Abstract
Perioperative hemodynamic optimisation improves postoperative outcome for patients undergoing high-risk surgery (HRS). In this prospective randomized multicentre study we studied the effects of an individualized, goal-directed fluid management based on continuous stroke volume variation (SVV) and stroke volume (SV) monitoring on postoperative outcomes. 64 patients undergoing HRS were randomized either to a control group (CON, n = 32) or a goal-directed group (GDT, n = 32). In GDT, SVV and SV were continuously monitored (FloTrac/Vigileo) and patients were brought to and maintained on the plateau of the Frank-Starling curve (SVV <10 % and SV increase <10 % in response to fluid loading). Organ dysfunction was assessed using the SOFA score and resource utilization using the TISS score. Patients were followed up to 28 days for postoperative complications. Main outcome measures were the number of complications (infectious, cardiac, respiratory, renal, hematologic and abdominal post-operative complications), maximum SOFA score and cumulative TISS score during ICU stay, duration of mechanical ventilation, length of ICU stay, and time until fit for discharge. 12 patients had to be excluded from final analysis (6 in each group). During surgery, GDT received more colloids than CON (1,589 vs. 927 ml, P < 0.05) and SVV decreased in GDT (from 9.0 to 8.0 %, P < 0.05) but not in CON. The number of postoperative wound infections was lower in GDT (0 vs. 7, P < 0.01). Although not statistically significant, the proportion of patients with at least one complication (46 vs. 62 %), the number of postoperative complications per patient (0.65 vs. 1.40), the maximum sofa score (5.9 vs. 7.2), and the cumulative TISS score (69 vs. 83) tended to be lower. This multicentre study shows that fluid management based on a SVV and SV optimisation protocol is feasible and decreases postoperative wound infections. Our findings also suggest that a goal-directed strategy might decrease postoperative organ dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background
Hypovolaemia with subsequent tissue hypoperfusion might occur during and after high-risk surgery (HRS). Hypovolaemia can remain undetected and may lead to postoperative complications including organ dysfunction, prolonged hospital stay and increased mortality [1–3]. HRS accounts for about 10 % of surgical procedures but for a prolonged hospital stay and more than 80 % of deaths, [4, 5] indicating a need for improving survival for patients undergoing HRS. The outcome of patients undergoing HRS improves by an intraoperative fluid management using goal-directed stroke volume (SV) optimisation [6–9]. Two meta-analyses demonstrated that intraoperative haemodynamic optimisation is effective in reducing both postoperative complications and mortality [10], particularly postoperative infections [11]. In addition, postoperative organ dysfunctions including gastrointestinal complications [9, 12] and renal impairment [13] can be reduced by a goal-directed approach. Since studies aiming at maximizing physiological variables (e.g. cardiac output, oxygen delivery, mixed venous oxygen saturation) had inconsistent results [14–17], a more individualised or patient-oriented approach has been advocated [9].
Hypovolaemia is the major reason for haemodynamic instability in the perioperative setting [18]. On the other hand, there is evidence that volume application may be dangerous [19]. But volume administration is required and is achieved by using so-called dynamic variables, i.e. stroke volume variation (SVV), pulse pressure variation or systolic pressure variation [20]. Fluid optimisation guided by SVV is associated with haemodynamic stability and decreased lactate levels as well as reduced postoperative organ complications [21].
The aim of this prospective randomised multicentre study was to create a database enabling a sample size calculation for a larger study and to evaluate whether a goal directed fluid optimisation algorithm based on targeting SV and SVV improves the outcome of high-risk patients undergoing HRS. We hypothesise that an individualised fluid optimisation protocol decreases the incidence of postoperative complications.
2 Methods
Ethical approval for this prospective randomized multicentre study was provided by the Ethical Committee of University Hospitals, Rostock, Germany (A 51-2008)
HRS patients were identified according to predefined criteria [4, 22, 23]. All patients provided written informed consent prior to inclusion.
The purpose of this preliminary study was to create a database enabling a robust sample size calculation for a larger follow-up study and to evaluate feasibility of the study protocol. Feasibility was assessed by the number of eligible patients per center, the inclusion rate of these patients per center, the ability of the Vigileo-FloTrac device to detect hypovolemia (i.e. SVV >10 %) and thus enable to guide fluid therapy, the lack of complications related to the device and/or the treatment algorithm, and the compliance of the participating anaesthetists to the study protocol.
