Abstract
A mathematical model of whole-body acid–base and fluid-electrolyte balance was used to provide information leading to the diagnosis and fluid-therapy treatment in patients with complex acid–base disorders. Given a set of measured laboratory-chemistry values for a patient, a model of their unique, whole-body chemistry was created. This model predicted deficits or excesses in the masses of Na+, K+, Cl− and H2O as well as the plasma concentration of unknown or unmeasured species, such as ketoacids, in diabetes mellitus. The model further characterized the acid–base disorder by determining the patient’s whole-body base excess and quantitatively partitioning it into ten components, each contributing to the overall disorder. The results of this study showed the importance of a complete set of laboratory measurements to obtain sufficient accuracy of the quantitative diagnosis; having only a minimal set, just pH and PCO2, led to a large scatter in the predicted results. A computer module was created that would allow a clinician to achieve this diagnosis at the bedside. This new diagnostic approach should prove to be valuable in the treatment of the critically ill.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Quantitative diagnosis and treatment of non-respiratory (metabolic) acid–base disorders, particularly metabolic acidosis, has long been an issue of misunderstanding and consequently, great controversy. Early studies in the twentieth century focused on blood, and most particularly on the bicarbonate-ion concentration in plasma ([HCO3 −]P), as a way of quantifying acid–base disorders. In 1917, Palmer and Van Slyke [1] proposed that the amount of absorbed or injected bicarbonate that raised [HCO3 −]P a specified amount could be determined knowing only the body weight of an individual. The proportionality factor proposed in their method is now more commonly called the bicarbonate space (BS), expressed in units of l/kg. BS and other variables derived from basic blood-chemistry measurements have been referred to as “screening parameters” by Siggaard-Andersen and Fogh-Andersen [2] or “scanning tools” by Morgan [3]. In the current study, they are called quantitative diagnostic variables (QDV) because they are used to quantitatively diagnose the numerous factors leading to acid–base disorders and/or suggest quantitative fluid-therapy treatment.
Base excess (BE) is a QDV that originated in 1963 from the seminal work of Siggaard-Andersen [4]. He measured the pH of blood in vitro after adding various amounts of acid or base while PCO2 was kept constant at various values. The original method used a nomogram to determine blood BE values (BEB, mEq/lB), and added another variable, the blood hemoglobin concentration ([Hb]B). A later effort produced the “Van Slyke” equation [5] which determines BEB from a linearized, algebraic description of the CO2 equilibration curve. More recently, this equation has been modified to extend it to the interstitial fluid by assuming that the “effective” [Hb] is 1/3 of that in blood for adults. This new QDV is called the standard BE (SBE) and has units of mEq/l, but the liters now are that of blood plus extracellular fluid (ECF) rather than just blood. It is clear that SBE is an approximation not derived from experimental measurements. These measures constitute the “acid–base status” of an individual, however, a drawback of both the BEB and SBE QDVs is that they do not directly point to the cause(s) of the disorder.
Recently, Wolf and DeLand [6] developed a model, based on the physico-chemical principles of fluid electroneutrality and osmotic equilibrium, that could predict the steady-state fluid and electrolyte distribution in the interstitial (I), plasma (P) and erythrocyte (E) fluid compartments. This IPE model accurately predicted changes in distribution due to acid–base disorders and other clinical abnormalities that did not substantially involve water or electrolyte movements into or out of cells. Subsequently, Wolf and Deland [7] used their model and laboratory blood-chemistry measurements in patients to predict changes in acid–base and fluid-electrolyte-system QDVs in these patients. An important outcome was a partitioning of the estimated IPE BE-value (BEIPE) in these patients into a number of quantitative components, each suggestive of a cause of the acid–base disorder.
Subsequently, Wolf [8] extended the IPE model to include a cellular (C) compartment. This addition was important since acid–base chemistry of cells plays a principle role in overall acid–base balance in the body. This new CIPE model allows determination of the whole-body BE (WBBE) and its partitioning into its component parts just as for the IPE model. The present study uses the new model to explore the details of WBBE as a major QDV and its use in providing further understanding of the causes of acid–base disorders and their treatment, particularly using NaHCO3 fluid therapy to treat metabolic acidosis. In addition to determining WBBE and its components, the CIPE model allows a more comprehensive understanding in that it not only characterizes the acid–base disorder, but also considers the effects of fluid-electrolyte distribution abnormalities.
Recently, BS has been proposed [9] as a QDV that can be used to predict the amount of NaHCO3 to give to children with metabolic acidosis. Although the concept of BS is rarely used in current clinical practice, it has important historical significance, hence, a further use of the model is to examine its BS predictions to see if they give valid information to NaHCO3 fluid-therapy specification or can be ignored in the face of the vast information contained in WBBE and its components.
2 Methods
2.1 The predictive CIPE model and its solution
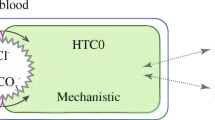
The CIPE model used in the present study has been described in detail previously [8]. Figure 1 shows a diagram of the model and its principle parts. Briefly, the CIPE model describes the steady-state distribution in four homogeneous fluid compartments of (1) water, (2) small ‘strong’ ions, Na+, K+, Cl− and lactate (lac−) that don’t interact with H+, (3) the ‘weak’ small ion inorganic phosphate (Pi−) that can chemically combine with H+, (4) impermeable proteins that interact with H+, hemoglobin in erythrocytes, albumin in plasma and interstitial fluid, globulins in plasma and a generalized species (mostly myosin and myoglobin) in the intracellular compartment and (5) metabolites, permeable urea and glucose in all compartments and impermeable ATP, GSH and 2,3 DPG in erythrocytes. Protein, metabolite and Pi− electrical valences are pH dependent. In each compartment, [HCO3 −] is determined from [H+] and dissolved [CO2] using the Henderson–Hasselbalch equation. All of these quantities are considered as model outputs or dependent variables.
