Abstract
A novel wavelet transform cardiorespiratory coherence (WTCRC) algorithm has been developed to measure the autonomic state. WTCRC may be used as a nociception index, ranging from 0 (no nociception, strong coherence) to 100 (strong nociception, low coherence). The aim of this study is to estimate the sensitivity of the algorithm to nociception (dental dam insertions) and antinociception (bolus doses of anesthetic drugs). WTCRC’s sensitivity is compared to mean heart rate (HRmean) and mean non-invasive blood pressure (NIBPmean), which are commonly used clinical signs. Data were collected from 48 children receiving general anesthesia during dental surgery. The times of dental dam insertion and anesthetic bolus events were noted in real-time during surgeries. 42 dental dam insertion and 57 anesthetic bolus events were analyzed. The change in average WTCRC, HRmean, and NIBPmean was calculated between a baseline period before each event and a response period after. A Wilcoxon rank-sum test was used to compare changes. Dental dam insertion changed the WTCRC nociception index by an average of 14 (82 %) [95 % CI from 7.4 to 19], HRmean by 7.3 beats/min (8.1 %) [5.6–9.6], and NIBPmean by 8.3 mmHg (12 %) [4.9–13]. A bolus dose of anesthetics changed the WTCRC by −15 (−50 %) [−21 to −9.3], HRmean by −4.8 beats/min (4.6 %) [−6.6 to −2.9], and NIBPmean by −2.6 mmHg (3.4 %) [−4.7 to −0.50]. A nociception index based on cardiorespiratory coherence is more sensitive to nociception and antinociception than are HRmean or NIBPmean. The WTCRC algorithm shows promise for noninvasively monitoring nociception during general anesthesia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Anesthesiology includes the practice of autonomic medicine. Noxious stimuli during surgery cause the autonomic nervous system (ANS) to invoke a stress response, increasing sympathetic tone and decreasing parasympathetic tone [10]. An excessive and prolonged sympathetic response increases the risk of suffering from peri-operative complications, delays recovery, and is a key factor in postoperative morbidity [12]. Anesthesiologists must therefore control the stress response (nociception) by administering analgesic drugs (antinociception).
The ANS is currently not routinely monitored. Anesthesiologists are guided by observation and interpretation of trends in patients’ vital signs, including heart rate (HR) and blood pressure. These are only indirect and insensitive measures of nociception or the ANS. Confounding factors such as pre-existing medical conditions and inter-patient variability cause difficulties in such indirect estimations. An automated nociception monitor that directly assesses ANS activity would be very useful for general anesthesia, providing anesthesiologists with feedback about the adequacy of antinociception. Heart rate variability (HRV) shows promise as a noninvasive nociception monitor [9, 15, 16].
HRV, which refers to fluctuations in the instantaneous HR over time, reflects autonomic state. Most of these fluctuations are too fast to be observed in the standard 5–10 s HR trends recorded in physiological monitors. HRV contains important information that is missing from the mean HR (HRmean). In particular, a component of HRV called respiratory sinus arrhythmia (RSA) has been shown to reflect the autonomic state [8, 17]. RSA is a healthy heart arrhythmia that is driven by the respiratory cycle. As the patient inspires and expires, the HR increases and decreases in synchrony. During periods of physiological stress, the coupling between the HR and respiration weakens and the RSA pattern is attenuated. In the extreme, the HR and respiration decouple and the RSA completely disappears. Cardiorespiratory coherence (CRC) can be used to estimate the strength of linear coupling between the HR and respiration [13].
We have developed a CRC-based algorithm to monitor nociception, called wavelet transform cardiorespiratory coherence (WTCRC) [6, 7]. WTCRC is a form of sensor fusion, combining information from the HR and respiration signals. The algorithm tracks the effect of respiration on the HR (i.e. RSA) as it moves in the time/frequency plane, by using the known respiratory frequency calculated from a respiration signal (e.g. CO2). We have previously shown that WTCRC detects patient movement during general anesthesia, which is a strong sign of nociception [7].
There is no noninvasive gold standard for comparing the performance of WTCRC in monitoring nociception. Anesthetized patients clearly cannot report their level of pain, and no algorithm has yet been proven to be an accurate measure of nociception. Changing levels of surgical stimuli and anesthetic drugs lead to variable levels of nociception during surgery, and their precise level at any given point in time is unknown. Nevertheless, it may be possible to infer the sensitivity of WTCRC by measuring its response to nociceptive and antinociceptive stimuli.
