Abstract
Intraoperative goal directed fluid therapy (GDT) guided by an arterial pressure-based cardiac output system has been reported to improve gastrointestinal (GI) recovery in high-risk patients. This study evaluates the impact of this approach on GI recovery in low to moderate risk patients undergoing major abdominal surgery. IRB approved randomized controlled trial in low to moderate risk adults scheduled for major surgery. Patients were randomized to standard (n = 20) or GDT (n = 18) groups, whose fluids were managed to maintain stroke volume variation (SVV) <12 %. The primary outcome measure was GI recovery. Additional measures included quality of recovery score. Continuous, non-normally distributed by Mann–Whitney test; ordinal and nominal by Chi square analysis. GDT patients had lower average intraoperative SVV. The GDT group had faster return of GI function (p = 0.004) and higher quality of recovery scores. In low to moderate risk patients undergoing major abdominal surgery, intraoperative GDT guided by SVV optimization was associated with faster restoration of GI recovery and higher quality of recovery scores. These results suggest that outcome benefits related to the use of an intraoperative goal directed fluid protocol guided by SVV are not limited to high-risk patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Background
Patients who undergo major abdominal surgery require judicious perioperative care, including optimization of fluid management. There is evidence that either too little or too much fluid administration during the perioperative period can worsen organ function [1], suggesting that the use of intraoperative goal directed fluid therapy (GDT) might improve patient outcome [2]. Individual response is variable, so patients may not respond to fluid boluses as expected [3, 4]. The variability is likely to be more pronounced in high-risk patients but it may be less prominent in low or moderate risk patients. Fixed volume strategies may be insufficient for perioperative fluid therapy in some patients [5]. Impaired tissue oxygenation events may not be revealed by heart rate, urine output, central venous pressure or blood pressure abnormalities [6]. Consequently, several authors have suggested intraoperative fluid administration may be better guided by flow related parameters [7, 8].
More recently, so called dynamic predictors of fluid responsiveness such as pulse pressure variation (PPV), stroke volume variation (SVV), or Pleth Variability Index (PVI) have been used in intraoperative GDT hemodynamic protocols [9]. These indices, which are influenced by cardiopulmonary interactions in patients under general anesthesia receiving mechanical ventilation, can accurately predict whether a patient is likely to be responsive or nonresponsive to volume expansion and indicates whether the patient is on the steep portion or on the plateau of the Franck Starling relationship [10–12]. Consequently, the concept of cardiac output maximization could be achieved using PPV or SVV minimization.
Intraoperative GDT is reported to improve outcome following surgery in high-risk patients (identified by risk index by Lee et al. [13]), by decreasing both morbidity and length of hospital stay [1, 2, 14, 15]. However, the vast majority of patients (more than 75 %) who undergo major abdominal surgery do not have multiple coexisting medical conditions [16]. As patients with fewer coexisting medical conditions may have more physiologic reserve than patients with more coexisting medical conditions, it is possible that intraoperative GDT would not contribute to improved outcomes following major abdominal surgery. We sought to assess the possible impact of intraoperative GDT on outcome following major surgery in low to moderate risk patients, who were defined as patients without preoperative diagnosis of any: coexisting ischemic heart disease; congestive heart failure; chronic lung disease; cerebrovascular disease; or renal or hepatic dysfunction (creatinine >50 % or liver enzymes >50 % of normal values).
The goal of this randomized study was to assess the impact of an intraoperative SVV guided GDT on postoperative gastrointestinal (GI) recovery as well as other postoperative recovery markers in low to moderate risk patients undergoing high risk abdominal surgery.