General model diagram of cell-interstitial-plasma-erythrocyte (CIPE) model of fluid-electrolyte balance. The standard-state masses of water, electrolytes and metabolites for a subject are distributed among these homogeneous fluid spaces according to the physico-chemical principles shown. The results of this distribution are model OUTPUTS: compartment volumes, species concentrations and pH values. INPUTS from external sources and internal organ function perturb this standard-state resulting in a new state for the subject
The model-output values are dependent upon the model inputs, which are the assumed volume of water and masses of all the chemical species in the body. Additional inputs are the values of the respiratory gases, PCO2 and PO2. The latter quantities are not truly inputs since they are outputs from the respiratory system, but, they are simulated as constant values because they are known from chemical measurement. The inputs are changed (perturbed) by infusion and intake and excretion of chemical species by various body organs and processes, including the respiratory system. Not shown is that new species can be created by metabolic processes. Using the simulated physico-chemical processes indicated, the simulation program (VisSim 8 simulation language, Visual Solutions, Westford, MA) distributes the volume of water and masses of all species throughout the four body compartments to form model outputs.
The standard-state solution of the CIPE model is that for a 70 kg, 175 cm tall adult male. As described previously [8], the standard volumes of water in each compartment are determined from anthropometric relationships dependent upon body size and subject gender. These standard volumes are changed automatically in the model for different subject gender and size. The standard concentrations of the chemical species in all compartments were determined from the literature as previously described [7, 8]. These concentrations are independent of gender or body size.
After a perturbation, the model solution predicts the changes from the standard-state values of all the dependent variables. This predictive solution is sometimes referred to as a forward solution to describe the directional information flow in the diagram of Fig. 1. A computer module was developed previously [8] that could be used by anyone, free of charge, to explore such predictions.
In the present study some of the model parameters have been updated (see Sect. 5) from that formulated previously [8] to provide improved prediction of experimental data. It is expected that further updates will be required for the model to describe various disease states, however, the basic physico-chemical principles will remain unchanged.
2.2 The diagnostic CIPE model and its solution
When the model is solved in reverse (inversely), some of the former output variables become inputs and some of the former input variables become outputs. When a subset of the model’s dependent variables are fixed (constrained) to desired values, i.e., particular laboratory blood-chemistry values, the inverse solution determines the values of specified unknowns (original independent variables), such as masses of ions, the change in the volume of H2O in the body and the value for [XA−]P, the plasma concentration of unmeasured or unknown ions (normally zero), that simultaneously satisfy these constraints as well as the model’s physico-chemical constraints (e.g., electrical neutrality and osmotic equilibrium). In a patient, the [XA−]P value can reflect the effects of the acid–base disorder, such as ketoacids in diabetes, but it also accounts for ions which may not be measured, lactate for example.
Using anthropometric data for a particular subject (patient) and their laboratory blood-chemistry values, the inverse solution results in a model for that subject. If any of the laboratory measurements are not available, unconstrained standard-state values are used in their place, however, the accuracy of prediction may be compromised. Another feature of the inverse solution is model prediction of variable values not normally measured, for example, interstitial volume, cell pH, etc., however, the accuracy of such predictions depends upon the number of chemical variables measured in blood. The most desirable complete set consists of venous-plasma concentrations (see Sect. 4) of Na+, K+, Ca++, Mg++, Cl−, Pi−, Lac−, Alb, pH, PCO2, urea, glucose, hemoglobin O2 saturation, the concentration of Hb in blood and/or hematocrit. An importance of the subject-model is that it can be used to predict the results of perturbations such as fluid infusions, urinary losses, etc. in that subject.
For a patient with an acid–base disorder, the inverse solution finds (diagnoses) the quantitative causes of the disorder. Determination of blood BE from measurement of only blood pH and PCO2 is a simple example of this concept, but determination of just this BEB value does not explain how this acid–base defect comes about. Partitioning of BEB into its components can quantitatively indicate the multiple causes of an abnormal BEB, however, to do this accurately, a complete set of blood-chemistry information is required; we showed previously [7] how this partitioning was accomplished for the IPE system. The present study demonstrates how the partitioning of WBBE is used for diagnostic purposes and specification of fluid therapy.
2.3 WBBE determination
An important possible outcome from the inverse solution is an estimation of WBBE, however, the values obtained depend upon its definition. If WBBE is defined similarly to BEB as the normalized mEq of acid or base necessary to return the subject’s blood to arterial pH (pHa) and PaCO2 values of 7.4 and 40 mmHg, respectively then the minimal number of required measurements are blood pH and PCO2 pre- and post-infusion. When the model variables are constrained to these values, the “minimal model” can be solved inversely to find the change (Δ) in the mass (M) of Cl− (HCl) required. Since WBBE is defined in terms of mEq, it is actually the mass times the electrical valence of Cl− (\({\text{M}}_{\text{Cl}}^{Z}\)) that is actually determined. It should be noted that in the present study, Cl− is considered as an acid along with any other anions. Likewise, Na+ is considered as a base along with any other cations. This view is similar to Stewart [10], but contrary to that of Siggaard-Andersen and Fogh-Andersen [2], (see Sect. 4).
The appropriate normalization factor for the whole body is kg of body weight (Wt), rather than liters of fluid as for BE or SBE, hence, WBBE = Δ \({\text{M}}_{\text{Cl}}^{Z}\)/Wt. As shown below, this estimated value of WBBE from the minimal model can be quite in error compared to the prediction where more subject information is known.
2.4 Relationship between WBBE, BEB and SBE
Siggaard–Andersen stated [4] that BEB for the whole organism (mEq/kg) was equal to 50 % of BEB (see Sect. 4). To test this assertion, the model was used to determine WBBE, SBE and BEB for either addition or subtraction of 1, 5 or10 mEq/kg of Cl− (HCl). The Van Slyke equation [5] was used to calculate BEB and SBE ([Hb]B/3) except that the 7.7 factor in the original equation was replaced [2] by 2.14 + 8*[Alb]P + 0.309*[Pi]P, where the 2.14 factor accounts for the assumed constant effect of serum globulins.