The aim of this study was to measure the response of the WTCRC nociception index to nociception and antinociception, and to compare it to the traditional clinical measures (HRmean and NIBPmean).
2 Method
2.1 Patients & anesthesia
This study was approved by the University of British Columbia Clinical Research Ethics Board. Parents of all subjects provided written informed consent to participate in the study. Subjects were receiving general anesthesia during routine dental surgery. All subjects were between 3 and 6 years of age, and had ASA physical status I or II. Exclusion criteria included cardiorespiratory disease, developmental delay, ANS dysfunction, neuromuscular disease, cutaneous disease, chronic pain, a history of head injury, contraindication to propofol or remifentanil, anticipated difficult airway management, or use of any medications that may alter ANS function (e.g. anticholinergics, alpha-agonistors, anticonvulsants). Subjects received standard preoperative oral analgesic medications of 15 mg/kg acetaminophen and 10 mg/kg ibuprofen.
Anesthesia was induced with a bolus dose of 1 % propofol based on a manually adjusted pediatric target controlled infusion (TCI) algorithm using the Paedfusor pharmacokinetic model [1]. A target plasma level of 4 μg/mL of propofol was initially selected. After induction of anesthesia, standard monitors were placed, including electrocardiogram, pulse oximetry, spirometry, capnometry, non-invasive blood pressure, and electroencephalogram. A bolus dose of 3 μg/kg remifentanil was then administered. The patient’s trachea was intubated with a nasal endotracheal tube (ET). No muscle relaxants were administered for intubation. Following insertion of the ET, the target propofol drug concentration was reduced to 3.5 μg/mL (maintenance hypnosis) and an infusion of remifentanil was initiated and manually adjusted to achieve a target blood concentration of 2 ng/mL (maintenance analgesia). Propofol and remifentanil were infused separately using Alaris (CareFusion, San Diego, CA, USA) automatic infusion pumps. Infusion rates were manually adjusted every 5 min in accordance with the pharmacokinetic model.
Cases provided multiple periods of nociception and antinociception. A dental dam was inserted at least once during each case, providing a strong nociceptive stimulus. Other nociceptive stimuli included tooth extractions, cavity drillings, and cap/crown insertions. Antinociception interventions often followed nociceptive periods. The protocol allowed the anesthesiologist to deliver rescue bolus doses of propofol (1 mg/kg) and/or remifentanil (0.5 μg/kg) at their discretion. The anesthesiologist could also deliver bolus doses of morphine and/or fentanyl towards the end of the case to assist with postoperative pain management.
Physiological data were recorded and annotated throughout each case. The electrocardiogram (ECG) and capnometry (CO2) waveforms, as well as the respiratory rate (RR) and mean non-invasive blood pressure (NIBPmean) trends, were recorded using S/5 Collect software (GE Healthcare, Helsinki, Finland). The ECG was recorded at 300 Hz, CO2 at 25 Hz, RR (derived from CO2, with a 60 s moving average filter) at 1/10 Hz, and NIBPmean at 1/180 Hz. A research assistant annotated the data in real-time with markers identifying significant clinical events, including dental dam insertions, surgical stimulation, and rescue bolus doses of anesthetics. Annotation times were accurate to within 10 s of the recorded time.
2.2 Cardiorespiratory coherence
2.2.1 Data preparation
All data preparations and analyses were performed using Matlab (The Mathworks, Natick, MA, USA). The HR, respiration (CO2), and RR signals were first prepared for coherence analysis. ECG R peaks were detected using a filter bank algorithm [2], and minor artifacts (false or missed beat detections) were manually corrected. Each R peak time series was converted into a HR series (tachogram), and then resampled onto an evenly-spaced 4 Hz grid using Berger’s algorithm [4]. The respiration signal was downsampled to 4 Hz using standard low pass filtering and decimation. The RR trend was upsampled to 4 Hz using a repeater. The HR, respiration, and RR signals then had a matching sampling frequency.