2 Materials and methods
This was a Departmentally sponsored, prospective, single blind randomized controlled trial approved by the Institutional Review Board for Human Studies at Loma Linda University and registered in ClinicalTrials.gov (NCT01082614). Patients scheduled for major abdominal, non-vascular surgery were assessed for eligibility. Abdominal procedures were considered major if listed for resection of urologic, gastrointestinal or gynecologic cancers with tumor debulking, staging or reconstruction with a risk for significant surgical blood loss. All surgeries were open laparotomy procedures, none were done laparoscopically. Exclusion criteria included age less than 18 years, coagulopathy, history of cerebrovascular disease, significant renal or hepatic dysfunction (creatinine >50 % or liver enzymes >50 % of normal values), history of congestive heart failure, ischemic heart disease, cardiac arrhythmias producing irregular rhythms, significant lung disease, and patient choice. Also, patients were excluded if they developed intraoperative arrhythmias of more than 4 non-sinus beats within a minute for a period of at least 5 min. Patients were excluded if they developed a condition requiring a second surgical procedure prior to return of GI function, since the urgent nature of the second surgery would interfere with interpretation of the impact of fluid management during the first surgery. Patient characteristics, current diagnosis, and a measure of physiologic and surgical risk (Portsmouth physiologic and operative severity score for the enumeration of mortality and morbidity score: P-POSSUM [17–19]) were collected.
A computerized algorithm was used to randomly assign patients to GDT or Control groups (www.randomization.com). Surgical teams, intraoperative and postoperative nursing staff and patients were blinded to group assignment, but the anesthesiologist was aware of group designation. All patients had routine intraoperative monitoring per American Society of Anesthesiologists Guidelines. All patients were ventilated at 8 mL/kg of ideal body weight and their respiratory rate was adjusted such that they had minute ventilation of approximately 7–8 L/min with an I:E ratio of 1:2. Radial arterial catheters were placed in all patients because of the risk for significant surgical blood loss, as is our practice for patients undergoing the surgical procedures performed in these subjects. The arterial catheters were connected to the cardiac output device transducer system and SVV data was collected throughout surgery in all patients. All transducers were zeroed to atmospheric pressure at the level of the right atrium. For this study the FloTrac/Vigileo system was used (FloTrac/Vigileo Version 3.02, Edwards Lifesciences, Irvine, CA, USA). This is an autocalibrated device that analyzes the arterial pressure waveform to calculate cardiac output, stroke volume and SVV.
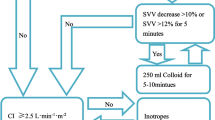
The fluid management protocol used is shown in Fig. 1. Control patients had fluid management guided by routine cardiovascular monitoring at the discretion of their Staff Anesthesiologist, who was blinded to SVV data. Control group anesthesiology teams were allowed to have the SVV information unblinded if needed for clinical decision-making but patients were removed from analysis if this occurred. In contrast, GDT patients were managed by an SVV guided protocol to maintain SVV <12 %. This percentage of SVV has previously been validated as a threshold above which fluid administration increases stroke volume [20], with an area under the receiver operating characteristics curve reported at 0.87 [11]. For the GDT group, if SVV remained above 12 % for at least 2 min, then a 250 mL bolus of 5 % albumin was given. SVV was assessed every 20 s via the FloTrac/Vigileo proprietary algorithm. However, our study protocol required that all treatment interventions were done after SVV values remained over 12 % for 2 min. Albumin was infused per protocol to a total of 20 mL/kg, based on the package insert recommendation of a dose of 1 g per kg (the 5 % albumin used in this study has a concentration of 1 gm per 20 mL). If additional fluid beyond the total allowed albumin dose was required then crystalloid was administered at a ratio of 3:1 for replacement of estimated surgical blood loss. Similar to other GDT studies, colloid, specifically albumin, was used for this algorithm secondary to the known effects of improved intravascular repletion and intravascular half life and the theoretical benefit of prolonged stroke volume optimization. In both groups, the administration of blood products was at the discretion of the anesthesiology team as clinically indicated. There was no standard protocol for crystalloid maintenance infusion for either group. The anesthesiologist and research team had no influence over postoperative care management. Physicians in charge of postoperative care were blinded to the allocation of patients.
