is used, it is possible that the patient may be exposed to the unnecessary complications related to the procedure, without receiving the benefits of the therapy [22]. There is data in other fields of medicine that prove the guidelines and protocol can improve patients’ outcome. For instance with a simple checklist, wrong site of surgery errors have been reduced in poor resource settings [23] and central venous line infections abolished in large teaching hospital [24]. In practice if no flow parameter and no protocol are used, then there is no real GDT [25]. Our results suggest a “reactive” rather than “preemptive approach” to hemodynamic optimization. This may explain why in our population the pulmonary artery catheter is used by 33 % of respondent to measure CO in high-risk surgical patients. It could be that respondents monitor CO only when the patient is extremely high-risk, or that a CO monitor is used when hemodynamic instability already occur. While we don’t have data to prove this point, we believe the data rise another important point about “timing”. The literature about GDT shows that it works when is implemented proactively based on risk identification and not based on the occurrence of hemodynamic instability. For instance waiting for hemodynamic instability to occur in the postoperative period before starting GDT may not work [26, 27]. The fact that no protocols are used and the Pulmonary Artery Catheter is considered the CO monitor or choice in more than 30 % of the cases suggest that many respondents use CO monitors to stabilize hemodynamically unstable high risk surgical patients, rather than preventing hemodynamic instability proactively. The fact that the majority of respondents stated that they optimize high risk surgical patients is in contrast with the poor use of monitors and protocols. This rises important questions about education in order to rise the awareness of what GDT is. On the other hand the availability of non invasive monitors for more than 60 % of the respondents suggest that there is a growing awareness that less invasive technologies may be available to optimize high risk surgical patients.
Despite the increasing evidence that hemodynamic optimization is beneficial in high-risk surgery patients, this strategy continues not to be applied in the clinical routine of many institutions. This can be due to the fact that some belief that the data from clinical studies are still not strong enough; others could not be convinced by the accuracy of the available studies or the practicability of the monitoring equipment used to measure CO or fluid responsiveness; many still avoid the potential extra costs for the necessary monitoring equipment and some may simply not be motivated enough to change their current clinical practice [28]. Interestingly, recently Ebm et al. [29] demonstrated that GDT for high risk surgical patients is also cost-effective, bringing economical benefit both to the hospital (decreased complications and length of stay) and to the society (increase in quality adjusted life years).
Our responders used more frequently crystalloids for plasmatic expansion and, among colloids, 140/04 hydroxyethyl starch was the agent of choice. However, for an appropriate interpretation of this result, it is important to outline that the survey was performed immediately before the starch-based products were withdrawn from the clinical use by the Italian National Drugs Agency.
Indeed, our survey has the limitations of being addressed only to the members of SIAARTI who responded in a limited number. Therefore it does not claim to mirror the attitudes and beliefs of the entire population of the Italian anesthesiologists. However, compared to previous similar experiences, it presents the data from the highest number of respondents so far.
In conclusion, we believe that we have highlighted an important gap between available evidence and clinical practice. Our results emphasize the need for a better awareness, more information and knowledge on the specific topic and the need for more education in order to provide patients with the treatment they deserve. Future resources should aim not only at delivering new research, but also at implementing in a cost-effective way more evidence at the bedside for high-risk surgery patients.
References
Funk DJ, Moretti EW, Gan TJ. Minimally invasive cardiac output monitoring in the perioperative setting. Anesth Analg. 2009;108:887–97.
Cove ME, Pinsky MR. Perioperative hemodynamic monitoring. Best Pract Res Clin Anaesthesiol. 2012;26:453–62.
Vincent JL, Rhodes A, Perel A, Martin GS, Della Rocca G, Vallet B, et al. Clinical review: update on hemodynamic monitoring—a consensus of 16. Crit Care. 2011;15:229.
Lobo SM, Rezende E, Knibel MF, Silva NB, Páramo JA, Nácul FE, et al. Early determinants of death due to multiple organ failure after non cardiac surgery in high-risk patients. Anesth Analg. 2011;112:877–83.