2.2.2 Wavelet transform cardiorespiratory coherence
The WTCRC algorithm calculates the continuous wavelet transform of the HR and the respiration signals. The wavelet transform is a mathematical technique that converts signals between the time and scale (frequency) domains, with varying degrees of localization in each domain. At any given scale, the wavelet transform is given by:
where x n is the input signal, n is the time index, s is the scale, δt is the sampling time, and the asterisk (*) is the complex conjugate operator. A complex Morlet wavelet is used as \(\Uppsi\), as its scales are directly related to Fourier frequencies. The HR and respiration signals are decomposed to 96 scales, at 12 scales per octave. The scales correspond to a range of analysis frequencies from 0.0404 to 1.936 Hz. The frequency resolution increases with decreasing analysis frequency, as is typical of wavelet analysis. These parameters provide good localization across the relevant time/frequency spectrum of HRV. The result is a 2D matrix of wavelet coefficients at different times and frequencies. The wavelet coefficients for the HR and respiration are denoted as W T n and W R n , respectively.
From the wavelet coefficients, the algorithm calculates the wavelet power spectrum for each signal, as well as the cross power spectrum:
Power spectra are then smoothed in time with a Gaussian window (\(e^{{-n}^{2}}/2s^{2}\)) and in scale with a rectangular window (length 0.6 × scale).
The algorithm uses the power spectra to calculate the coherence estimator:
where the angled brackets (〈〉) denote the smoothing operator. The coherence estimator is a 2D matrix of coherence values at different times and frequencies. It was calculated using crosswavelet and wavelet coherence software provided by A. Grinsted.
Finally, the algorithm extracts the coherence values at the known respiratory frequency at each point in time, using RR values calculated a priori from the respiration signal. WTCRC outputs a 1D vector of time-varying coherence values at the respiratory frequency, denoted \(\hat{C}^2\). WTCRC is strictly bounded between 0 (no coherence) to 1 (strong coherence).
2.2.3 Nociception index
The nociception index is defined as a dimensionless linear scale bounded from 0 (no nociception) to 100 (strong nociception). This definition is similar to indices of consciousness reported by devices such as the BIS (Aspect Medical Systems Inc., Newton, MA, USA), Entropy (GE Healthcare, Helsinki, Finland), and NeuroSense (NeuroWave Systems Inc., Cleveland Heights, OH, USA).
The nociception index is derived from the WTCRC as:
2.3 Response to nociception
Dental dam insertions were used as indicators of nociception. A dental dam inserted into the mouth is a strongly nociceptive stimulus. Dental dams are inserted prior to dental surgery in anesthetized children to stop debris from entering the airway and to keep the teeth dry. The jaw is widely distracted with a retractor and a rubber dam is clamped to the teeth and gums with steel clips. The dental dam insertion is expected to follow a period of low nociception (such as performing dental X-rays), and to lead to a period of increased nociception. The WTCRC nociception index should increase during the dental dam insertion compared to baseline before insertion.
Case annotations were searched to find the first dental dam event in each case. The first dental dam always occurred during the maintenance phase of anesthesia, and the patient was always mechanically ventilated. Dental dam insertion always occurred at least 10 min after intubation. Events were only retained for analysis when the ECG or respiration signals were free of significant artifacts and when the RR was not being changed.
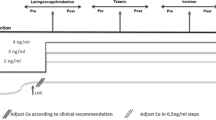
Data segments were extracted around each dental dam event (see Fig. 1). Each segment comprised a baseline period and a response period. The response period consisted of a 60 s analysis window immediately following the start of the dental dam event marker time. The baseline period consisted of a 60 s analysis window ending 60 s before the start of the dental dam event. This allowed for a 60 s buffer between the periods, to ensure the analysis was not corrupted by cross contamination. The analysis windows were padded with 120 s of data on each end, to ensure the analysis was not corrupted by edge artifacts.
2.4 Response to antinociception
Bolus doses of anesthetics were used as indicators of strong antinociception. A bolus dose of anesthetic drug (e.g. propofol, remifentanil, fentanyl, morphine) is a strongly antinociceptive stimulus. Bolus doses of anesthetics are typically given to rescue the patient from a strong sympathetic response to nociception. As such, rescue boluses are expected to follow a period of strong nociception, and to lead to a period of decreased nociception. The WTCRC nociception index should decrease after a bolus dose of anesthetic drugs compared to baseline before the bolus.
Case annotations were searched to find all anesthetic bolus events. Bolus doses of propofol, remifentanil, fentanyl, and morphine were considered to have antinociceptive properties. While propofol is not by itself antinociceptive, its synergistic interactions with remifentanil augment antinociception. Multiple bolus doses of anesthetics delivered in quick succession (<120 s apart) were considered to constitute a single bolus event. Events were only retained for analysis if they occurred during the maintenance phase of anesthesia, when the patient was mechanically ventilated, when the ECG and respiration signals were free of significant artifacts, and when the RR was not being changed.