The primary outcome measure was GI recovery defined as the time between the end of the surgery and the first bowel movement (assessed by morning and evening RN interview) and postoperative day (POD) soft diet was tolerated (defined by >50 % consumption of breakfast and lunch or lunch and dinner). Secondary outcome measures included postoperative hospital length of stay and quality of recovery score. The quality of recover score was calculated based on a previously validated questionnaire (QoR Score-“Appendix”) [21] on postoperative day 2 and day 4. Change in hemoglobin concentration was calculated based on the difference between preoperative hemoglobin and hemoglobin on postoperative day 1. Intraoperative volumes of crystalloid and colloid administered were compared. Postoperative fluid management was left up to the surgical team and was not regulated or recorded. In addition, the total amount of morphine equivalent analgesia was compared through postoperative day 2 for all patients who did not receive epidural analgesia. Two of the control patients and three of the treatment patients did receive epidurals. These patients postoperative pain control were controlled by the anesthesiology pain service and their postoperative narcotic requirements were not included in the analysis of the study (Table 3). The surgical teams were blinded to the patient’s randomization.
2.1 Statistical analysis
Sample size for the study was calculated based on prior studies using GDT therapy guided by esophageal Doppler [14]. Specifically, we used the data from prior studies [22, 23] to estimate our sample size. In order to detect a 3-day mean length of stay difference [23] between the two groups, with a standard deviation of 3 days in each group [22], a two tailed alpha of 0.05 and power of 0.80, we calculated that a minimum of 17 subjects were required in each group. We assumed that a similar sample size would be needed to detect a similar difference in time to return of GI function. Also, since we expected similar results between arterial pressure waveform analysis technology used for this study and the esophageal Doppler technology used for these referenced studies we felt that this estimation was appropriate.
Statistical analysis was performed using computerized software (SPSS for Windows version 12.0). For data that was non-normally distributed a Mann–Whitney test was used and normally distributed data were compared using the Student T test. Ordinal and nominal data were compared using Chi square analysis. A p value ≤0.05 was considered significant.
3 Results
3.1 Patient inclusion and demographics
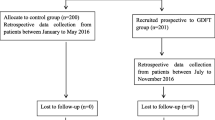
A total of 46 patients were consented for the study. Of these, eight patients were not included in analysis. Two GDT patients developed an irregular cardiac arrhythmia during surgery that interfered with SVV calculation and thus prevented use of SVV to guide fluid therapy. In one GDT patient, equipment malfunction during surgery prevented use of SVV to guide fluid therapy. One Control patient was excluded since the surgical procedure did not meet the definition for inclusion in this study as the surgery did not proceed as scheduled but was terminated following diagnostic laparoscopy. Another Control patient was not included as the anesthesiologist requested unblinding of SVV data during surgery to guide clinical management. Three patients (2 GDT, 1 Control) were excluded for developing conditions that required second look surgery prior to return of GI function during the same hospital stay. Thus 38 patients were included in final data analysis (18 GDT and 20 Control; CONSORT diagram, Fig. 2).
Table 1 summarizes demographic and surgical characteristics of the patients. There were no statistically significant intergroup differences in physiologic or operative P-POSSUM scores, body mass index, or gender. The Control group was older than the GDT group. Surgical procedures were for resection of various urologic, gastrointestinal or gynecologic cancers, with similar distribution of surgical procedures in the groups, as summarized in Table 2.
3.2 Intraoperative data
Perioperative characteristics are shown in Table 3. There were no statistically significant intergroup differences in colloid, crystalloid, or PRBC administration. Also, there were no statistically significant intergroup differences in urine output, surgery time, or change in hemoglobin concentration. However, the GDT group did receive albumin boluses (13/18) more frequently than the control group (8/20) as would be expected from the fluid administration protocol (p = 0.003). Prokinetic agents were given after surgery to 3 GDT and 5 Control patients (p = 0.40). Also, there was no statistically significant difference in opioid use through postoperative day 2 between groups (p = 0.32). Secondary analysis showed no significant difference in LOS between patients that received colloid (n = 21, LOS = 7.81 days) and those that did not (n = 17, LOS = 8.3; p > 0.05) regardless of study group.