Pearse RM, Harrison DA, James P, Pearse RM, Harrison DA, James P, et al. Identification and characterisation of the high-risk surgical population in the United Kingdom. Crit Care. 2006;10:R8.
Jhanji S, Thomas B, Ely A, Watson D, Hinds CJ, Pearse RM. Mortality and utilisation of critical care resources amongst high-risk surgical patients in a large NHS trust. Anaesthesia. 2008;63:695–700.
Lobo SM, de Oliveira NM. Clinical review: what are the best hemodynamic targets for noncardiac surgical patients? Crit Care. 2013;17:210.
Bakker EJ, Ravensbergen NJ, Poldermans D. Perioperative cardiac evaluation, monitoring, and risk reduction strategies in noncardiac surgery patients. Curr Opin Crit Care. 2011;17:409–15.
Della Rocca G, Pompei L. Goal-directed therapy in anesthesia: any clinical impact or just a fashion? Minerva Anestesiol. 2011;77:545–53.
Vallet B, Blanloeil Y, Cholley B, Orliaguet G, Pierre S, Tavernier B. Guidelines for perioperative haemodynamic optimization. Ann Fr Anesth Reanim. 2013;32:454–62.
Cecconi M, Corredor C, Arulkumaran N, Abuella G, Ball J, Grounds RM, et al. Clinical review: goal-directed therapy—what is the evidence in surgical patients? Eff Differ Risk Groups Crit Care. 2013;17:209.
Giglio M, Dalfino L, Puntillo F, Rubino G, Marucci M, Brienza N. Haemodynamic goal-directed therapy in cardiac and vascular surgery. a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2012;15:878–87.
Aya HD, Cecconi M, Hamilton M, Rhodes A. Goal-directed therapy in cardiac surgery: a systematic review and meta-analysis. Br J Anaesth. 2013;110:510–7.
Cannesson M, Pestel G, Ricks C, Hoeft A, Perel A. Hemodynamic monitoring and management in patients undergoing high risk surgery: a survey among North American and European anesthesiologists. Crit Care. 2011;15:R197.
Pierrakos C, Velissaris D, Scolletta S, Heenen S, De Backer D, Vincent JL. Can changes in arterial pressure be used to detect changes in cardiac index during fluid challenge in patients with septic shock? Intensive Care Med. 2012;38:422–8.
Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134:172–8.
Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med. 2013;41:1774–81.
Pearse R, Dawson D, Fawcett J, Rhodes A, Grounds RM, Bennett E. Early goal-directed therapy after major surgery reduces complications and duration of hospital stay. A randomised, controlled trial. Crit Care. 2005;9:R687–93.
Boyd O, Grounds RM, Bennett ED. A randomized clinical trial of the effect of deliberate perioperative increase of oxygen delivery on mortality in high-risk surgical patients. JAMA. 1983;270:2699–707.
Noblett SE, Snowden CP, Shenton BK, Horgan AF. Randomized clinical trial assessing the effect of Doppler-optimized fluid management on outcome after elective colorectal resection. Br J Surg. 2006;93:1069–76.
Sandham JD, Hull RD, Brant RF, Knox L, Pineo GF, Doig CJ, et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003;348:5–14.
Harvey S, Harrison DA, Singer M, Ashcroft J, Jones CM, Elbourne D, et al. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomised controlled trial. Lancet. 2005;366:472–7.
Haynes HB, Weiser TG, Berry WR, Lipsitz SR, Breizat As, Dellinger EP, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491–9.
Berenholtz SM, Pronovost PJ, Lipsett PA, Hobson D, Earsing K, Farley JE, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32:2014–20.
Cecconi M, Bennett D. Should we use early less invasive hemodynamic monitoring in unstable ICU patients? Crit Care. 2011;15:173.
Gattinoni L, Brazzi L, Pelosi P, Latini R, Tognoni G, Pesenti A, Fumagalli R. A trial of goal-oriented hemodynamic therapy in critically ill patients. SvO2 Collaborative Group. N Engl J Med. 1995;333:1025–32.
Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. 1994;330:1717–22.
Reuter DA. Pragmatic fluid optimization in high-risk surgery patients: when pragmatism dilutes the benefits. Crit Care. 2012;16:106.
Ebm C, Ceconi M, Sutton L, Rhodes A. A cost-effectiveness analysis of postoperative goal-directed therapy for high-risk surgical patients. Crit Care Med. 2014 (epub ahead of print).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Appendix: Full questionnaire and definitions
Appendix: Full questionnaire and definitions
1.1 Definition of high risk surgery patient
A patients aged 18 years or older presenting for major surgery expected to last more than 1.5 h and having at least two of the following criteria:
-
1.
Cardiac or respiratory illness resulting in functional limitation
-
2.
Extensive surgery planned for carcinoma involving bowel anastomosis
-
3.
Predictable acute massive blood loss (>2.5 l)
-
4.
Aged over 70 years with functional limitation of one or more organ systems
-
5.
Septicemia (positive blood cultures or septic focus)
-
6.
Respiratory failure (PaO2 < 8 kPa on FiO2 > 0.4, that is, PaO2:FiO2 ratio <20 kPa or ventilation >48 h)
-
7.
Acute abdominal catastrophe (for example, pancreatitis, perforated viscous, gastro-intestinal bleed)
-
8.
Acute renal failure (urea >20 mmol/l, creatinine >260 μmol/l)
-
9.
Surgery for abdominal aortic aneurysm
-
10.
Disseminated malignancy
1.2 Definition of hemodynamic optimization
Fluid administration (and/or dobutamine) aimed at achieving a fixed hemodynamic goal (target): goal direct therapy
1.3 Questions
-
1.
How many times in a typical working week do you provide or directly supervise anesthesia for a high risk surgery patient?
-
Rarely or never
-
1–5 times a week
-
6–10 times a week
-
More than 11 times a week
-
-
2.
Which statement best describes your practice setting?
-
University hospital
-
Regional hospital
-
Province hospital
-
Private hospital
-
Other
-
-
3.
Does your institution or group have a written protocol, care guide, or statement concerning hemodynamic management in this setting?
-
Yes
-
No
-
Do not know
-
-
4.
What hemodynamic monitoring do you routinely use for the management of high risk surgery patients? (please, mark all that apply)
-
Invasive arterial pressure
-
Central venous pressure (CVP)
-
Cardiac output (CO)
-
Pullmonary capillary wedge pressure (PCWP)
-
Central venous saturation (ScvO2)
-
Stroke volume variation (SVV)
-
Systolic pressure variation (SPV)
-
Pulse pressure variation (PPV)
-
Mixed venous saturation (SvO2)
-
Non invasive arterial blood pressure (NIBP)
-
Transoesophageal echocardiography (TEE)
-
Global end diastolic volume (GEDV)
-
Intrathoracic blood volume (ITBV)
-
Extra vascular lung water (EVLW)
-
Near infrared spectroscopy (NIRS)
-
Plethysmographic Variation Index (PVI)
-
Flow time corrected (FTc) (Oesophageal doppler)
-
-
5.
Do you usually perform hemodynamic optimization in your high risk surgical patients?
-
Yes
-
No
-
-
6.
If you answered “YES” to the previous question, which parameter do you use?
-
Blood pressure
-
CVP
-
PVC
-
CO
-
ScvO2
-
SvO2
-
SVV
-
Urine output
-
Other
-
-
7.
When, in your opinion, is hemodynamic optimization most valuable?
-
Before anesthesia induction
-
Immediately after anesthesia induction
-
During surgery
-
After surgery
-
-
8.
How do you usually assess PPV?
-
Manual calculation
-
Automatic calculation through a monitor’s dedicated software
-
Visual estimation on the monitor’s trace
-
-
9.
What technique do you use to monitor cardiac output? (please, mark all that apply)
-
Pulmonary artery catheter
-
LiDCO monitor
-
PiCCO monitor
-
Transoesophageal echocardiography
-
Thoracic bioimpedance
-
Oesophageal doppler
-
Vigileo monitor
-
PRAM-Mostcare monitor
-
-
10.