Data segments were extracted around each anesthetic bolus event (see Fig. 2). Each segment comprised a baseline period and a response period. The baseline period consisted of a 60 s analysis window immediately preceding the anesthetic bolus event marker time. The bolus dose of anesthetic drug was given a buffer period of 60 s to take effect. The response period consisted of a 60 s analysis window immediately following the buffer period. The analysis windows were padded with 120 s of data on each end, to ensure the analysis was not corrupted by edge artifacts.
2.5 Analysis
Forty-eight pediatric subjects were included in the study. The sample size was based on the precision of a receiver operating characteristic (ROC) curve that has not been utilized in this analysis. The data analyzed in this study have been included in previous publications [6, 7]. This is the first study to directly investigate the effects of nociceptive and antinociceptive events on WTCRC.
The responses of the WTCRC nociception index, HRmean, and NIBPmean were measured for all dental dam and anesthetic bolus events. WTCRC and HR were calculated for each data segment, then averaged over the baseline and response periods. Since NIBPmean was only sampled every 180 s, the last sample in or before the baseline period and the first sample in or after the response period were used. The change from the baseline to the response period was calculated for all measures. Changes were averaged over all dental dam and anesthetic bolus events, to arrive at an overall average nociception and antinociception response for each of the measures. Percent responses to nociception and antinociception were calculated from the mean baseline and change results.
The analysis makes no assumptions about the potency of the stimuli or about other events occurring simultaneously during surgery. Dental dam insertions will induce variable levels of nociception, owing to differences in tooth spacing, clamp placement, and individual oral health. Bolus doses of anesthetics will likewise induce variable levels of antinociception. In particular, the different anesthetic agents are not equipotent. Confounding events during surgery may serve to lessen or even invert the response to individual nociception/antinociception events. Such confounding events may include dental work performed prior to dam insertion, or increasing levels of surgical stimulation following a bolus dose of anesthetics. The experiment only requires that all dental dams be nociceptive, and all bolus doses of anesthetics be antinociceptive. Differences in the potency of stimuli and confounding events are expected to average out in the overall result.
A 95 % confidence interval was estimated about the mean response, using a corrected percentile bootstrapping method (the bootci function in Matlab). A Wilcoxon rank-sum test was applied to estimate the statistical significance of the mean responses to nociception and antinociception. Responses below the 5 % level were considered statistically significant.
3 Results
The 48 subjects (22 male and 26 female) had a median (IQR [full range]) age of 3.7 (0.68 [3.0–6.8]) years, body weight of 16 (3.0 [12–24]) kg, and body height of 101 (6.5 [92–114]) cm.
Forty-two dental dam events were analyzed. Five were excluded due to significant ECG artifacts, where the HR was impossible to discern, and one was excluded due to a lost CO2 waveform. Two additional events were excluded from NIBPmean analysis, because of a blood pressure cuff artifact.
Fifty-seven anesthetic bolus events were analyzed. One was excluded due to significant ECG artifacts, where the HR was impossible to discern, three were excluded due to significant CO2 artifacts, two were excluded due to a baseline or response period that extended outside the maintenance phase of anesthesia, two were excluded because they occurred in conjunction with a change in respiratory rate (causing unreliable WTCRC measurements), and two were missing due to a failure of the data recording equipment.
The WTCRC nociception index increased significantly in response to nociception, and decreased significantly in response to antinociception. WTCRC was more sensitive to nociception and antinociception than were HRmean or NIBPmean. Dental dam insertion changed WTCRC by an average of 14 (82 %) [95 % CI from 7.4 to 19] P < 0.0002, HRmean by 7.3 beats/min (8.1 %) [5.6–9.6] P < 0.004, and NIBPmean by 8.3 mmHg (12 %) [4.9–13] P < 0.002. A bolus dose of anesthetics changed the WTCRC by −15 (−50 %) [−21 to −9.3] P < 0.00002, HRmean by −4.8 beats/min (4.6 %) [−6.6 to −2.9] P < 0.05, and NIBPmean by −2.6 mmHg (3.4 %) [−4.8 to −0.50] P > 0.1. An example anesthetic bolus event analysis is shown in Fig. 3.