2.5 Partitioning of WBBE
To partition WBBE among its many components, requires a determination in the model of the change in WBBE due to each component with the other components fixed to their normal values as described previously [7]. This value, determined for each component, is commonly called a sensitivity and is mathematically referred to as a partial derivative. These sensitivities are determined from the forward-model as the value of ΔMCl required to return the model pHv back to the normal venous state (7.37) divided by the imposed change in the component; linearity is assumed in these determinations (see Sect. 4). PCO2 is not an issue as it is maintained at its normal-state value when these sensitivity determinations are made. Model sensitivities are given in Table 1. As seen, even the non-ion, glucose, has a sensitivity, but its effect would only become appreciable at high glucose concentrations due to glucose impermeability into cells in diabetes mellitus, for example. This result is mainly due to the water shift out of cells, leading to an acidosis as per the present definition.
2.6 Bicarbonate space (BS) and apparent BS (ABS)
Historically, BS has been used to estimate the amount of NaHCO3 required to correct a metabolic acidosis [1, 9, 11], however, BS has been defined in many ways. The distribution space of a chemical species in the body is usually defined as the ratio of the amount of the substance injected divided by the concentration (or change in concentration) of the substance after a steady-state concentration has been achieved. If the substance distributes in more than one body compartment, as in most cases, the concentration may not be uniform and the concentration to use in the calculation is not clear. This description is true for [HCO3 −] because its concentration is different in each of the four compartments, but only the concentration in plasma is determined. Sometimes this concentration is converted to the value in plasma water because this concentration is closer to that assumed for interstitial fluid, hence, extracellular water. Following this approach the formula for BS is,
where the units of BS are lP/kg of body weight (Wt). This definition ignores the fact that [HCO3 −] is much lower in cells. Consequently, when values of BS are determined in the literature, it is not clear the relation between the value calculated and some anatomic space. For example an alternative formulation to predict BS in clinical situations from Fernandez et al. [12] is,
where ABS is the apparent BS value. The 0.4 constant refers to the HCO3 − volume/kg in some anatomical space (see Sect. 4). Although the units given by these authors is Wt%, the actual units are the same as for BS, above. In the present study, the predictions of these formulas will be determined from both experimental [HCO3 −] data and corresponding model data under a number of situations.
2.7 Experimental data from human studies
There are two studies where human subjects were infused with NaHCO3 solutions and extensive measurements made. These studies will be used as a first step in comparing experimentally determined and model-predicted plasma-chemistry values as well as predictions of WBBE, BS and ABS. Singer et al. [13] infused 0.89 M NaHCO3 (~2 mEq/kg) into seven subjects; one was used twice. Measurements of pHa, PaCO2, [Na+]P, [K+]P and [Cl−]P were done before the infusion (pre-infusion state) and for periods up to 156 min post-infusion. The data point closest to the 30-min post-infusion time will be used for the calculations and comparisons in the present study because steady state is presumed [14] to occur after this time. Urinary excretions of Na+, K+ and Cl− were also measured after the infusion. Cumulative post-infusion excretion amounts were summed up to the time of the post-infusion plasma-chemistry determination. Mellemgaard and Astrup [14] infused 0.595 M NaHCO3 (~2 mEq/kg) into seven subjects and made the same measurements as Singer et al. [13]. In both of these studies, subject gender and weight were given so that reasonable anthropometric estimates of their normal compartment volumes could be obtained (see below). In contrast, in another study, Russell et al. [15] measured just arterial pH and PCO2 before and 30 min after infusions of from 1 to 4 mEq/kg of 0.895 M NaHCO3, hence, only the minimal-model approach could be used to estimate WBBE. The latter study was done while the 12 patients were undergoing cardiac surgery with the support of extracorporeal circulation. Presumably, they were anesthetized; no other measurements were made.
3 Results
3.1 Use of WBBE to predict the amount of NaHCO3 to infuse in subjects (patients)
3.1.1 Subject-model prediction of plasma data
A test of the subject-model was to see if it could accurately predict the plasma pH and critical electrolyte changes due to a NaHCO3 infusion in humans in the two studies, above. In the Mellemgaard and Astrup [14] study, measured data pre-infusion and 30-min post-infusion are shown in Table 2 for one female subject (PJ). As seen, infusion of 2.1 mEq/kg of NaHCO3 solution resulted in an increased arterial pH from 7.38 to 7.44; [Na+]P increased as expected because of the high [Na+] of the infused fluid, whereas [K+]P and [Cl−]P both decreased because of dilution by the water infused and also for K+ because of its entry into cells. Arterial PCO2 rose slightly, presumably to compensate for the increased pH. The post-infusion urinary excretions of H2O, Na+, K+ and Cl− are shown towards the bottom of the Table. Net cumulative base excretion post-infusion was calculated from the excretions of Na+ + K+–Cl−.
A subject model was created for the pre-infusion state of this individual as described above. The compartment volumes were estimated from the subject’s lean body mass (LBM) as calculated from her height (Ht) and weight (Wt) using anthropometric equations as described previously [7]. This forward, subject-model was perturbed by changing PCO2 to the measured post-infusion value of 38 mmHg, by adding the volume of water infused (0.21 l) and the mass of Na+ infused (126 mEq) and by subtracting the volume of water excreted and the masses of the ions excreted. Then, the model was solved again. As seen, the subject-model reasonably-closely predicted (model data in italics) the increases in pH and [Na+]P, and the decreases in [K+]P and [Cl−]P resulting from this relatively small infusion. The same procedure was used for the remaining experiments in the Mellemgaard and Astrup [14] study and for those in the Singer et al. [13] study. Over the 15 experiments, the mean differences between model predictions and experimental measurements for pH, [Na+]P, [K+]P and [Cl−]P were 0.002 ± 0.022 (SD) pH units and 0.94 ± 2.06, 0.06 ± 0.39 and 1.05 ± 2.3 (SD) mEq/lP, respectively. None of these differences was statistically significant (p > 0.05). The relatively large SD values likely reflect that the data were for only a single measurement in each experiment in the transient period post-infusion.