The primary objective is to evaluate the proportion of patients developing post-operative complications in each study arm. The secondary objective will be to evaluate information regarding the complications and their consequences by the following endpoints:
-
maximum sequential organ failure assessment (SOFA) score during stay in intensive care unit (ICU)
-
cumulative therapeutic intervention scoring system (TISS-28) score during ICU stay (cost analysis)
-
duration of mechanical ventilation
-
time until fit for discharge from ICU according to the following criteria:
-
Spontaneous breathing with oxygen <3 l min−1 and SpO2 > 92 %
-
Haemodynamic stability (systolic blood pressure >100 mmHg) without vasoactive or inotropic support
-
Normothermia (central temperature between 36 and 38.5 °C)
-
-
ICU Length Of Stay (LOS) (in hours)
-
Mortality rate at day 28
Patients aged 18 and older scheduled for HRS and postoperative intensive care were eligible for participation in the study. Inclusion criteria were ASA III or IV score, an arterial and central venous line planned for pressure monitoring during surgery, a preoperative decision that postoperative care will be undertaken in the ICU because of co-morbidities and/or the surgical procedure, and a thoracic epidural anaesthesia. Exclusion criteria were: age below 18 years, cardiac arrhythmia, body mass index >40, scheduled procedure for surgery with an open thorax, neurosurgery, hepatic or emergency surgery.
Patients were randomised either to a control group (CON) or to a goal-directed group (GDT). Randomization was performed by closed envelopes in each centre containing the treatment arm.
2.1 Data acquisition
Patients of both groups were connected to the FloTrac System and recorded from skin incision until end of skin closure by a Multi Data Logger (MDL). The haemodynamic values collected were heart rate (HR), mean arterial pressure (MAP), arterial oxygen saturation by pulse oximetry (SpO2), central venous pressure (CVP), stroke volume variation (SVV), and stroke volume (SV). The data from the FloTrac System (SVV and SV) in the control group were recorded, but made inaccessible for the investigator by using an intransparent cover sheet for the monitor. Patients from this group received treatment according to a standardized approach. In the GDT group, the haemodynamic data given by the FloTrac System were available for the investigator and used every 10 min during surgery to apply the fluid optimisation protocol (Fig. 1). This protocol was based on the Frank–Starling mechanism of the heart and assumed that subjects benefit from volume application with an increase in SV until they reach the top (flat part) of the curve. If indicated, 200 ml of 6 % hydroxyethylstarch (HES 130/0.4, Voluven®, Fresenius Kabi, Graz, Austria) were infused over 10 min.
Data were collected in case report forms and fed into a relational Oracle database on a central server, networked to data entry and data analytical workstations.
Subjects were followed regarding infectious, cardiac, respiratory, renal, hematologic and abdominal post-operative complications for 28 days or until hospital discharge. To avoid any bias, doctors performing this evaluation were blinded concerning the patient’s treatment group. Ventilation was in a volume controlled mode with a tidal volume of >7 ml kg−1 [24].
2.2 Statistical analysis
Data from this preliminary study were used for a sample size calculation for a follow-up multicentre trial. In addition, we performed an outcome analysis. Data were tested for normality (Kolmogorov–Smirnov test) and are presented as mean (SD) unless otherwise stated. For data representation, the frequency and percentage of patients developing post-operative complications are reported for each randomization group. Comparison of groups was by using Fisher’s Exact Test.
The secondary outcome variables (total number of complications developed post surgery, maximum SOFA score and cumulative TISS score during ICU stay, duration of mechanical ventilation, length of ICU stay, and time until fit for discharge) are reported for each randomization group. The comparison was performed either by using t test or, if the data deviated from normal distribution, by the Wilcoxon–Mann–Whitney test.
For variables other than the primary and secondary endpoints, descriptive statistics were provided. For continuous data, mean and standard deviation are presented, for dichotomous and categorical data, frequency and percentage are provided. Comparisons were performed either by using t test or, if the data deviated from normal distribution, by the Wilcoxon–Mann–Whitney test. Binary and qualitative data were compared using Fisher’s Exact test.