Example anesthetic bolus event analysis with WTCRC. Plots from top to bottom: a heart rate; b capnogram; c cardiorespiratory coherence map. Bright areas indicate high coherence. Horizontal continuous line (green) indicates the respiratory frequency obtained from b; d WTCRC nociception index. Vertical dashed lines (- -) denote clinical events and the dot-dash line (- - .) denotes a rezeroing artifact in the capnogram. The patient movement event is a sign of strong nociception. The nociception index is high in the period preceding the bolus dose of anesthetic drugs (baseline period, highlighted in red), and low following it (response period, highlighted in green). The capnometer rezeroing artifact leads to a transient false increase in the nociception index
WTCRC increased in response to most of the dental dam insertions (33/42 events, 79 %), and decreased in response to most of the bolus doses of anesthetic (43/57 events, 75 %). These results are evident in Figs. 4 and 5, which illustrate the WTCRC nociception index response to individual dental dam and anesthetic bolus events. The overall statistics of the nociception measures both before and after the nociceptive and antinociceptive stimuli are shown in the boxplots in Figs. 6 and 7. The mean responses to nociception and antinociception are shown in Fig. 8.
WTCRC nociception index response to nociception for each dental dam event, sorted by response strength. Arrows indicate the direction of the response from the baseline period to the nociceptive period. Upward arrows (blue) indicate a change in the expected direction (increase), and downward arrows (red) indicate a change in the opposite direction (decrease)
WTCRC nociception index response to antinociception for each anesthetic bolus event, sorted by response strength. Arrows indicate the direction of the response from the baseline period to the antinociceptive period. Downward arrows (blue) indicate a change in the expected direction (decrease), and upward arrows (red) indicate a change in the opposite direction (increase)
Boxplots of responses to nociception for WTCRC, HRmean, and NIBPmean. The central red bar represents the median (second quartile). The box edges are the first and third quartiles. The whiskers extend 1.5× the interquartile range (IQR) beyond the box edges. Points beyond the whiskers are drawn as red plus symbols (+), and may be considered outliers. Such possible outliers were not excluded from analysis
4 Discussion & conclusion
The results of this study demonstrate that a nociception index based on cardiorespiratory coherence is more sensitive to nociception and antinociception than are HRmean or NIBPmean.
The WTCRC nociception index can be used to improve targeted anesthetic drug delivery. Using WTCRC, anesthesiologists can monitor the balance between nociception and antinociception during surgery, receiving feedback about the adequacy of antinociception. This information is not readily observed with changes in heart rate and blood pressure. The analgesic drug delivery could be specifically targeted to the individual patient and to the level of surgical stimulation. Moreover, as the level of surgical stimulation changes over the course of surgery, the analgesic drug delivery could be changed in step with the degree of response to maintain an adequate nociception/antinociception balance.
Other HRV-based devices have previously been described for monitoring nociception. The PhysioDoloris Analgesia Nociception Index (ANI) (MetroDoloris SAS, Loos, France) measures changes in the magnitude of RSA. The algorithm applies a wavelet bandpass filter to the HR to isolate the RSA, then calculates the area under the RSA curve (AUC). The ANI is calculated as a weighted fraction of the smallest short-term AUC to longer-term AUC [15]. The Surgical Stress Index (SSI) (GE Healthcare, Helsinki, Finland) measures changes in both RSA and the photoplethysmogram (PPG). It uses the RSA amplitude as a measure of parasympathetic tone, and the PPG amplitude as a measure of sympathetic tone [16]. Neither the ANI nor the SSI incorporate information from the respiration signal.
WTCRC is unique in combining information from the HR and respiration signals, operating as a form of sensor fusion. Most methods of time/frequency HRV analysis assume that the RSA will exist within a fixed frequency band, typically from 0.15 to 0.4 Hz (9–24 breaths/min). Others have investigated dynamic frequency band limits, though not in the context of nociception monitoring [3]. WTCRC goes further, completely eliminating the concept of fixed frequency bands. The algorithm tracks the RSA as it moves in time and frequency using the respiratory rate calculated from the respiration signal (e.g. CO2). WTCRC has information on the exact location of the RSA. We have previously shown that WTCRC continues to function when the RR is low (8 breaths/min), while another algorithm that assumes the standard frequency band fails [6]. Indeed, many of the events in this study occurred during periods of low RR, including 36 of the dental dam events and 18 of the anesthetic bolus events. While we did not test the ANI and SSI directly, we believe that they will fail when the RSA exists outside a fixed frequency band. WTCRC obtains further benefits from analyzing the shape of the respiration signal. During periods of strong nociception the patient may struggle against the mechanical ventilator, leading to irregularities in the shape and frequency content of the respiration waveform. This feature is evident in Fig. 3 in the period from t = 75–175 s. WTCRC measures these changes, leading to improved sensitivity in detecting nociception/antinociception. Indeed, we have previously shown that WTCRC is sensitive to patient movement events, which are a sign of strong nociception [7]. Sensor fusion between HR and respiration has a drawback, however. Unlike other methods, WTCRC requires a clean respiration signal. Dependence on multiple signals increases the risk of artifacts affecting the measurements. A good example of a common artifact in the CO2 waveform is shown in Fig. 3 at around t = 330 s. The capnometer periodically rezeros itself to ensure accurate measurements, and this feature can lead to false detections of nociception. Appropriate artifact handling will be essential for clinical implementation.