3.2 Model prediction of WBBE
Shown towards the bottom of Table 2 in this subject, is that 0.33 mEq/kg of the infused base was excreted 30 min post-infusion, leaving 1.77 mEq/kg retained. This excretion is about 15 % of the base infused. Using the subject-model determined from the initial measured chemical state of this subject, the predicted change (Δ) in WBBE is 1.84 mEq/kg (right part of Table), which is about equal to the measured value of 1.77 mEq/kg. The ratio of predicted ΔWBBE to measured retained base is 1.04 in this experiment (bottom-right of Table). Table 3 shows the mean values for this ratio for each study and the cumulative result. Over the total of 15 experiments, this ratio is not statistically significantly different from 1.0, suggesting that individual subject-models can accurately predict changes in the whole-body, acid–base status of an individual under these conditions.
An alternative approach to estimating WBBE is to use only a minimal amount of subject information, initial and post-infusion measurements of just pH and PCO2. Assuming that changes in Cl− mass (HCl) account for the difference between the two states, the model predicts a minimal-model ΔWBBE of 1.99 mEq/kg (right-most column of Table 2) for this subject, which is much farther from the measured value than was the subject-model prediction.
The minimal-model method was used to predict ΔWBBE for the Russell et al. [15] experimental data and compare this value to the actual amount of NaHCO3 infused. The model ΔWBBE results were similar to those given by Russell et al. who used the much earlier steady-state fluid and electrolyte model of DeLand and Bradham [16]. The present results are shown by the filled triangles in Fig. 2. The large SD of these data around the identity line is apparent. For the 12 experiments, the ratio of predicted minimal-model ΔWBBE to infused NaHCO3 was 1.1 ± 0.35 SD.
Model-predicted whole-body base excess (WBBE) values using just arterial pH and PCO2 measurements (minimal-model) in three human studies where NaHCO3 solution was infused. The regression line shown is for the data in all three studies. Its slope is not significantly different than that of the identity line
To put the Russell et al. [15] data in perspective, the minimal-model ΔWBBE data also were calculated for the other two studies. These data are shown by the open symbols. It is clear that the scatter in predicted ΔWBBE values is much greater using the minimal model than when using the subject model, however, the regression line calculated for all the data (dashed line) is close to the identity line.
3.3 Relationship between WBBE, BEB and SBE
Table 4 shows the model results for the relationship between ΔWBBE, ΔBEB and ΔSBE under both metabolic alkalosis and acidosis conditions. As seen, ΔWBBE is not a constant fraction of either ΔBEB or ΔSBE during changing acid–base conditions. The fractions become progressively smaller going from alkalotic to acidotic conditions. As seen, the relationship between ΔWBBE and ΔSBE varies over a wider range, but the standard value of about 0.33 is almost the same as for ΔBEB, except that the units of the constant are now lIPE/kg.
3.4 BS and ABS
The values of BS and ABS were calculated (Eqs. 1 and 2) for the three experimental studies, above. Figure 3 shows the results for BS (left axis, filled symbols) and ABS (right axis, open symbols). As seen, there is no apparent correlation for either variable with the weight-normalized amount of NaHCO3 infused, which contrasts with the WBBE results (Table 2; Fig. 3). There may be many reasons for this result, but an important one is that both BS and ABS vary with PCO2 in contrast to WBBE (see Sect. 4).
3.5 Comprehensive diagnosis (prediction of both fluid-electrolyte balance and WBBE)
Critically ill patients who have severe acid–base disorders also have significant fluid-electrolyte abnormalities. A comprehensive diagnosis requires knowledge of both of these conditions. To prove that the CIPE model is able to accurately diagnose the complexity of these disorders first requires proof of the models capabilities.
3.5.1 Proof of concept
The first task is to prove that the inverse model will return the same input values used to perturb the forward model. Table 5 shows the forward-model output values for the required complete set of measured dependent variables, (1) for the venous standard state of a 70 kg, 175 cm height male, (2) after addition of 5.33 mEq/kg of 0.5 M HCl to simulate metabolic acidosis; this amount was selected because it yielded a pHa of 7.233 (pHv = 7.203) at a PaCO2 of 23.5 mmHg, which were the mean values found by Albert et al. [17] for fully compensated metabolic acidosis in humans and (3) after further addition of 5.33 mEq/kg NaHCO3, the amount expected for full compensation, i.e., to bring pHa back to the normal value at an expected normal final PaCO2 of 40 mmHg.
The number of significant figures displayed in Table 5, in some cases, is beyond that of today’s laboratory instruments, but this number was used to assess the ability of the inverse model to accurately predict the values of the original disturbance(s). As shown in the middle column, the infusion of 0.5 M HCl to simulate metabolic acidosis caused decreases in all model-output quantities, other than [Cl−]P, because of the dilution by the volume of water added (0.746 l or 10.7 ml/kg). Similarly, the therapeutic, compensatory infusion of 0.5 M NaHCO3 (right column) further decreased all output quantities, other than [Na]P and pHv, due to the additional dilution. The value of pHv returned towards normal, but not totally, because the model acidifying effects of dilution and HCl addition are stronger than the basifying effect of NaHCO3 (see below).
Figure 4 shows a screen-shot of the model predictions for the HCl infusion. The displayed results are in a similar format as the screen-shots previously shown [7] for the IPE model using the Vissim-Viewer program. The left column shows model-input data, taken from the middle column of Table 5; [Na+]P = 138 mM, for example. The input variables (normally dependent) are constrained in the model to equal these values. Detailed model-output values are shown in the other columns. Much information is shown, but, for now, the CIPE model solution at the top of the middle column is of most interest. The values of these five selected variable values (unknowns) are determined through a complex optimization process inside the Vissim-program software. As seen, these values very closely mimic the perturbation of 10.7 ml/kg of water and 5.33 mEq/kg of only Cl− added; the optimization process was able to discern that no amounts of Na+ or K+ were added and that there were no unmeasured ions ([XA−]P = 0). Figure 5 shows similar results after the NaHCO3 infusion. As above, the corresponding unknown values shown in the Fig. now closely reproduce the 21.3 ml/kg of water and 5.33 mEq/kg of both Na+ and Cl− added to the model.
