Abstract
Considering the exclusive environmental conditions and geological characteristics, Indian flora is extensive and rich in medicinal plants. From primeval times, plant parts and their metabolites have been widely explored for various practices including medicinal as well as culinary. The phytochemicals present in these plants are potential reducing agents for the bio-fabrication of these nanoparticles. The non-toxic nature and combination of the plant phytochemicals with precursor ions act as key aspects for synthesized nanoparticles. The present review highlights the potential applications of Inorganic nanoparticles synthesized from 148 traditionally used medicinal plants present in the Indian geographical region. In addition, parameters that influence the green synthesis of Inorganic nanoparticles such as the extraction methods, solvents used for extraction, the concentration of precursor and plant phytochemicals, pH, temperature, reaction time, and characterization techniques of the nanoparticles are discussed. Thus, the review provides information on the research that has been done in the area of green synthesis using Indian medicinal plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
India is known to have rich biodiversity, with more than 8000 species of medicinal plants. The Himalayas and Tropical forests of the Western Ghats are the hotspots of traditional medicinal plants. About 1800 species of medicinal plants are extensively available for their use in traditional healing practices such as Ayurveda, Siddha, Tibetan, and Unani [1]. Extensive information about the properties and uses of these plants has been well documented in ancient Indian monumental works like Charaka Samhita and Susruta Samhita. The climatic conditions and geographical location significantly support the maintenance of these medicinal plants and thus help in the sustenance of the same in traditional medications. Fruit, roots, leaves, bark, and sometimes whole plants are used in the preparation of traditional medicines [2, 3]. Studies have revealed the abundance of vast varieties of phytochemicals like phenolic acids, flavonoids, etc. in these medicinal plant species which is the major factor that makes use of these medicinal plants in different medicinal practices [4].
Recent researches try to explore the practicability of the utilization of phytochemicals in the field of nanotechnology as a result of advancements in the field of medicinal research. Nano in Greek means "dwarf", but nano is infinitely smaller than a dwarf. The nanoworld deals with tiny objects which are nanometric (10–9 m) in size at least in one dimension. Nanoparticles (NPs) have a maximum size of 100 nm. These particles exist in the nanometer range. The nanometer dimension gives them their unique properties. The newly synthesized NPs can have variations in their size, shape, and even in their distribution [5]. The science of nanomaterials deals with their generation and the properties exhibited by them because of their small size. The subject of nanoscience has gained great importance because of its promising applications in various areas such as the chemical and textile industry, material industry, medical diagnostics, drug delivery, and electronics [6].
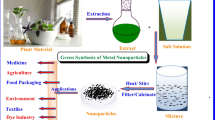
Diverse methods for the fabrication of NPs are getting popularized. Nanofabrication by chemical and physical synthesis methods utilizes unsafe chemicals and requires high energy utilization, which affects the environmental conditions. The bio-fabrication techniques of NPs synthesis include the practice of using biological agents including carbohydrate sources, plant extracts, and microorganisms as reducing and capping agents [7]. Those methods which utilize plants for NP synthesis have supremacy over other synthesis methods as they are uncomplicated, one-step, worthwhile, ecological, and reproducible. Often results are in more safe and steady materials. Microorganisms are also utilized to synthesize NPs, but at the same time, the rate of NP synthesis is slow in comparison with green synthesis, due to long incubation periods [8]. The plant-mediated green synthesis is obtaining more attention, as these processes are flexible, in-expensive and non-toxic than other methods. The phytochemicals present in the plant extracts act as both reducing and capping agents in the NPs synthesis [9]. The authors compile and summarize the current knowledge about the use of phytochemicals from Indian medicinal plants in the synthesis of NPs, optimization parameters, characterization methods, and its applications.
Nanoparticle Synthesis
General Mechanism of NP Synthesis
Top-down and bottom-up approaches are two types of NPs synthesis. The top-down approach uses macroscopic particles as the starting blocks of the synthesis procedure. In these methods, bigger-sized particles are reduced to small-sized NPs through a series of reactions like grinding of bulk materials to smaller-sized particles and further to nano-sized particles. Physical and Chemical vapor deposition, Ion implantation, Electron beam, and X-ray lithography utilize the top-down approach [10]. In bottom-up methods, NPs are synthesized from molecules at the atomic level and these units are clustered to get stable nanostructures. Sol–gel, Colloidal precipitation, Hydrothermal, Organometallic chemical, and electro-deposition utilize the bottom-up approaches [11].