If you do not monitor cardiac output routinely in these patients, what are the main reasons for not monitoring it? (please, mark all that apply)
-
Cardiac output monitoring does not provide any additional clinically relevant information in this setting
-
I use SvO2 and/or ScVO2 as surrogates for cardiac output monitoring
-
I use dynamic parameters of fluid responsiveness (pulse pressure variations, systolic pressure variations, plethysmographic waveform variations) as surrogates
-
Available cardiac output monitoring solutions are unreliable
-
Available cardiac output monitoring solutions are too invasive
-
-
11.
What are your indicators (diagnostic tools)? for volume expansion in the setting of the high risk surgical patient (please, mark all that apply)
-
Clinical experience
-
Stroke volume variation
-
Cardiac output
-
Urine output
-
PPV, SVV o SPv
-
Pulmonary capillary wedge pressure
-
Plethysmographic Waveform Variation
-
Central venous pressure
-
Central venous saturation (SvO2)
-
Blood pressure
-
Mixed venous saturation (ScvO2)
-
Global end diastolic volume
-
Intrathoracic blood volume (ITBV))
-
Transesophageal echocardiography
-
FTc (flow time corrected, oesophageal doppler)
-
-
12.
How do you routinely assess the hemodynamic effects of volume expansion in the setting of the high risk surgical patient ?
-
Decrease in stroke volume variation
-
Increase in cardiac output
-
Decrease in plethysmographic waveform variation
-
Increase in central venous saturation (SvO2)
-
Decrease in pulse pressure variation or systolic pressure variation
-
Decrease in heart rate
-
Increase in urine output
-
Increase in blood pressure
-
Increase in mixed venous saturation (SvO2)
-
Decrease in blood lactates
-
-
13.
In your opinion, what best predicts an increase in cardiac output following volume expansion?
-
Blood pressure
-
Pulse pressure variation or systolic pressure variation
-
Central venous pressure
-
Pulmonary capillary wedge pressure
-
Mixed venous saturation (ScvO2)
-
Stroke volume variation
-
Central venous saturation (SvO2)
-
Plethysmographic waveform variations
-
Transesophageal echocardiography
-
Global end diastolic volume
-
Clinical experience
-
Cardiac output
-
-
14.
What is your first choice solution for volume expansion?
-
130/0.4 hydroxyethyl starch solutions
-
Blood derived products
-
Human albumin
-
Crystalloids
-
Dextrans
-
Gelatin
-
-
15.
Do you believe that providing the best possible oxygen delivery to the tissues is of major importance in patients during high risk surgery?
-
Yes
-
No
-
-
16.
Do you have available invasive pressure monitoring in the theatre where you usually work?
-
Yes
-
No
-
-
17.
Do you have available cardiac output monitoring in the theatre where you usually work?
-
Yes
-
No
-
-
18.
Do you think that the monitoring techniques you have currently available in your clinical setting could be significantly improved ?
-
Yes
-
No
-
-
19.
Which statement best describes you?
-
I am an anesthesiologist predominantly caring for abdominal surgery patients
-
I am an anesthesiologist predominantly caring for cardiac surgery patients
-
I am an anesthesiologist predominantly caring for thoracic (lungs) surgery patients
-
I am an anesthesiologist predominantly practicing intensive care
-
I am an anesthesiologist predominantly caring for patients not having neither abdominal or other specialist surgery
-
-
20.
When did you get your post-degree specialization diploma in anesthesiology?
-
After year 2000
-
1990–1999
-
1980–1989
-
Before year 1980
-
Rights and permissions
About this article
Cite this article
Biancofiore, G., Cecconi, M. & Rocca, G.D. A web-based Italian survey of current trends, habits and beliefs in hemodynamic monitoring and management. J Clin Monit Comput 29, 635–642 (2015). https://doi.org/10.1007/s10877-014-9646-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-014-9646-7