Screen-shot of Vissim-program results for the diagnostic model. The input blood-chemistry values (left column) were from a forward solution of the model (Table 5, metabolic acidosis). The CIPE model solution (upper-middle column) shows that the model can accurately predict the results for infusion of 5.33 mEq/kg of 0.5 M HCl into the standard subject (male, 70 kg, 175 cm in height). Model WBBE predictions (upper-right column) show a diagnosis of whole-body metabolic acidosis with a gain of Cl− (HCl) as the principle cause, but with negligible effects by other factors. The screenshot shows other results not normally measured, cell pH, for example, and results for other diagnostic approaches, Anion gap, Siggaard-Andersen (traditional) and that of Stewart
The individual WBBE values (each equal to 0 in the standard state) determined from changes in various dependent variables are shown on top of the right-most column in Figs. 4 and 5. As seen in Fig. 4, the primary contributor was the increased amount of Cl−, amounting to a −5.3 mEq/kg acidifying effect, whereas changes in the other quantities contributed much smaller amounts; the decrease in albumin concentration actually produced a small basifying effect. Similar WBBE effects for the combined acid and base additions are seen in Fig. 5. Again, the Cl− addition produced the primary acidifying effect, whereas, now, the Na+ addition produced the primary basifying effect. It is important to note that the difference between the Cl− and Na+ effects, 0.5 mEq/kg, resulted in a net acidification. Consequently, equal amounts of Cl− and Na+ additions, such as produced from saline addition, result in a net acidification in the model.
3.5.2 Actual patient diagnosis
An example of the use of this comprehensive diagnostic model is shown in Fig. 6. The case is a 41 year old female with diabetic ketoacidosis (DKA) and septicemia. Laboratory-chemistry data were taken from the AcidBase.org website (case 3,288). No other clinical information was given so that it was not known if the standard clinical treatment of saline infusion was begun prior to the blood sampling for this chemistry data Since normal data for this subject were not available, a subject-model was derived for this patient from her gender and assumed initial weight, and height coupled with the normal electrolyte values in the model (see Table 5, column 1). The initial measured laboratory data (assumed to be arterial) for this patient are shown in the left column of Fig. 6. Notable are the acidemia with low PCO2 (2.3 kPa, 17.3 mmHg), presumably due to respiratory compensation, high plasma concentrations for K+, Cl−, lactate− and glucose and low values for Na+ and Pi−. Also important are the low values for concentrations of plasma albumin and blood hemoglobin. Data for Ca++, Mg++, Pi− and urea were measured about 16 h later, which illustrates the difficulty of obtaining a complete set of laboratory measurements at the same point in time.
Screen shot for a patient with a severe diabetic-ketoacidosis, acid–base disorder with blood chemistry data (left column) taken from the website AcidBase.org. The model diagnosis (middle column) shows a high degree of intracellular dehydration, a large deficit of K+, the presence of a high concentration of unmeasured ions (XA−) and Cl− retention. The net metabolic-acidosis WBBE effect of −10.8 mEq/kg (right column) is predominantly due to the latter three factors, however, other factors, some producing alkalotic effects, contribute to this net value
As seen from the inverse CIPE-model solution (upper-middle column), the model fluid-electrolyte diagnosis is a H2O deficit of about 30 ml/kg (1.8 l), an unmeasured-ion excess of −13.5 mEq/lP and a K+ deficit of 6.6 mEq/kg, all characteristic of DKA. Plasma concentrations of Cl− and Na+ show excesses of 7.4 and 3.4 mEq/kg, respectively, consistent with the high plasma Cl− concentration, but at variance with the low Na+ concentration, hence, one cannot tell from plasma concentrations, alone, net excesses or deficits in ionic masses.
Notable in the CIPE-model variables (middle part of middle column) are that osmolarity is high and there are model-predicted increases in fluid volume in all compartments other than cells, which is not always the case in DKA, as often, extracellular fluid volume is reduced. In contrast, cell volume was reduced by ~5 l. These quantitative fluid changes would be difficult to predict from the basic data. As expected, there is an intracellular acidosis and cell [K+] is greatly increased (lower part of middle column), which could lead to cardiovascular consequences.
The model-predicted WBBE values for this case are shown in the upper-right column. The net WBBE of −10.7 mEq/kg, is primarily a consequence of the Cl− excess, the unmeasured–ion presence and the loss of K+, which are only partially offset by the excess of Na+.
QDV data from other diagnostic approaches are shown at the bottom of the right column. The increases in all anion gap (AG) variables suggest a metabolic acidosis with the presence of unmeasured ions of about the same magnitude as the CIPE-model diagnostic prediction. The large negative values for both BEB and SBE from the Siggaard-Andersen approach indicate a severe metabolic acidosis, but do not indicate the cause(s). As seen, in the Stewart QDVs, the decrease in measured strong-ion difference (SID) values suggest again a severe metabolic acidosis with a large “weak-ion” effect (Atot). The large strong-ion gap (SIG) value, suggests the presence of a large concentration of unmeasured anions at about the same value as the CIPE model prediction.
The conclusion that can be reached by the diagnoses, above, is that the QDV values of the various diagnostic techniques proposed previously each give both qualitative and some quantitative information to the clinician as to the characterization of the acid–base disorder in this patient. In contrast, the comprehensive diagnostic procedure proposed in the present study gives a much more complete quantitative picture to the clinician that can lead to a better understanding of the disorder and an improved rationale for treatment.
Similarly to our previous study [7] with the IPE diagnostic model, a computer module was constructed that can diagnose whole-body acid–base and fluid-electrolyte disorders. The module uses the Vissim-Viewer program that can be obtained free of charge (see Sect. 6 for details).