Retrospective analysis of SVV at 5-min intervals showed successful application of the fluid administration protocol in the GDT group during the surgical procedure. Average SVV was similarly elevated at baseline (Control 18.6 ± 5.7 %, GDT 19.4 ± 3.0 %; p = 0.30 Mann–Whitney test). Figure 3 shows mean SVV values along with standard error of the mean (SEM) error bars for the first 200 min of intraoperative time. The average SVV decreased following initiation of the fluid protocol in GDT patients, and remained below 12 % from surgical incision to closing. The average SVV in Control patients remained higher, with more readings between 12 and 20 % than found in GDT patients. We observed more frequent early optimization of SVV (less than 12 %) with 77 % of the GDT group achieving this within the first 10 min of arterial line monitoring compared to 19 % in the Control group, p = 0.001 by Chi square. Interestingly, the control group took approximately 60 min for the average SVV to go below 12 %, while this occurred within the first 10 min in the treatment group.
Average intraoperative stroke volume variation. SVV average at 5-min intervals following surgical incision, shown as average with standard error of the mean (SEM) error bars. Comparison shows higher average SVV and more readings between 12 and 20 % in control patients compared to intraoperative maintenance of SVV <12 % in GDT patients
3.3 Outcome data
Table 4 summarizes outcome characteristics between groups. The GDT group had earlier return of GI function (3 vs. 4 days, p = 0.004) and PO intake (4 vs. 5 days, p = 0.004) compared to the Control group. There was a trend for median duration of hospital stay to be shorter in the GDT group (5 days) compared to the control group (7.5 days, p = 0.04). In addition, the GDT group had significantly higher quality of recovery score on postoperative day 2 (p = 0.05) and 4 (p = 0.03).
4 Discussion
This study shows that intraoperative GDT based on SVV minimization may improve gastrointestinal function in low to moderate risk patients undergoing major surgery. This approach, in this specific group of patients, may have the ability to significantly impact postoperative outcome and length of stay in the hospital. Interestingly, we found that despite a similar amount of fluid received in the control group and in the GDT group, SVV was lower in the GDT compared to the control group. Moreover, we observed an earlier optimization of SVV in the GDT group than in the control group, suggesting the importance of timing on the impact of hemodynamic optimization.
The use of flow-related parameters to guide intraoperative goal-directed fluid therapy has appeal as these parameters provide a numeric representation of the patient’s volume status, which can be difficult to ascertain using standard monitors, urine output or even CVP [3, 4, 6]. Intraoperative GDT guided by various monitors of flow-related parameters has shown benefit in a number of clinical settings. Importantly there may be a long-term survival benefit for high-risk surgical patients who are managed with intraoperative GDT [24].
Previously published studies have shown decreased complications and hospital length of stay in high-risk patients undergoing major abdominal surgery with SVV guided GDT therapy [25, 26]. In addition, Cecconi et al. [27] recently showed a decrease in postoperative complications in non-high risk surgical patients undergoing elective total hip arthroplasty under regional anesthesia. Major abdominal surgery is often performed in patients who are not high-risk from their comorbidities [16]. Our patients’ P-POSSUM mortality risk falls in the expected range of low to moderate risk with a mean predicted mortality rate of 1.4 %. Our patients showed similar statistically significant improvements in return of GI function, PO intake, and quality of recovery score as previously reported for high-risk patients undergoing similar types of surgery. In addition, our data showed a decreased length of stay in the treatment group compared to the control, which although limited by small sample size, supports the potential benefit of SVV guided GDT therapy for low to moderate risk patients undergoing high risk surgery. However, we do not claim that the use of SVV is only modality that may improve postoperative morbidity in these low to moderate risk patients. Rather this study suggests that the use of a dynamic parameter, such as SVV, to provide structured GDT therapy may be beneficial for patients of low to moderate risk undergoing major abdominal surgery. However, in practices in which monitoring of patients undergoing major abdominal surgery does not routinely include arterial catheterization, other dynamic parameters such as pulse pressure ventilation (PPV) or pleth variability index (PVI) may provide equally useful information. Further research would be reasonable to assess this potential.