3 Results
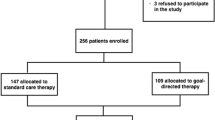
Sixty-four HRS patients were included between September 2008 and August 2009 (Fig. 2). 12 patients (6 in each group) were excluded because of protocol violation (n = 8), not meeting inclusion criteria (n = 2), or arrhythmia during or shortly after surgery (n = 2), resulting in 52 patients (26 in each group) included in the final analysis (Fig. 2). Protocol violation included mechanical ventilation with tidal low volumes (<7 ml kg−1, n = 6) and recording of SV and SVV values in the CRF in the CON group (n = 2).
The two groups of patients were comparable for ASA score and co-morbidity before surgery (Table 1). However, patients from the GDT group were younger (68(9) vs. 73(9) years) and had a slightly higher haemoglobin level (12.8(2.2) vs. 11.2(1.7) g dl−1) than those of the CON group. Type and duration of surgery (275(98) vs. 280(107) min) and blood loss (1,118(1,057) vs. 984(647) ml) were comparable in both groups of patients (Fig. 3).
During surgery, both groups of patients received a similar amount of fluids (GDT 4,477 (2,107) ml vs. CON 4,528 (2,387) ml, P = 0.86) (Fig. 3). The GDT group received however more colloids than the control group (1,589(1,283) vs. 927(845) ml, P < 0.05), while the control group tended to receive more red blood cells (685(832) vs. 319(495) ml, P = 0.063) (Fig. 3). This resulted in similar haemoglobin levels at the end of surgery (10.0(1.2) versus 9.8(1.5) g dl−1 in the CON and GDT group, respectively). More colloids were given early during surgery in the GDT group (Fig. 4), whereas fluid administration was equally distributed over the whole intraoperative period in the control group (data not shown).
At baseline, heart rate, mean arterial pressure, CVP, SV and SVV were comparable between groups (Table 2). SVV decreased in the GDT group (from 9.1 (2.5) to 8.0 (4.3) %, P = 0.048) but not in the control group (8.9 (2.9) vs. 8.8 (2.7) %). The time that SVV was <10 % (area under the curve) tended to be greater in the GDT group (69 ± 10 vs. 61 ± 15.9 %, P = 0.41). Cardiac output increased in both groups to a similar extent (Table 2). Vasoactive support did not differ between groups (norepinephrine dosage 0.05(0.05) vs. 0.04(0.06) μg kg−1 min−1 in groups CON and GDT, respectively).
The number of post-operative wound infections was significantly lower in the GDT group (0 vs. 7, P = 0.01). Although not statistically significant, the proportion of patients with at least one complication (46 vs. 62 %), the number of post-operative complications per patient (0.65(0.85) vs. 1.40(1.9)), the maximum ICU SOFA score (5.9(3.9) vs. 7.2(4)), and the cumulative ICU TISS score (69(47) vs. 83(66)) were also lower in the GDT group (Fig. 5). In this study, the postoperative duration of mechanical ventilation (2.4(3.6) vs. 4.8(10.4) h), the time until fit-for-discharge (19(19) vs. 28(21) h) and the actual ICU length of stay (30(29) vs. 42(52) h) tended to be shorter in the GDT group, although no significant differences occurred. While all patients of the GDT group survived, 2 patients (7.7 %) died in the control group (P = 0.49). There were no complications related to the FloTrac/Vigileo device or the treatment algorithm.
4 Discussion
This multicentre study shows that the chosen treatment algorithm is feasible and that about 250 patients should be included in a follow-up study to get a significant difference in the main study endpoint, i.e. the proportion of subjects developing post-operative complications in each study arm. Furthermore, it demonstrates that fluid management based on SVV and SV optimisation decreases post-operative wound infections. Although the two groups of patients were not perfectly matched, our findings suggest that a goal-directed strategy might decrease post-operative organ dysfunction and thus resources utilization.
HRS patients account for a majority of perioperative morbidity and mortality [4, 25]. The reasons for perioperative morbidity and mortality and potential strategies for prevention and intervention have been summarised [26]. Numerous single-centre trials showed that a goal-directed approach of haemodynamic optimisation reduces both postoperative complications and mortality in HRS patients, regardless of the monitoring method or target variable chosen [10, 27]. Furthermore, in a 15-year follow-up patients for whom haemodynamics had been optimised survived on average 3 years longer than those who did not receive such goal directed therapy [28]. This long-term survival benefit was also observed in those patients of the protocol group who developed complications after surgery [28].