4 Discussion
After over 100 years of scientific discovery, there remains a great controversy concerning the quantitative diagnosis and treatment of non-respiratory (metabolic) acid–base disorders. The “bicarbonate-centered” approach posits that if the distribution of bicarbonate is known in a patient, then the acid–base disorder can be classified and presumably treated. Palmer and VanSlyke [1] were the original proponents of this idea and it has been perpetuated to the present by the “Boston or bedside” rules [18]. A strong dissenter to this approach is Siggaard-Anderson [18] who proposed the blood base excess (BEB) approach [4] and the more recent blood-extracellular fluid, standard base excess (SBE) approach [2]. A more recent addition to the debate is the so called “Stewart” approach [10] which takes a more physico-chemical view to describing acid–base balance, however, this view is not new as DeLand and Bradham [16] used such an approach to construct a whole-body fluid-electrolyte model many years earlier. Siggaard-Andersen [2] vehemently opposed this approach because Stewart did not cite that his strong-ion difference (SID) QDV was equivalent to the earlier defined buffer base QDV and because Stewart’s definition of an acid did not agree with the classical definition, among other issues.
In the current study, this author’s approach is from a physico-chemical, mathematical-modeling standpoint, hence, it did not purposefully side with any of these approaches. The goal was to use a mathematical model of whole-body human acid–base and fluid-electrolyte balance [8] to quantitatively diagnose and aid in quantitative fluid-therapy specification of complex acid–base disorders in the critically ill. This study followed from a similar earlier study [7], however, that model was incomplete in that it did not contain a cellular compartment. Criticisms that can always be made about the original DeLand and Bradham [16] model and the current one is that the fluid compartments, particularly cells, are not truly homogeneous and that the non-ideal behavior of ions (activity, rather than concentration should be used) should be described. These details can always be added to the model if it is deemed necessary to describe conditions with absolute accuracy. The alternative view of this author is that the predictions of the model are not absolute and should be used as guides and with caution by the clinician just as the predictions of any other theoretical diagnostic procedure.
The QDV we used previously and the one in the present study [7] were derived from the BE approach [4]. The whole-body BE (WBBE) QDV used was arbitrarily selected in the present study because it was clear and simple to determine using the model. Whole-body SID could have been used, but this QDV is more difficult to define, even though Wooten [19] had defined an approximate value for the IPE fluid system. His quantitative SID definition for the IPE system was an attempt to reconcile the divergent approaches to characterizing acid–base disorders.
Inherent in the Siggaard-Andersen BE approach [2] is that a H+-donating substance, such as HCl, decreases pH when added to a fluid, whereas a H+-accepting substance, such as NaOH, increases pH when added to the fluid. The effect of protein anions in the solution were not considered in this definition. In contrast, when writing the physico-chemical equations describing a chemical system, one cannot make such a distinction, hence, the buffering effects of protein anions are just as important on the pH outcome as are the additions of HCl or NaOH. Furthermore, when one writes these equations, it becomes clear that the consequences of the concentrations of both H+ and OH− are negligible, hence adding HCl is the equivalent of adding just Cl− and adding NaOH is equivalent to adding only Na+. Similarly, in the open system of the body, adding NaHCO3 is equivalent to adding only the Na+ part.
The WBBE value determined by the model depends upon the changes from the standard state of the masses, concentrations and the volume of ten individual components. Its net value is the algebraic sum of the values of each of these ten. Each value was determined from its sensitivity (partial derivative) with the assumption of linearity (see Table 1), however, these sensitivities were only approximately linear over the expected wide range of change of each component. It would have been possible to fit the more exact sensitivity changes with polynomial functions, for example, but this increase in complexity was not deemed necessary for the present study.
In the present study, the finding was that the change in WBBE could closely predict the measured weight-normalized values of retained base in human studies (see Tables 2, 3) even though relatively small amounts of concentrated NaHCO3 solutions were infused. The information needed for WBBE determination were measurements in these subjects of a sufficient number of pre-infusion blood-chemistry values. The finding of the present study is that with this amount of information, the model could accurately predict the mass of NaHCO3 to add to a subject to change their acid–base status a designated amount. In contrast, if only a minimal amount of information were available, for example, just pH and PCO2 as used for BE or SBE determinations, the results shown in Table 2 and Fig. 3 clearly indicate a wide scatter in minimal-model WBBE predictability, to the extent that the confidence would be low for any single prediction. One of the objectives of the present study was to see if BS or ABS QDVs can yield such important information (see below).
In 1963, Siggaard-Andersen [4] used available literature data to suggest a relationship between BE (in whole organism) to BEB. He used the results of Palmer and VanSlyke [1] who found with some assumptions that,
where BE is in mEq, total CO2 is in volume % and Wt is body weight in kg. Assuming that at constant PCO2, total CO2 is approximately equal to 0.8 times standard HCO3 −, Siggaard-Andersen [4] obtained,
Or, in present terms, WBBE = 0.5 × BE B where the constant has units of lB/kg. Siggaard-Andersen [4], in his experiments with dogs, found a value between 0.4 and 0.5 for this constant.
To Palmer and VanSlyke [1], the 0.7 constant in their equation suggested that the added base distributed in the total body fluids since these fluids are about 70 % H2O. Of course, this suggestion ignores the actual units of the constant. Nonetheless, their study began the discussion of a distribution volume for HCO3 −, or BS, as it is now more customarily called. In 1955, Swan et al. [20] used radioactive tracers to estimate compartment volumes to determine the distribution of HCO3 − in nephrectomized dogs infused with NaHCO3 solution Their results showed that about three-fourths of the infused HCO3 − remained in extracellular fluid (about 20 % of Wt), suggesting that BS is far less than the value assumed previously [1]. A later study by Russell et al. [21] in nephrectomized dogs suggested that BS was constant at about 0.3 lP/kg under alkalotic conditions, but could increase to be >1 under severe metabolic-acidosis conditions. Russell et al. [21] also reintroduced the idea of WBBE and derived a nomogram to predict it using the physico-chemical model of DeLand and Bradham [16].
Fernandez et al. [12] postulated that BS (apparent BS, ABS by their definition) consisted of an anatomical body space plus an additional space due to the titration of non-bicarbonate buffers (see Eq. 2). They assumed that ABS went from 0.4 under alkalotic conditions up to a maximum of 0.7 (70 % of body weight) under severe acidotic conditions, which led to the constants in Eq. 2. These constants are close to the value found in dogs by Garella et al. [11].