Our findings appear to be related to the maintenance of SVV below 12 % resulting from the SVV guided GDT protocol. While initial SVV was similarly elevated (>18 %) in all patients, Control patients had average SVV above the SVV threshold used in the GDT group until nearly the end of surgery. In contrast, GDT patients had SVV decreased and largely maintained below the 12 % threshold for the duration of surgery. This implies that Control patients had a relative intravascular volume deficit during a larger portion of their surgical procedure than GDT patients. Also GI recovery and hospital length of stay was not correlated to administration of colloid when analyzed separately from the GDT protocol. Therefore, it is possible that GDT benefits are secondary to appropriate timing of fluid administration, guided by SVV, which may provide improved intraoperative tissue oxygen delivery in patients undergoing major abdominal surgery, which could then impact postoperative recovery. We suggest that the difference in SVV is secondary to non-standardization of fluid management in the control group. The SVV protocol alerted the clinician when intravascular repletion could improve oxygen delivery, potentially providing more effective timing of fluid administration. This improved standardization of fluid administration was not present in the control group as seen by the longer duration of SVV above 12 %. It is possible that fluid loading at the beginning of the surgery may decrease SVV throughout the procedure secondary to a rightward shift of the Frank-Starling curve and a decrease in unstressed volume. Finally, 8 of 20 control patients received colloid boluses compared to 13 of the 18 in the GDT group. While there was no intergroup difference in net amount of fluid administration, the frequency of the colloid boluses was different, but this study was not designed to assess the relative impact of colloid or crystalloid volume replacement. In summary, this observation seems to emphasize the importance of timing in hemodynamic optimization and the early implementation of GDT strategies.
Recently Challand et al. [28] published results suggesting that SV maximization in aerobically fit patients undergoing major colorectal elective surgery was associated with an increased length of stay. While this appears to be in direct opposition to the findings in the study we believe the difference lies in the method of GDT therapy. Specifically Challand et al. used SV maximization in which boluses of colloid were given if SV increased by 10 % while we used SV optimization in which we only gave a bolus of colloid when the patient manifested a SVV difference of 12 %. In other words Challand’s algorithm was proactive with SV maximization while we were reactive with SVV optimization. In patients with normal cardiac function the ability to increase SV above the point of a euvolemic state may exist. This is supported by the fact that Challand showed a significant increase in the amount of colloid administered to the GDT group versus our study, which showed no difference in net amounts of colloid or crystalloid. Thus we suggest that by using SVV optimization in which we titrated for reduction in SVV to below 12 % we kept our patients at the precipice of their Frank Starling curve continuously throughout the surgery without increasing the SV past the point in which the complications of hypervolemia may manifest. Therefore, the choice to design our study as an SVV minimization strategy versus cardiac output optimization, which has been supported in the literature [29] was based on the fact that our patient population had normal cardiac outputs and therefore we expected SVV to be a more sensitive indicator for decreased end organ oxygenation delivery.
Intraoperative fluid GDT guided by esophageal Doppler technology is reported to improve GI recovery [14]. Esophageal Doppler has limitations related to probe positioning and the possible need for probe manipulation during the surgical procedure. Similarly, postoperative GDT guided by an arterial lithium indicator dilution cardiac output system decreased length of stay and complication rate in high-risk general surgery patients [30]. Similar results have been reported in high-risk surgery patients with intraoperative GDT therapy guided by an automated arterial pulse pressure calculation [31] and arterial pressure cardiac output systems [26]. The advantages of the device used in this study are that it is less invasive than those used for thermodilution, is not subject to changes induced by probe movement and requires no calibration.