NP synthesis methods are classified into physical, chemical, and biological. Physical methods require high energy and are suitable for small-scale purposes only. Chemical synthesis methods involve the usage of chemicals that may produce toxic byproducts damaging the environment. Biological methods explore the use of plant materials and microorganisms for synthesis [10]. Biological methods of synthesis are more advantageous over other methods as they are economical, nontoxic, low energy consumption, less consuming, and easily scalable as the raw materials are easily available in the environment. These methods are highly suitable for biological applications and in vivo applications such as drug delivery and can be used as bioactive agents in biological reactions [12]. Biological methods that utilize plant parts are collectively called green synthesis methods. Green synthesis utilizes phytochemicals, like flavonoids, phenolic acids, terpenoids, proteins, organic acids, and alkaloids as reducing and capping agents [13].
Green Synthesis of NPs from Indian Medicinal Plants
The huge number of phytochemicals present in medicinal plants make them potential agents for the green synthesis of NPs. Studies report the extensive utilization of various Indian medicinal plants as reducing agents in the reduction of various kinds of metallic, metal oxide, bimetallic as well doped NPs. Commonly available medicinal plants such as Azadirachta indica, Aloe vera, and Phyllanthus emblica are comprehensively practiced in the biosynthesis of NPs [14, 15, 16]. Studies have reported the reducing potential of Acalypha indica plant extract during the synthesis of Silver, Copper oxide, and Tin oxide NPs. FTIR studies of the plant extracts support the influence of phytochemicals on the bio-reduction process as well as the properties of the synthesized NPs [17, 18, 19, 20]. Ag NPs have been reported using the plant extracts of Aloe Vera, Andrographis paniculata, and Annona muricata [14, 21, 22, 23]. All these plants are potential medicinal plants known for their potential pharmaceutical properties. Various reports have proven the potential of different medicinal plants as reducing and capping agents in the synthesis of different NPs. (Table 1).
Types of Nanoparticles
Based on the chemical composition, NPs are mainly categorized as organic, inorganic, and carbon-based NPs. Organic NPs include liposomes, micelles, ferritin, dendrimers, etc., and these are widely used in biological systems mainly for drug delivery purposes as they are efficient in targeted drug delivery. Most plant-based NPs are inorganic. This category includes metal NPs, metal oxide NPs, bimetallic NPs, and doped NPs. Carbon-based NPs include different nanomaterials of various shapes and sizes. Fullerenes, graphene, carbon nanotubes, carbon nanofibers, and carbon black are examples of carbon-based NPs (Table 1) [24].
Metal NPs
Silver (Ag), Gold (Au), Zinc (Zn), Copper (Cu), Cobalt (Co), Aluminium (Al), Iron (Fe), Cadmium (Cd), Lead (Pb), Selenium (Se) are generally used as precursors for metal NPs synthesis. During the green synthesis of metallic NPs, the crude extract phytochemicals act as reducing agents in the bio-reduction of metal ions. The successful synthesis of Ag NPs was reported using leaf extracts of Ocimum gratissimum and Ocimum sanctum [20]. Spherical-shaped Se NPs were synthesized using Withania somnifera plant leaves extract. The synthesized NPs were reported to have potential biological applications such as antimicrobial and photocatalytic activities [25]. Numerous studies have reported the green synthesis of metal NPs to possess potential biological activities.
Metal Oxide NPs
Metals react with atmospheric oxygen to produce metal oxides and show more reactivity than metals. Metal oxide NPs are generally modifications of metal NPs. These oxides of NPs possess good biological and catalytic activities that can be potentially used in environmental applications [17]. Cerium oxide (CeO2), Aluminium oxide (Al2O3), Iron oxide (Fe2O3), Silicon oxide (SiO2), Zinc oxide (ZnO), Copper oxide (CuO), and Titanium oxide (TiO2) are generally synthesized metal oxide NPs.