However, impressive results obtained in single-centre trials, where dedicated teams follow a strict, well-established treatment protocol, may not be reproduced in a multicentre setting [29]. Just monitoring cardiac output without using a treatment algorithm is ineffective in facilitating haemodynamic stabilization or affecting outcome [30]. There is only one other multicentre-study showing reduced morbidity by intraoperative optimisation therapy [31]. In that study, the oxygen extraction ratio was used as a goal and was aimed to decrease below 27 %. The authors reported a reduction in organ failure (−40 %) and in length of hospital stay (−16 %).
Fluid administration is a mainstay of GDT optimising cardiac preload [25]. However, since excessive fluid administration is associated with excess mortality [32–34], an individualised assessment of fluid responsiveness is advocated [9].
With regard to the assessment of fluid responsiveness, dynamic changes in arterial waveform-derived variables such as pulse pressure variation, systolic pressure variation and SVV have been suggested [20]. Among these, SVV appears to be the most relevant variable with regard to fluid responsiveness. We chose an algorithm addressing SVV and SV in order to assess the individual patient’s Frank-Starling curve allowing us to titrate fluid therapy according to individual patient’s needs.
Heart rate and blood pressure were not much affected by the optimised fluid regimen, despite major differences in outcome in favour of GDT. This might be explained by the fact that the total amount of fluids given was similar in both groups. Nevertheless, the timing of fluid administration may be important, since patients in the GDT group received their colloid boluses early during surgery, suggesting an earlier optimisation of tissue perfusion [35, 36]. Furthermore, given the low SVV already at the start of surgery which was below the intervention threshold of 10 % and the small differences observed at the end of surgery, even the control group seems to be almost optimised by the standard therapy applied in the participating centres. Thus, one might expect even more reductions in complications in a setting where standard therapy is less extensive. The amount of volume given resulted in similar decreases in haemoglobin levels in both groups.
Independent of preoperative patient risk, a 30-day complication in patients after major surgery reduced median patient survival by 69 % [37] and increased their hospital length of stay [38]. Complications following surgery can be related to a patient’s age, preoperative comorbidities, and the type of surgery [39, 40]. Regarding the primary endpoint (percentage of patients with at least one complication), the benefit was 46 % relative reduction instead of 32 % in our study. Of particular interest is the reduction in postoperative wound infections in our GDT group (0 vs. 7 in the control group). These findings are supported by a meta-analysis including 26 randomised controlled trials and more than 4,000 patients [11]. Intraoperative haemodynamic optimisation leads to improved tissue perfusion and oxygenation and thus prevents infectious complications. The greater amount of transfused blood in control group patients might have contributed to differences in complications, since blood transfusions are immunosuppressive and might increase postoperative infections [41].
We found tendency of 17 % reduction of the cumulative TISS-28 score in our GDT group compared to control patients (Fig. 5). Since one TISS-28 point equals 10.6 min of an ICU nurse’s working time [42] or 25 pound sterling, [43] almost 2.5 h (148 min) or 350£ ICU costs per patient were saved if patients received the goal-directed fluid optimisation protocol intraoperatively. In addition, patients from the GDT group tended to be earlier fit for discharge from the ICU and were discharged by trend earlier (−33 and −28 % compared to control). Furthermore, the cost reduction outweighs the additional costs of intraoperative SV monitoring and additional colloids in accordance with cost analyses showing that perioperative optimisation not only improves patient outcome but also provides cost saving [44, 45].
We observed 2 deaths in our 52 HRS patients within 28 days (3.8 %), both occurring in the control group. Medicare data from the US showed that risk-adjusted mortality in HRS patients ranges from 3.7 % (cystectomy) to 8.9 % (oesophagectomy) [46]. A multicentre observational study of HRS patients from 21 Brazilian hospitals reported ICU and hospital mortality rates of 15 and 20.6 %, respectively, with multiple organ failure being the main cause of death [47]. In this study, the SOFA score proved to be a good predictor of hospital mortality with an area under the ROC curve of 0.805 [47]. We found an 18 % reduction of the maximum SOFA score (although not significant) in HRS patients receiving the individualised fluid optimisation protocol. Based on our findings we can calculate that a sample size of at least 232 HRS patients will be necessary to achieve significant results for the primary endpoint in a confirmatory study.