Repetto et al. [9] recently found that in dehydrated children with metabolic acidosis, the measured BS values (Eq. 1) after NaHCO3 infusion were correlated (r = 0.67) with calculated ABS (Eq. 2). In contrast, the present BS and ABS results of Fig. 3 showed no apparent pattern when plotted against the amount of NaHCO3 infused. A possible reason was that all of the Repetto et al. [9] patients underwent full respiratory compensation, whereas it was not clear that the subject and patient data of Fig. 3 were in that state. These contradictory results highlight the issue that the [HCO3 −] data used in BS and ABS determinations are PCO2 dependent, which is one reason that WBBE is a more reliable indicator of the acid–base status of a patient.
The results of Fig. 4 show that the model can correctly diagnose the metabolic acidosis caused by an increase in HCl in the body. The excess Cl− was predicted along with no loss or gain of Na+ or K+; losses of the latter two ions would also lead to a metabolic acidosis. The model also determined the negative value of WBBE, providing information that can lead to an estimate of the amount of NaHCO3 to administer (see Fig. 5) if this therapy is the one selected by the clinician.
The results of Fig. 6 show the model’s diagnosis of a complex acid–base disorder in an actual patient with DKA. There is no way to prove that these diagnostic predictions are correct and useful, only a clinician at the bedside could make this determination, however, the predictions of a large plasma concentration of unmeasured anions, a K+ deficit and cellular dehydration all agree with clinical diagnoses.
There are some issues with the WBBE estimation procedure. (1) The model simulates venous blood, rather than arterial because it is venous plasma that is in equilibrium with the interstitial fluid in the body, hence, measurements of venous blood values are preferred. If arterial-blood values are measured, model pHa is decreased by 0.03 units from the measured value to obtain the value of model pHv. Estimating model PvCO2 from measured PaCO2 is more complex and is described in Sect. 5 along with other model refinements. (2) ΔMCl, hence, minimal-model WBBE, may be positive if the subject is acidotic or negative if alkalotic. Alternatively, the model could use the same procedure using the mass of Na+ (NaOH) as the independent variable, however, ΔMNa will change in the opposite direction. Furthermore, the value for WBBE will be different because, in contrast to BEB, the differing distributions of Cl− and Na+ in the body lead to different changes in their masses required to return pH and PCO2 to the normal state. These differences can have some quantitative effects on the results of this study, but have little effect on the conclusions reached.
There are caveats that should be recognized when using the proposed diagnostic approach. First, Morgan [22] suggested that it may be difficult for clinicians to believe that body-fluid volume changes from normal in a subject can be accurately predicted from a single blood chemistry determination. The answer from Figs. 4 and 5 is that it possible if these chemistry measurements are made with high accuracy and there is a complete set of measurements, however, there is another requirement that was implicit in the model; erythrocyte hemoglobin and plasma albumin must also be conserved. As a consequence, situations such as excess bleeding or extra-corporeal circuits could seriously affect the fluid volume predictions, but if the magnitude of these effects were known, by blood volume measurement, for example, it would be possible to simulate them and restore confidence in model predictions. Even if the estimated fluid volume changes are in error, the predicted acid–base effects, such as WBBE, are little effected as these two parts of the predictions are only slightly coupled. Second, the model is based on using venous blood, but arterial blood is most often measured in critically-ill patients. The model corrects for this effect by converting measured arterial values to venous. The corrections are minimal for the small ions in plasma and the subtraction of 0.03 pH units to convert the arterial value to venous is consistent with clinical findings. The assumption that could cause the greatest error is the conversion of arterial PCO2 to the venous value. Under conditions where there is adequate blood flow, the method of conversion described in Sect. 5 (item 6) is acceptable, however, under conditions of low cardiac output, the venous value could be as much as 30–40 mmHg higher than the arterial value. Consequently, the diagnostic predictions must be evaluated in terms of the total picture of the clinical state of the patient, just as the use of any other diagnostic predictor.
In conclusion, this study presents a new diagnostic tool for the clinician to use to diagnose and treat complex acid–base disorders. Part of this tool is determination of the WBBE QDV and its components. This information will prove to be a valuable aid in describing a patient’s complete acid–base status and it can provide important quantitative knowledge to specification of appropriate fluid-therapy treatment.
Abbreviations
- Atot:
-
Electrical charge of plasma “weak” ions (mEq/lP)
- ABS:
-
Apparent bicarbonate space (lP/kg)
- AG:
-
Anion gap (mEq/lP)
- AGadj :
-
AG adjusted for albumin effect
- AGc :
-
AG corrected for albumin and Pi effects
- Alb:
-
Serum albumin
- B:
-
Blood
- BE:
-
Base excess (mEq/l)
- BS:
-
Bicarbonate space (lP/kg)
- CIPE:
-
Refers to cell-interstitial-plasma-erythrocyte model compartments
- E:
-
Erythrocyte
- ECF:
-
Extracellular fluid
- Ht:
-
Height (cm)
- IPE:
-
Refers to interstitial-plasma-erythrocyte model compartments
- Hb:
-
Hemoglobin
- I:
-
Interstitial
- LBM:
-
Lean body mass (kg)
- M:
-
Mass (mmol)
- MZ :
-
Mass times electrical valence (mEq)
- P:
-
Plasma
- Pi− :
-
Phosphate ions
- SBE:
-
Standard base excess (mEq/lB+ECF)
- SID:
-
Strong ion difference (mEq/lP)
- SIG:
-
Strong ion gap (mEq/lP)
- QDV:
-
Quantitative diagnostic variable
- Wt:
-
Weight (kg)
- WBBE:
-
Whole-body base excess (mEq/kg)
- XA− :
-
Net charge of undetermined or unmeasured ions in plasma (mEq/lP)
- a:
-
Arterial
- v:
-
Venous
- [i]:
-
Concentration of species i
- Δ:
-
Change
References
Palmer WW, Van Slyke DD. Studies of acidosis: IX. Relationship between alkali retention and alkali reserve in normal and pathological individuals. J Biol Chem. 1917;32:499–507.