Bimetallic NPs
Combinations of different metals are also used in the synthesis of NPs. Different metal solutions are mixed using a bio-reducing agent like plant extracts for the formation of bimetallic NPs. Plant extracts of ginger rhizomes have shown efficient bio-reducing activity in the synthesis of three bimetallic Cu–Ag, Cu–Ni, and Ni–Ag NPs [26]. Combinations of metals like Au–Cu, Ni–Cu, Ag–Ni, and ZnO–Ag have generally been used for NP preparations. The combinations of these metals show synergistic effects in combined nanostructure form.
Doped NPs
The improved efficiency of doped semiconductor NPs has been widely studied by recent research developments. Combinations of organic nanostructures such as polymeric NPs, Carbon-based NPs as well as inorganic NPs are intensively applied for formulating doped NPs. Carbon-doped ZnO NPs have reported the construction of wurtzite crystal-structured NPs with improved magnetic properties [27]. Nitrogen-doped TiO2 NPs with increased photocatalytic activity were synthesized by the thermal deposition method [28]. Metal doped, as well as organic material-doped NPs, have been extensively studied for their increased biological, thermal, catalytic, magnetic, and other optical properties [28, 29, 30].
Optimization of Green Synthesis
The bio-construction of metallic NPs takes place by reducing metal ions by the phytochemicals existing in plant extracts. This bio-reduction process can be considered the initial step of the NP synthesis process. After the initialization of the green synthesis, phytochemicals also act as crucial agents in stabilizing and regulating the morphological characteristics of the synthesizing NPs [31
Extraction Methods and the Solvents Used
The extraction methods for crude extract preparation are crucial for the quality of plant phytochemicals extracted. Commonly used method for extraction involves boiling, Soxhlet extraction, reflux extraction, and maceration among others. Boiling is a simple method that is used in the extraction of crude extracts using plant materials. However, the chances of phytochemical loss during this extraction are high. Maceration is a conventional extraction method that uses smaller-sized plant material followed by the application of pressurized conditions with subsequent filtration of the plant extract. Soxhlet extraction is a widely used extraction technique in the extraction of bioactive compounds from plant materials. [33]. Advanced extraction techniques such as Ultrasound-assisted extraction, Pulsed-electric field extraction, Enzyme-assisted extraction, Microwave-assisted extraction, Pressurized liquid extraction, and Supercritical fluid extraction can also be used for the extraction of bioactive phytochemicals [33, 34].
The solvents play an important role in the optimization of green synthesis. As the number of phytochemicals is a leading factor in the green synthesis efficiency the maximum number of phytochemicals acquired during the extraction will increase the optimization. Methanol, ethanol, and water are good solvents that can be used for the extraction of the maximum number of bioactive molecules. Other solvents such as chloroform, dichloro-methanol, ether, and acetone are also used for the extraction procedure [33]. Emblica officinalis fruit extract was obtained through the boiling method and subsequent filtration process. The study has reported the formation and efficient production of Ag NPs using the resulting aqueous fruit extract [35]. In another study, methanolic extract of Diospyros paniculata root was obtained through the soxhlet extraction technique for the bioreduction of Ag ions to Ag NPs [36]. Table 1 mentions the different extraction methods and solvents used for extraction from different plants.
Precursor Concentration
Various precursors are utilized for NPs synthesis. Ag NO3 is the extensively used precursor for the synthesis of Ag NPs. In the green synthesis of Ag NPs, the frequently reported metal ion concentration was 1 mM. Also, other concentrations such as 1, 2, 3, 4, 5, 8, 10, and 50 mM are used by researchers [18, 37, 38, 39, 40, 41, 42, 43, 44]. It is observed that higher metal ions concentrations help in the reduction of reaction time. Researchers have used 1, 5, and 10 mM Ag NO3 solutions for the bio-synthesis of Ag NPs using mangrove plant extracts. Among these concentrations of AgNO3 solutions, higher concentrations have produced Ag NPs in lesser time [45]. Studies have proved that changing concentrations of Ag ions during the green synthesis of Ag NPs significantly influence the morphological characteristics of the resulting NPs. Similarly, Chloroauric acid (HAuCl4) is used as the precursor for the green synthesis of Au NPs [46]. (Cu(NO3)2), for CuO NPs, Zn(NO3)2 6H2O for ZnO NPs, SnCl2.2H2O for SnO2 NPs, Hg(CH3COO)2 for HgO NPs, TiO(OH)2 for TiO2 NPs, Pb(CH3COO)2 for Pb NPs were efficiently utilized in the green synthesis studies [19, 47, 48, 49, 50, 51, 52].