Limitations of this study include known limitations of the arterial pressure cardiac output device, especially the inaccurate determination of SVV in the presence of irregular cardiac rhythms, which thus excludes a subset of patients. Based on this point, it is possible that the monitoring of sudden unexpected alterations in SVV along with the alarm that this triggers on the device used in the study did cause the providers in the treatment to be more vigilant in the detection of arrhythmias. In addition, bolus vasoactive medication administration has been reported to affect the accuracy of this arterial pressure cardiac output method [32], and was not prohibited by the study protocol. However, bolus vasoactive medication administration was rarely used in any patient, and no patient was placed on continuous intravenous infusion of vasoactive agents during this study. In addition, the sample size of this study is less than other randomized GDT studies and was calculated based on esophageal Doppler studies that used a different primary outcome. However, it is likely that hospital discharge depends on return of GI function, and thus a similar sample size would be needed to detect a similar difference in time to return of GI function. Thus we feel that our results for improved GI recovery are strong enough (p = 0.004) to warrant further research in this area. Another potential confounder is the age difference between Control patients and GDT patients. However, the study was designed to assess differences in baseline demographics using the P-POSSUM operative morbidity score, which does include age in its algorithm. P-POSSUM scores have good predictive value for postoperative mortality [33], and was found to better discriminate postoperative mortality than the Lee index [16] as well as less subjectivity compared to ASA physical status [18, 34–36]. In addition, further analysis of our data showed that there was one patient in the treatment group that was 18 years of age (13 years younger then any other patient) and if this patient was removed there is no difference in age between the groups. Also, this patient’s hospital stay and time of GI recovery was longer then the median for the treatment group. Therefore, if we removed this patient from the study it would have improved our results and supports the concept that the P-POSSUM scores correctly depicted that there were no differences in predicated operative morbidity between the two groups. Also, postoperative care was at the discretion of the surgical teams, no attempt was made to standardize pain control therapy. On average GDT patients had return of GI function on postoperative day 3. Since no intergroup differences were found in morphine equivalent administration through postoperative day 2, it is unlikely that the later return of GI function in Control patients was related to opioid administration. Also, fluid administration was managed by the anesthesia care providers who were aware of patient’s randomization. However, all providers were instructed to administer crystalloid and blood products as they would regularly. The difference for the treatment group is that they were asked to follow the SVV guided protocol, in addition, to their standard practice. This is likely why there was not a difference between total colloid or crystalloid administered. In essence, the goal of this study was to examine if the simply addition of a SVV guided colloid administration protocol to standard anesthetic management of these cases would impact postoperative outcomes. Finally, we were only able to blind the Staff Anesthesiologists caring for patients in the study to SVV data but not the arterial pressure waveform. Visual analysis of the arterial pressure waveform for pulse pressure variability can be used by experienced anesthesiologists as an indicator of fluid responsiveness. However, as SVV remained above the 12 % threshold for much longer in Control patients (Fig. 3), it appears that visual inspection of the arterial waveform is not as accurate a guide to fluid responsiveness as the autocalibrated system used in GDT patients.
5 Conclusion
Use of an intraoperative GDT fluid management strategy in low to moderate risk patients undergoing major abdominal surgery was associated with faster restoration of GI function, faster return of PO intake and higher quality of recovery scores. These results suggest that outcome benefits related to the use of an intraoperative goal directed fluid protocol guided by an autocalibrated arterial pressure cardiac output system may not be limited to high-risk patients.
Abbreviations
- GDT:
-
Goal directed fluid therapy
- SVV:
-
Stroke volume variation
- P-POSSUM:
-
Portsmouth physiologic and operative severity score for the enumeration of mortality and morbidity score
- ASA:
-
American Society of Anesthesiologists
- LOS:
-
Length of hospital stay
- POD:
-
Postoperative day
References
Bundgaard-Nielsen M, Holte K, Secher NH, Kehlet H. Monitoring of peri-operative fluid administration by individualized goal-directed therapy. Acta Anaesthesiol Scand. 2007;51(3):331–40.
Rahbari NN, Zimmermann JB, Schmidt T, Koch M, Weigand MA, Weitz J. Meta-analysis of standard, restrictive and supplemental fluid administration in colorectal surgery. Br J Surg. 2009;96(4):331–41.
Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134(1):172–8.
Gelman S. Venous function and central venous pressure: a physiologic story. Anesthesiology. 2008;108(4):735–48.