Andersen O, Fogh-Andersen N. Base excess or buffer base (strong ion difference) as measure of non-respiratory acid–base disturbance. Acta Anesthesiol Scand. 1995;39 (Supplementum 106):123–8.
Morgan TJ (2009) Unmeasured ions and the strong ion gap. In: Kellum JA, Elbers PWG (eds) Stewart’s textbook on acid–base. 2 edn. pp 323–337.
Siggaard-Andersen O. The acid–base status of the blood. Copenhagen: Munksgaard; 1963.
Siggaard-Andersen O. The van slyke equation. Scand J Clin Lab Invest. 1977;37 (Suppl. 146):15–20.
Wolf MB, DeLand EC. A mathematical model of blood-interstitial acid–base balance: application to dilution acidosis and acid–base status. J Appl Physiol. 2011;110 (April):988–1002.
Wolf MB, DeLand EC. A comprehensive, computer-model based approach for diagnosis and treatment of complex acid–base disorders in critically-ill patients. J Clin Monit Comput. 2011;25(6):353–64.
Wolf MB. Whole body acid–base and fluid-electrolyte balance: a mathematical model. Am J Physiol: Renal Physiol. 2013;305:F1118–31.
Repetto HA, Penna R. Apparent bicarbonate space in children. Sci World J. 2006;6:148–53.
Stewart PA. How to understand Acid–base. North Holland, New York: Elsevier; 1981.
Garella S, Dana CL, Chazan JA. Severity of metabolic acidosis as a determinant of bicarbonate requirements. N Engl J Med. 1973;19(July):121–6.
Fernandez PC, Cohen RM, Feldman GM. The concept of bicarbonate distribution space: the crucial role of body buffers. Kidney Int. 1989;36:747–52.
Singer RB, Clark JK, Barker ES, Crosley AP Jr, Elkinton JR. The acute effects in man of rapid intravenous infusion of hypertonic sodium bicarbonate solution. Medicine. 1955;34(1):51–95.
Mellemgaard K, Astrup P. The quantitative determination of surplus amounts of acid or base in the human body. Scand J Clin Lab Invest. 1960;12:187–99.
Russell CD, Roeher HD, DeLand EC, Maloney JV Jr. Acute response to acid–base stress. Ann Surg. 1978;187(4):417–22.
DeLand EC, Bradham GB. Fluid balance and electrolyte distribution in the human body. Ann NY Acad Sci. 1966;128(3):795–809.
Albert MS, Dell RB, Winters RW. Quantitative displacement of acid–base equilibrium in metabolic acidosis. Ann Int Med. 1967;66(2):312–22.
Severinghaus JW. Siggaard–Andersen and the “great trans-atlantic acid–base debate”. Scand J Clin Lab Invest Suppl. 1993;214:99–104.
Wooten EW. The standard strong ion difference, standard total titratable base, and their relationship to the Boston compensation rules and the Van Slyke equation for extracellular fluid. J Clin Monit Comput. 2010;24:177–88.
Swan RC, Axelrod DR, Seip M, Pitts RF. Distribution of sodium bicarbonate infused into nephrectomized dogs. J Clin Invest. 1955;34(12):1795–801.
Russell CD, Illickal MM, Maloney JV Jr, Roeher HD, DeLand EC. Acute response to acid–base stress in the dog. Am J Physiol. 1972;223(3):689–94.
Morgan TJ. Partitioning standard base excess: a new approach. J Clin Monit Comput. 2011;25(6):349–52.
Wolf MB, Garner RP. A mathematical model of human respiration at altitude. Ann Biomed Eng. 2007;11:2003–22.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
The model used in the present study has been updated from that formulated previously [8] in the following ways:
-
1.
The pK for intracellular proteins has been changed from 6.5 to 5.5 to achieve more realistic buffering.
-
2.
The standard-state interstitial albumin concentration was set to the concentration that would achieve a standard-state colloid osmotic pressure of 14 mmHg. This value resulted from a total albumin concentration of 28.8 g/l corrected to 38.4 g/l because of a 25 % protein exclusion volume in the standard-state interstitial space.
-
3.
Changing the pK and standard-state interstitial colloid osmotic pressure, above, required solving the model again to achieve the standard state. As described previously [8], the new values of six constants had to be determined to be consistent with the model changes. Table 6 shows the new values.
Table 6 Selected standard-state data -
4.
Net unmeasured anions (XA−) were added to the model. The concentration in interstitial fluid was assumed to be the plasma concentration as corrected for the plasma protein volume and the Donnan-distribution effect. These effects amounted to about a 12 % interstitial concentration increase above that in plasma in the standard state.
-
5.
Plasma glucose concentrations above the standard value of 5.3 mmol/lW resulting from glucose impermeability in diabetes mellitus were assumed to osmotically draw water from cells.
-
6.
A model of human respiratory physiology [23] was used to generate data which led to a mathematical relationship (SigmaPlot computer program. Systat Software, Point Richmond CA) that was used to convert arterial PCO2 values to those in venous blood. The relationship was,
$$PvCO_{2} = - 0.0000291 \times X^{3} + 0.00586 \times X^{2} + 0.67 \times X + 0.586$$where X stands for PaCO2.
Appendix 2
The screenshots of Figs. 4, 5, 6 were from a computer module that a scientist/clinical diagnostician can obtain from this author and run free of charge using downloadable Vissim-Viewer software from Visual Solutions Inc. The subject information and laboratory-chemistry values are entered in the left column. Model solution results and normal values are shown in the middle column. WBBE values and QDVs from other diagnostic approaches are shown in the right column.
Instructions for using the software and program will be provided with the software. All that is required are that the values for the patient’s laboratory chemistry be entered into the left column of the display.
Rights and permissions
About this article
Cite this article
Wolf, M.B. Comprehensive diagnosis of whole-body acid–base and fluid-electrolyte disorders using a mathematical model and whole-body base excess. J Clin Monit Comput 29, 475–490 (2015). https://doi.org/10.1007/s10877-014-9625-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-014-9625-z