The high concentrations of metal ions effectively produce more NPs within less time. It is vital in the case of green synthesis, which utilizes plant extract with less amount of phytochemicals. The deficiency of the phytochemicals/effective reducing agents can be satisfied by using high metal ion concentrations, thereby helping in the subsequent reduction of reaction time [18, 38, 41
The Concentration of Plant Phytochemicals
Plant extract phytochemicals such polyphenols, alkaloids, tannins, flavonoids, terpenoids, ketones, aldehydes, amides, and carboxylic acids serve as reducing and capping agents for the bio-reduction of NPs in the biological synthesis [17, 41, 53,54,55]. These biomolecules donate electrons for the reduction of precursor molecules. Studies have proved the efficiency of plant-mediated reducing agents, including terpenoids and flavonoids, in the bio-reduction of Ag salt to Ag NPs [56]. FTIR studies on Annona muricata plant-mediated Ag NP synthesis have reported the utilization of plant phytochemicals such as alkaloids, polyphenols, carbohydrates, glycosides, and flavonoids in the bioreduction of NPs. These biomolecules influence the antimicrobial and antioxidant properties of the established NPs [21]. Studies have used varying concentrations of crude extracts to analyze the significance of phytochemical concentrations on the bioreduction process [17, 40, 57, 58]. The quantitative and qualitative analysis of phytochemicals carried out in different nanofabrication studies has proved the importance of the presence and concentration of these biomolecules in the bioreduction of NPs [25, 35, 59,60,61,62](Table 2).
The Effect of pH
pH is crucial for NPs synthesis as it affects the morphological characteristics of the synthesized NPs. The pH of the reaction mixture influences the redox reaction and thereby the binding among the metal ions and the plant phytochemicals that act as capping agents by altering the charge on metabolites during the nanofabrication process. Consequently, the stability of the NPs is also influenced by the pH of the reaction medium [32]. Few researchers have demonstrated the impact of pH on the NP's green synthesis. The study has proposed two reaction pathways to demonstrate the formation of Ag NPs based on pH changes that develop by the addition of NaOH during green synthesis. Silver nitrate, glucose, sodium hydroxide (NaOH), and starch, respectively were used as precursors, reducing agents, accelerators, and stabilizers for the reduction synthesis of Ag NPs. pH was reduced to a minimum value and then increased with the consequent addition of NaOH. pH performs an influential part in the nano-synthesis mechanism by influencing the rate of the reduction process, consequently affecting the topology of the synthesizing NPs [32, 63
In a similar study, Seriphidium quettense mediated green synthesis of biogenic nanoparticles, the optimization of the synthesis was done by optimizing the plant extract pH. They used crude extract at various pH of 4, 5, 6, 7, 8, and 9 and proved the importance of pH in the bioreduction of NPs. It was observed that the increased pH has increased the rate of NPs formation and alkaline pH synthesized stable NPs. At the same time, acidic pH has produced aggregates of NPs. Studies have proved that the basic pH supports the synthesis of smaller-sized NPs than acidic pH [64].
The Effect of Temperature
The temperature at which the bioreduction of the NPs takes place is a crucial factor in the green synthesis of nanomaterials. Most researchers synthesize NPs at room temperature (RT). At the same time, it has been also reported that higher temperatures can reduce the reaction time and ease the green synthesis process. It is evident from the studies that temperature is an influential factor that can affect the size, shape, yield, and stability of green synthesized NPs. The NPs synthesis at RT is considered a simple and natural method of green synthesis. The stability of the plant phytochemicals is a crucial as well as advantageous factor in the green synthesis of Inorganic NPs at RT. But it may cause an increase in the reaction time. Green synthesis of Ag NPs using Artemisia vulgaris was established at room temperature with stirring conditions for 18 h. The resulting Ag NPs have reported λmax at 427 nm, and the TEM analysis showed a globular structure having a size of 20–50 nm [43]. In a concurrent study, Ag NPs synthesis using Gmelina arborea extracts was done at 60 °C with a continuous stirring at 1000 rpm. The study reported the change from colorless to yellowish-brown within 5 min implying the development of Ag NPs [54].