Some limitations of the presented multicentre study have to be considered: First, the overall compliance to the study protocol was only 75 %. The main reasons for not following the protocol were applicability (i.e. the short time interval of 10 min between successive interventions) and scepticism of the attending anaesthetist about the necessity or benefit of either the requested minimal tidal volume (6 patients had to be excluded afterwards for this reason) or further fluid administration (although the protocol suggested to give fluid). One might speculate that the results of our study might have been even better if doctors had followed the protocol more strictly. Second, the study was not double-blinded, i.e. the physicians were aware in which group the patients were randomised, which may be a reason for modified handling and awareness. However, one of the goals was to evaluate the efficiency of goal-directed versus non-directed fluid management on the detected parameters; hence, no alternative approach seemed to be feasible. Third, many patients had to be excluded during the screening due to pre-existing arrhythmia, which, however, is present in a considerable partition of HRS patients. Efforts are currently made to implement new software for the used monitoring device which is able to handle even data from patients with arrhythmias in optional follow-up studies. Fourth, postoperative fluid management in the ICU was not standardized, so that we cannot exclude that a poor postoperative fluid management derogated the positive effect of an intraoperative fluid optimization. Finally, the sample size is too low to make any definite conclusions on the efficiency of the goal-directed approach.
5 Conclusions
In summary, our multicentre study shows that an individualised fluid management based on a SVV and SV optimisation protocol is feasible and decreases post-operative wound infections. Our findings also suggest that such a goal-directed strategy seems to decrease other post-operative complications such as organ dysfunction. The intraoperative use of minimal invasive monitoring of SV and SVV and a fluid optimisation protocol might be cost-effective.
References
Thom O, Taylor DM, Wolfe RE, Myles P, Krum H, Wolfe R. Pilot study of the prevalence, outcomes and detection of occult hypoperfusion in trauma patients. Emerg Med J. 2010;27(6):470–2. doi:10.1136/emj.2009.073254.
Davies SJ. Wilson RJ (2004) Preoperative optimization of the high-risk surgical patient. Br J Anaesth. 2004;93(1):121–8. doi:10.1093/bja/aeh164.
Bennett-Guerrero E, Welsby I, Dunn TJ, Young LR, Wahl TA, Diers TL, Phillips-Bute BG, Newman MF, Mythen MG. The use of a postoperative morbidity survey to evaluate patients with prolonged hospitalization after routine, moderate-risk, elective surgery. Anesth Analg. 1999;89(2):514–9.
Pearse RM, Harrison DA, James P, Watson D, Hinds C, Rhodes A, Grounds RM, Bennett ED. Identification and characterisation of the high-risk surgical population in the United Kingdom. Crit Care. 2006;10(3):R81. doi:10.1186/cc4928.
Jhanji S, Thomas B, Ely A, Watson D, Hinds CJ, Pearse RM. Mortality and utilisation of critical care resources amongst high-risk surgical patients in a large NHS trust. Anaesthesia. 2008;63(7):695–700. doi:10.1111/j.1365-2044.2008.05560.x.
Gan TJ, Soppitt A, Maroof M, el-Moalem H, Robertson KM, Moretti E, Dwane P, Glass PS. Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology. 2002;97(4):820–6.
McKendry M, McGloin H, Saberi D, Caudwell L, Brady AR, Singer M. Randomised controlled trial assessing the impact of a nurse delivered, flow monitored protocol for optimisation of circulatory status after cardiac surgery. BMJ. 2004;329(7460):258. doi:10.1136/bmj.38156.767118.7C.
Grocott MP, Mythen MG, Gan TJ. Perioperative fluid management and clinical outcomes in adults. Anesth Analg. 2005;100(4):1093–106. doi:10.1213/01.ANE.-0000148691.33690.AC.
Bundgaard-Nielsen M, Holte K, Secher NH, Kehlet H. Monitoring of peri-operative fluid administration by individualized goal-directed therapy. Acta Anaesthesiol Scand. 2007;51(3):331–40.
Hamilton MA, Cecconi M, Rhodes A. A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg. 2011;112(6):1392–402. doi:10.1213/ANE.0b013e3181eeaae5.
Dalfino L, Giglio MT, Puntillo F, Marucci M, Brienza N. Haemodynamic goal-directed therapy and postoperative infections: earlier is better. A systematic review and meta-analysis. Crit Care. 2011;15(3):R154. doi:10.1186/cc10284.
Giglio MT, Marucci M, Testini M, Brienza N. Goal-directed haemodynamic therapy and gastrointestinal complications in major surgery: a meta-analysis of randomized controlled trials. Br J Anaesth. 2009;103(5):637–46. doi:10.1093/bja/aep279.