Bundgaard-Nielsen M, Secher NH, Kehlet H. ‘Liberal’ vs. ‘restrictive’ perioperative fluid therapy—a critical assessment of the evidence. Acta Anaesthesiol Scand. 2009;53(7):843–51.
Howell MD, Donnino M, Clardy P, Talmor D, Shapiro NI. Occult hypoperfusion and mortality in patients with suspected infection. Intensive Care Med. 2007;33(11):1892–9.
Benes J, Chytra I, Altmann P, Hluchy M, Kasal E, Svitak R, Pradl R, Stepan M. Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: results of prospective randomized study. Crit Care. 2010;14(3):R118.
Forget P, Lois F, de Kock M. Goal-directed fluid management based on the pulse oximeter-derived pleth variability index reduces lactate levels and improves fluid management. Anesth Analg. 2010;111(4):910–4.
Bendjelid K, Romand JA. Fluid responsiveness in mechanically ventilated patients: a review of indices used in intensive care. Intensive Care Med. 2003;29(3):352–60.
Cannesson M. Arterial pressure variation and goal-directed fluid therapy. J Cardiothorac Vasc Anesth. 2010;24(3):487–97.
Cannesson M, Musard H, Desebbe O, Boucau C, Simon R, Henaine R, Lehot JJ. The ability of stroke volume variations obtained with Vigileo/FloTrac system to monitor fluid responsiveness in mechanically ventilated patients. Anesth Analg. 2009;108(2):513–7.
Michard F. Changes in arterial pressure during mechanical ventilation. Anesthesiology. 2005;103(2):419–28. (quiz 449–415).
Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, Sugarbaker DJ, Donaldson MC, Poss R, Ho KK, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043–9.
Abbas SM, Hill AG. Systematic review of the literature for the use of oesophageal Doppler monitor for fluid replacement in major abdominal surgery. Anaesthesia. 2008;63(1):44–51.
Giglio MT, Marucci M, Testini M, Brienza N. Goal-directed haemodynamic therapy and gastrointestinal complications in major surgery: a meta-analysis of randomized controlled trials. Br J Anaesth. 2009;103(5):637–46.
Noordzij PG, Poldermans D, Schouten O, Bax JJ, Schreiner FA, Boersma E. Postoperative mortality in The Netherlands: a population-based analysis of surgery-specific risk in adults. Anesthesiology. 2010;112(5):1105–15.
Copeland GP, Jones D, Wilcox A, Harris PL. Comparative vascular audit using the POSSUM scoring system. Ann R Coll Surg Engl. 1993;75(3):175–7.
Copeland GP. The POSSUM system of surgical audit. Arch Surg. 2002;137(1):15–9.
Prytherch DR, Whiteley MS, Higgins B, Weaver PC, Prout WG, Powell SJ. POSSUM and Portsmouth POSSUM for predicting mortality. Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity. Br J Surg. 1998;85(9):1217–20.
Derichard A, Robin E, Tavernier B, Costecalde M, Fleyfel M, Onimus J, Lebuffe G, Chambon JP, Vallet B. Automated pulse pressure and stroke volume variations from radial artery: evaluation during major abdominal surgery. Br J Anaesth. 2009;103(5):678–84.
Myles PS, Hunt JO, Nightingale CE, Fletcher H, Beh T, Tanil D, Nagy A, Rubinstein A, Ponsford JL. Development and psychometric testing of a quality of recovery score after general anesthesia and surgery in adults. Anesth Analg. 1999;88(1):83–90.
Gan TJ, Soppitt A, Maroof M, el-Moalem H, Robertson KM, Moretti E, Dwane P, Glass PS. Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology. 2002;97(4):820–6.
Noblett SE, Snowden CP, Shenton BK, Horgan AF. Randomized clinical trial assessing the effect of Doppler-optimized fluid management on outcome after elective colorectal resection. Br J Surg. 2006;93(9):1069–76.
Rhodes A, Cecconi M, Hamilton M, Poloniecki J, Woods J, Boyd O, Bennett D, Grounds RM. Goal-directed therapy in high-risk surgical patients: a 15-year follow-up study. Intensive Care Med. 2010;36(8):1327–32.