During the green synthesis of gold nanoparticles (Au NPs), chloroauric acid (HAuCl4) was reduced by plant extracts of Angelica archangelica, Hypericum perforatum, and Hamamelis virginiana was done at RT, and a pH of 8. The study has produced Au NPs of 4–8 nm and appeared in various structures such as spherical, and polyhedral and also reported the formation of aggregates of Au NPs [65]. Abroma augusta (L.) bark extract mediated biological synthesis of Au NPs at RT took several hours for the formation of Au NPs [66]. In another study, nettle (Urtica dioica L.) leaves mediated bio construction of Au NPs was done at 650 C within 15 min [67]. In the case of zinc nanoparticles (Zn NPs), in Cassia fistula and Melia azadarach mediated bio fabrication of Zn NPs from 0.01 M, Zinc acetate dihydrate was done at 700 C reporting the formation of small-sized NPs at high temperatures [68]. Fe NPs were synthesized at RT using 0.1 M FeCl3 solutions in a proportion of 2: 1 with plant extract and the synthesized Fe NPs showed potential degradation of arsenic in wastewater [69]. Unlike this, the Eclipta prostrata mediated synthesis of Fe NPs using 5 g of precursor ion and 50 ml plant extract at 70 °C, took 45 h to complete the reaction [70
Effect of Reaction Time
Reaction time is another influential aspect in the formation of NPs. It influences their shapes, sizes, and stabilities. Considering the plant-mediated synthesis of NPs, the clearest advantages are the mild processes involved and the less time the process takes. As soon as the precursor solutions are mixed with plant extracts, usually, a color change takes place, indicating the formation of NPs. The size of the synthesized NPs increases with reaction time. Though the time needed for reaction varies depending on other factors of synthesis like reaction temperature, the concentration of plant extracts, pH, and type of plant extracts, usually the reaction requires shorter periods. At the same time, it is to be noted that some researchers reported that the entire transformation of Ag+ and the formation of stable Ag NPs required several days [71
Separation and Purification of Green Synthesized NPs
NP characterizations and applications require separation and purification processes. The centrifugation method is an extensively used practice to remove unreacted elements and by-products from the reaction mixture. Mainly green synthesized NPs are purified by the centrifugation method. In this method, the synthesized NPs are centrifuged at a high rpm (10,000 rpm) for a fixed time to remove the unreacted enzymes and proteins, and the resulting pellet was washed with deionized water [18, 76].
Methods such as oven drying and calcinating in a muffle furnace are also used for the purification of green synthesized NPs. Purification techniques such as precipitation methods, electrophoretic deposition methods, and chromatographic methods can also be used in the post-synthesis processing of the NPs. As these methods require additional solvents for the purification process, the use of green nontoxic solvents in the bioreduction of NPs will be more economical and can be practiced for environmental applications [77]
Characterization of the Synthesized Nanoparticles
Particle and pore sizes, shapes, surface area, fractal dimensions, and crystallinity describes the NPs. There are varieties of microscopic, spectroscopic, optical, thermodynamic, and, x-ray diffraction analysis methods for NPs characterization. UV–visible Spectrophotometry (UV–vis), Scanning Electron Microscopy (SEM), X-ray Diffraction (XRD), Transmission Electron Microscopy (TEM), Auger Electron Spectroscopy (AES), Zeta Potential, Dynamic Light Scattering (DLS), Fourier Transform Infrared Spectroscopy (FTIR), Energy Dispersive Spectroscopy (EDS), Atomic Force Microscopy (AFM), Scanning Tunneling Microscopy (STM), etc. are the commonly used methods [32].
UV–Visible Spectrophotometry (UV–vis)
One of the essential tools that are used to identify, characterize and analyze nanomaterials is UV–vis spectroscopy. Light waves of 300–800 nm can be used to demonstrate distinct metal NPs in the size range of 2–100 nm [78]. Shape, size, and interaction of the particles with the medium influence the Surface Plasmon Resonance (SPR) bands in UV–vis spectrophotometry.