Brienza N, Giglio MT, Marucci M, Fiore T. Does perioperative hemodynamic optimization protect renal function in surgical patients? A meta-analytic study. Crit Care Med. 2009;37(6):2079–90. doi:10.1097/CCM.0b013e3181a00a43.
Gattinoni L, Brazzi L, Pelosi P, Latini R, Tognoni G, Pesenti A, Fumagalli R. A trial of goal-oriented hemodynamic therapy in critically ill patients. N Engl J Med. 1995;333(16):1025–32. doi:10.1056/NEJM199510193331601.
Heyland DK, Cook DJ, King D, Kernerman P, Brun-Buisson C. Maximizing oxygen delivery in critically ill patients: a methodologic appraisal of the evidence. Crit Care Med. 1996;24(3):517–24.
Ziegler DW, Wright JG, Choban PS, Flancbaum L. A prospective randomized trial of preoperative “optimization” of cardiac function in patients undergoing elective peripheral vascular surgery. Surgery. 1997;122(3):584–92.
Sandham JD, Hull RD, Brant RF, Knox L, Pineo GF, Doig CJ, Laporta DP, Viner S, Passerini L, Devitt H, Kirby A, Jacka M. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003;348(1):5–14. doi:10.1056/NEJMoa021108.
Kreimeier U. Pathophysiology of fluid imbalance. Crit Care. 2000;4(Suppl 2):S3–7. doi:10.1186/cc968.
Holte K, Sharrock NE, Kehlet H. Pathophysiology and clinical implications of perioperative fluid excess. Br J Anaesth. 2002;89(4):622–32.
Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009;37(9):2642–7. doi:10.1097/CCM.0b013e3181a590da.
Benes J, Chytra I, Altmann P, Hluchy M, Kasal E, Svitak R, Pradl R, Stepan M. Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: results of prospective randomized study. Crit Care. 2010;14(3):R118. doi:10.1186/cc9070.
Shoemaker WC, Appel PL, Kram HB. Hemodynamic and oxygen transport responses in survivors and nonsurvivors of high-risk surgery. Crit Care Med. 1993;21(7):977–90.
Boersma E, Kertai MD, Schouten O, Bax JJ, Noordzij P, Steyerberg EW, Schinkel AF, van Santen M, Simoons ML, Thomson IR, Klein J, van Urk H, Poldermans D. Perioperative cardiovascular mortality in noncardiac surgery: validation of the Lee cardiac risk index. Am J Med. 2005;118(10):1134–41. doi:10.1016/j.amjmed.2005.01.064.
De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent JL. Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med. 2005;31(4):517–23. doi:10.1007/s00134-005-2586-4.
Lees N, Hamilton M, Rhodes A. Clinical review: goal-directed therapy in high risk surgical patients. Crit Care. 2009;13(5):231. doi:10.1186/cc8039.
Kehlet H, Mythen M. Why is the surgical high-risk patient still at risk? Br J Anaesth. 2011;106(3):289–91. doi:10.1093/bja/aeq408.
Tote SP, Grounds RM. Performing perioperative optimization of the high-risk surgical patient. Br J Anaesth. 2006;97(1):4–11. doi:10.1093/bja/ael102.
Rhodes A, Cecconi M, Hamilton M, Poloniecki J, Woods J, Boyd O, Bennett D, Grounds RM. Goal-directed therapy in high-risk surgical patients: a 15-year follow-up study. Intensive Care Med. 2010;36(8):1327–32. doi:10.1007/s00134-010-1869-6.
Takala J. Highs and lows in high-risk surgery: the controversy of goal-directed haemodynamic management. Crit Care. 2005;9(6):642–4. doi:10.1186/cc3929.
Takala J, Ruokonen E, Tenhunen JJ, Parviainen I, Jakob SM. Early non-invasive cardiac output monitoring in hemodynamically unstable intensive care patients: a multi-center randomized controlled trial. Crit Care. 2011;15(3):R148. doi:10.1186/cc10273.
Donati A, Loggi S, Preiser JC, Orsetti G, Munch C, Gabbanelli V, Pelaia P, Pietropaoli P. Goal-directed intraoperative therapy reduces morbidity and length of hospital stay in high-risk surgical patients. Chest. 2007;132(6):1817–24. doi:10.1378/chest.07-0621.
Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR, Payen D. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34(2):344–53. doi:10.1097/01.CCM.0000194725.-48928.3A.
Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot–Olupot P, Akech SO, Nyeko R, Mtove G, Reyburn H, Lang T, Brent B, Evans JA, Tibenderana JK, Crawley J, Russell EC, Levin M, Babiker AG, Gibb DM. Mortality after fluid bolus in african children with severe infection. N Engl J Med. 2011;364(26):2483–95. doi:10.1056/NEJMoa1101549.
Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12(3):R74. doi:10.1186/cc6916.
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–77. doi:10.1056/NEJMoa010307.
Noblett SE, Snowden CP, Shenton BK, Horgan AF. Randomized clinical trial assessing the effect of Doppler-optimized fluid management on outcome after elective colorectal resection. Br J Surg. 2006;93(9):1069–76. doi:10.1002/bjs.5454.
Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242(3):326–41.
Snowden CP, Prentis JM, Anderson HL, Roberts DR, Randles D, Renton M, Manas DM. Submaximal cardiopulmonary exercise testing predicts complications and hospital length of stay in patients undergoing major elective surgery. Ann Surg. 2010;251(3):535–41. doi:10.1097/SLA.0b013e3181cf811d.
Noordzij PG, Poldermans D, Schouten O, Bax JJ, Schreiner FA, Boersma E. Postoperative mortality in The Netherlands: a population-based analysis of surgery-specific risk in adults. Anesthesiology. 2010;112(5):1105–15. doi:10.1097/ALN.-0b013e3181d5f95c.
Rhodes A, Moreno RP, Metnitz B, Hochrieser H, Bauer P, Metnitz P. Epidemiology and outcome following post-surgical admission to critical care. Intensive Care Med. 2011;37(9):1466–72. doi:10.1007/s00134-011-2299-9.
Madjdpour C, Spahn DR. Allogeneic red blood cell transfusions: efficacy, risks, alternatives and indications. Br J Anaesth. 2005;95(1):33–42. doi:10.1093/bja/aeh290.
Miranda DR, de Rijk A, Schaufeli W. Simplified Therapeutic Intervention Scoring System: the TISS-28 items–results from a multicenter study. Crit Care Med. 1996;24(1):64–73.
Dickie H, Vedio A, Dundas R, Treacher DF, Leach RM. Relationship between TISS and ICU cost. Intensive Care Med. 1998;24(10):1009–17.
Guest JF, Boyd O, Hart WM, Grounds RM, Bennett ED. A cost analysis of treatment policy of a deliberate perioperative increase in oxygen delivery in high risk surgical patients. Intensive Care Med. 1997;23(1):85–90.
Fenwick E, Wilson J, Sculpher M, Claxton K. Pre-operative optimisation employing dopexamine or adrenaline for patients undergoing major elective surgery: a cost-effectiveness analysis. Intensive Care Med. 2002;28(5):599–608. doi:10.1007/s00134-002-1257-y.
Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364(22):2128–37. doi:10.1056/NEJMsa1010705.
Lobo SM, Rezende E, Knibel MF, Silva NB, Paramo JA, Nacul FE, Mendes CL, Assuncao M, Costa RC, Grion CC, Pinto SF, Mello PM, Maia MO, Duarte PA, Gutierrez F, Silva JMJ, Lopes MR, Cordeiro JA, Mellot C. Early determinants of death due to multiple organ failure after noncardiac surgery in high-risk patients. Anesth Analg. 2011;112(4):877–83. doi:10.1213/ANE.0b013e3181e2bf8e.
Acknowledgments
The authors wish to thank Pascal Gandolfi for help in statistical analysis.
Conflict of interest
This work was supported by Edwards Lifesciences Corporation (sponsored research). All authors received honoraria for lectures from Edwards Lifesciences Corporation.
Author information
Authors and Affiliations
Corresponding author
Additional information
For commentary, please refer Niels H. Secher (doi: 10.1007/s10877-013-9462-5).
Rights and permissions
About this article
Cite this article
Scheeren, T.W.L., Wiesenack, C., Gerlach, H. et al. Goal-directed intraoperative fluid therapy guided by stroke volume and its variation in high-risk surgical patients: a prospective randomized multicentre study. J Clin Monit Comput 27, 225–233 (2013). https://doi.org/10.1007/s10877-013-9461-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-013-9461-6