A WTCRC nociception monitor would integrate well with existing monitors in the operating room. The required ECG and CO2 signals are ubiquitous during general anesthesia. Unlike traditional measures of HR or blood pressure, WTCRC naturally produces a bounded, dimensionless index of nociception ranging from 0 (no nociception) to 100 (strong nociception). This index is similar to existing indices of hypnosis that are already widely used in monitors of consciousness such as the BIS or Entropy monitors. The nociception index will thus be familiar and intuitive to anesthesiologists.
Our experiments were performed only on healthy pediatric subjects in a narrow age range. Children have a more active RSA, and the RSA response to changing autonomic balance has been shown to decrease with age [14]. Further studies will be required to confirm these results in adult subjects, and in subjects with diseases affecting autonomic function (e.g. diabetes mellitus) or medications (e.g. atropine). We have recently shown that WTCRC responds to circadian changes in autonomic balance in nine healthy adult subjects aged 26.3 ± 4.6 years [5]. The response of WTCRC to these autonomic changes provides evidence that it may be used to monitor nociception in adult subjects.
Our experiments underestimate the true sensitivity of all three measures. We assume that insertion of a dental dam will always increase the patient’s level of nociception, and that a bolus dose of anesthetic drugs will always decrease it. However, these assumptions may not always hold. For example, in some cases the surgical stimulation can be stronger before the dental dam than during its insertion. This appears to be the situation in dental dam event number 1 (see Fig. 4). In this event, WTCRC and NIBPmean both reported a decrease in nociception. Likewise, a bolus dose of anesthetic may be insufficient to counteract increasingly strong nociceptive stimuli. The patient may experience stronger nociception after the anesthetic bolus event than before it. This appears to be the situation in anesthetic bolus event numbers 1 and 2 (see Fig. 5). In these events, all three measures reported an increase in nociception. These are likely to be the correct responses, but since they act in the wrong direction they serve to decrease the reported overall mean sensitivity.
Direct comparisons between WTCRC, HRmean, and NIBPmean are imperfect. The sparse NIBPmean measurements resulted in baseline and response periods that did not necessarily align with the other measures. Dynamic surgical conditions, which introduced noise into the measurements, may have been slightly different for NIBPmean. Furthermore, the peak effect of anesthetic agents (particularly propofol) on NIBPmean was likely delayed relative to the other measures [11]. As a result, the anesthetic bolus response period may not have captured the peak effect on NIBPmean. We may have observed a stronger response with a longer delay. However, a longer delay would have increased the misalignment with the other measures.
Our experiments could not measure the specificity of the nociception index. We have used dental dam insertions and bolus doses of anesthetics as indicators of true positive responses to nociception and antinociception, respectively. No corresponding indicator of true negative responses exists in our data, so measuring specificity is beyond the scope of this study.
Future work will involve adapting the WTCRC algorithm for real-time analysis. The method presented here, based on the continuous wavelet transform, is a useful tool for exploring the relationship between HR and respiration in time and frequency. It is a resource intensive calculation, however, and most of the information calculated is eventually discarded. We are thus developing an optimized method, based on the same underlying theory, for estimating cardiorespiratory coherence in real-time. Future work will also involve improving artifact detection and correction. These steps were performed manually in the current study, but will need to be automated in a future real-time nociception monitor.
WTCRC shows promise as a monitor of nociception during general anesthesia. We have shown that the WTCRC nociception index responds to both nociception and antinociception, and that WTCRC is more sensitive than HRmean or NIBPmean. In the future, WTCRC could provide anesthesiologists with feedback about the adequacy of antinociception in real time, increasing patient safety during surgery.