Lees N, Hamilton M, Rhodes A. Clinical review: Goal-directed therapy in high risk surgical patients. Crit Care. 2009;13(5):231.
Mayer J, Boldt J, Mengistu AM, Rohm KD, Suttner S. Goal-directed intraoperative therapy based on autocalibrated arterial pressure waveform analysis reduces hospital stay in high-risk surgical patients: a randomized, controlled trial. Crit Care. 2010;14(1):R18.
Cecconi M, Fasano N, Langiano N, Divella M, Costa MG, Rhodes A, Della Rocca G. Goal-directed haemodynamic therapy during elective total hip arthroplasty under regional anaesthesia. Crit Care. 2011;15(3):R132.
Challand C, Struthers R, Sneyd JR, Erasmus PD, Mellor N, Hosie KB, Minto G. Randomized controlled trial of intraoperative goal-directed fluid therapy in aerobically fit and unfit patients having major colorectal surgery. Br J Anaesth. 2012;108(1):53–62.
Hamilton MA, Cecconi M, Rhodes A. A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg. 2011;112(6):1392–402.
Pearse R, Dawson D, Fawcett J, Rhodes A, Grounds RM, Bennett ED. Early goal-directed therapy after major surgery reduces complications and duration of hospital stay. A randomised, controlled trial [ISRCTN38797445]. Crit Care. 2005;9(6):R687–93.
Lopes MR, Oliveira MA, Pereira VO, Lemos IP, Auler JO Jr, Michard F. Goal-directed fluid management based on pulse pressure variation monitoring during high-risk surgery: a pilot randomized controlled trial. Crit Care. 2007;11(5):R100.
Eleftheriadis S, Galatoudis Z, Didilis V, Bougioukas I, Schon J, Heinze H, Berger KU, Heringlake M. Variations in arterial blood pressure are associated with parallel changes in FlowTrac/Vigileo-derived cardiac output measurements: a prospective comparison study. Crit Care. 2009;13(6):R179.
Donati A, Ruzzi M, Adrario E, Pelaia P, Coluzzi F, Gabbanelli V, Pietropaoli P. A new and feasible model for predicting operative risk. Br J Anaesth. 2004;93(3):393–9.
Chandra A, Mangam S, Marzouk D. A review of risk scoring systems utilised in patients undergoing gastrointestinal surgery. J Gastrointest Surg. 2009;13(8):1529–38.
Luna A, Rebasa P, Navarro S, Montmany S, Coroleu D, Cabrol J, Colomer O. An evaluation of morbidity and mortality in oncologic gastric surgery with the application of POSSUM, P-POSSUM, and O-POSSUM. World J Surg. 2009;33(9):1889–94.
Ptok H, Marusch F, Schmidt U, Gastinger I, Wenisch HJ, Lippert H. Risk adjustment as basis for rational benchmarking: the example of colon carcinoma. World J Surg. 2010;35:196–205.
Acknowledgments
This study was supported by the Department of Anesthesiology, Loma Linda University School of Medicine, Loma Linda, CA, USA.
Conflict of interest
D.R. received speaking fees from Edwards Lifesciences, Irvine, CA, USA. M.C. is consultant and/or speakers for Edwards Lifesciences, Covidien, Masimo, Fresnius Kabi, ConMed, and Bmeye. R.A. currently principal investigator for ongoing study for which the Department of Anesthesiology, Loma Linda University School of Medicine is sponsored by Edwards Lifesciences, Irvine, CA, USA.
Author information
Authors and Affiliations
Corresponding author
Appendix
Rights and permissions
About this article
Cite this article
Ramsingh, D.S., Sanghvi, C., Gamboa, J. et al. Outcome impact of goal directed fluid therapy during high risk abdominal surgery in low to moderate risk patients: a randomized controlled trial. J Clin Monit Comput 27, 249–257 (2013). https://doi.org/10.1007/s10877-012-9422-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-012-9422-5