References
Absalom A, Amutike D, Lal A, White M, Kenny GNC. Accuracy of the 'Paedfusor’ in children undergoing cardiac surgery or catheterization. Br J Anaesth. 2003;91(4):507–513.
Afonso VX, Tompkins WJ, Nguyen TQ, Luo S. ECG beat detection using filter banks. IEEE Trans Biomed Eng. 1999;46(2):192–202.
Bailón R, Laguna P, Mainardi L, Sörnmo L. Analysis of heart rate variability using time-varying frequency bands based on respiratory frequency. In: Conf proc IEEE Eng Med Biol Soc, vol. 29, 2007. pp. 6674–7.
Berger RD, Akselrod S, Gordon D, Cohen RJ. An efficient algorithm for spectral analysis of heart rate variability. IEEE Trans Biomed Eng 1986;33(9):900–4.
Boudreau P, Brouse CJ, Dumont GA, Boivin DB. Sleep-state and circadian variation of cardiorespiratory coherence. In: Conf proc IEEE Eng Med Biol Soc, in press 2012.
Brouse CJ, Dumont GA, Myers D, Cooke E, Ansermino JM. Wavelet transform cardiorespiratory coherence for monitoring nociception. In: Computing in cardiology, 2010. p. 713–6.
Brouse CJ, Karlen W, Myers D, Cooke E, Stinson J, Lim J, Dumont GA, Ansermino JM. Wavelet transform cardiorespiratory coherence detects patient movement during general anesthesia. In: Conf proc IEEE Eng Med Biol Soc, vol. 33, 2011. p. 6114–7.
Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution and biobehavioral functions. Biol Psychol 2007;74(2):263–85.
Huhle R, Burghardt M, Zaunseder S, Wessel N, Koch T, Malberg H, Heller A. Effects of awareness and nociception on heart rate variability during general anaesthesia. Physiol Meas 2012;33(2):207–17.
Johnson J, Grecu L, Lawson N. Autonomic nervous system: physiology and pharmacology. In: Clinical anesthesia. 6th edn., chap. 15. Philadelphia: Lippincott Williams & Wilkins; 2009. p. 326–69
Kazama T, Ikeda K, Morita K, Kikura M, Doi M, Ikeda T, Kurita T, Nakajima Y. Comparison of the effect-site keO s of propofol for blood pressure and EEG bispectral index in elderly and younger patients. Anesthesiology 1999;90(6):1517–27.
Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth 1997;78:606–17.
Keissar K, Davrath LR, Akselrod S. Coherence analysis between respiration and heart rate variability using continuous wavelet transform. Phil Trans R Soc A. 2009;367:1393–406.
Laitinen T, Niskanen L, Geelen G, La E. Age dependency of cardiovascular autonomic responses to head-up tilt in healthy subjects. J Appl Physiol. 2004;96:2333–2340.
Logier R, Jeanne M, Dassonneville A, Delecroix M, Tavernier B. PhysioDoloris: a monitoring device for analgesia/nociception balance evaluation using heart rate variability analysis. In: Conf proc IEEE Eng Med Biol Soc, 2010. p. 1194–7.
Seitsonen E, Korhonen I, van Gils M, Huiku M, Lötjönen J, Korttila K, Yli-Hankala A. EEG spectral entropy, heart rate, photoplethysmography and motor responses to skin incision during sevoflurane anaesthesia. Acta Anaesth Scand 2005;49:284–92.
Taylor JA, Myers CW, Halliwill JR, Seidel H, Eckberg DL. Sympathetic restraint of respiratory sinus arrhythmia: implications for vagal-cardiac tone assessment in humans. Am J Physiol Heart Circ Physiol. 2001;280(6):2804–14.
Acknowledgments
This work was supported in part by the Michael Smith Foundation for Health Research (MSFHR) and the Natural Sciences and Engineering Research Council of Canada (NSERC). The authors acknowledge the contributions of Carolyne Montgomery to the clinical study design, and the Department of Pediatric Anesthesia at BC Children’s Hospital for its assistance in conducting the study. Special thanks to the dental operating room staff for their boundless patience (and patients!).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brouse, C.J., Karlen, W., Dumont, G.A. et al. Monitoring nociception during general anesthesia with cardiorespiratory coherence. J Clin Monit Comput 27, 551–560 (2013). https://doi.org/10.1007/s10877-013-9463-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-013-9463-4