It allows rapid recognition and demonstration of metal NPs. The counteraction between the light and mobile surface electrons of Ag NPs produces stable absorbance bands called, SPR in 400–500 nm wavelength. Many research studies have shown that, through the phenomenon called the ‘Excitation of the LSPR,’ AgNPs shows an SPR peak in 380–450 nm [18]. It is also to be noted that AuNPs of 5–50 nm produces a sharp SPR peak at 520–530 nm, but AuNPs less than 5 nm do not give any LSPR absorption peaks in this region. The wavelength of LSPR is based on the shape, size, and chemical composition of NPs [79]. One of the universal approaches to tracking the production and stability of metal NPs in an aqueous solution is UV–Vis absorption spectroscopy. Particle shape, size, and particle–particle interaction (agglomeration) with the medium are some of the factors that influence the absorption spectrum of metal NPs.
The majority of the SPR peaks of the synthesized AgNPs are within the desired wavelength range of 400–500 nm. Some green synthesis studies have recorded SPR peaks below 400 nm during the characterization process [80
The particle size and shape influence the specific vibration modes of the electrons. Hence size, the frequency, as well as width of the SPR peaks, confides in the size and shape of the NPs synthesized. The surrounding medium and dielectric constant of the precursor also play an influential role [32]. Similarly, the concentration of the precursor ions can also influence the SPR peak. In a green synthesis study, the UV visible spectrum was analyzed during the bioreduction process using 1 mM metal ion solution, the synthesized NPs have shown SPR peak at 440 nm, and at the same time 2 mM solution has given a peak at 445 nm; also 3 mM, 4 mM, and 5 mM have shown peaks at 448 nm, 463 nm, and 476 nm respectively. During the study, the resulting spectrum has shown a redshift with a gradual increase in the molar concentration of metal ion solution. The increment occurs in the mean size of the Ag NPs as the concentration of the metal ion solution increases [39, 71
The researchers have proved that the biosynthesized metallic NPs have greater electromagnetic absorption in the visible spectrum as a result of their SPR. Similarly, Au NPs have shown SPR peaks between 520 nm – 550 nm with the most repeated peak at 540 nm [46, 65, 83, 84]. ZnO NPs have reported SPR peaks in a range of 300–372 nm [44, 86]. Cu NPs and Fe NPs have reported SPR peaks between 255 nm-535 nm and 230 nm-370 nm respectively [69, 87].
Fourier Transform Infrared Spectroscopy (FTIR)
FTIR, a crucial approach in the characterization of NPs is used to analyze the IR spectrum of a compound’s absorption/emission, and collectively assemble huge spectral information [88]. The outcome of the FTIR spectroscopy analysis enables us to determine the functional groups of the crude extracts along with the synthesized NPs using those extracts.
FTIR allows the study of absorptive and emissive properties of the materials. It utilizes IR radiations to study the chemical bonds between atoms. During the FTIR analysis, both plant extracts and the synthesized NPs undergo the assay, and the IR spectra produced by both samples are compared to verify the formation of NPs. The differences between the peaks formed in both spectra and the specific peaks formed due to the existence of NPs validate the formulation of NPs during the analysis [89, 90].
Research has reported that plant phytochemicals act as capping agents at the time of NP synthesis and cause the reduction of the precursor ions during the reaction. FTIR studies have demonstrated phytochemicals like flavonoids, alkaloids, tannins, terpenes, and quinones are the principal agents of bioreduction [44, 59, 92, 93]. Amino acids, Saponins, Alkaloids, flavonoids, Terpenoids, Glycosides, Cardiac glycosides, Carbohydrates, Steroids, Lipids, Proteins, Carbohydrates, Glycosides, and Phenols also help in the green synthesis of NPs [21, 94].
FTIR analysis done using the Indigofera aspalathoides mediated biosynthesis of Ag NPs has proved the importance of phytochemicals like Carbohydrates, Alkaloids, Amino acids, Terpenoids, Saponins, Tannins, Lipids, Steroids, Flavonoids, Glycosides on the bioreduction of silver during the reaction [95]. The presence of Flavonoids such as rutin, hesperidin, quercitrin, and kaempferol-3-glucoside has been investigated during the Nycanthes arbor-tristis mediated green synthesis of Ag NPs [96]. A green synthesis study has examined the role of plant phytochemicals in NP synthesis through FTIR analysis. They proved the presence of phenolic compounds in crude plant extracts which participate in the bioreduction of metal ions to metal NPs [97]. In a similar study, green synthesis of ZnO NPs, Phenols, Anthocyanin, Flavonoids, Tannin, Carbohydrate, and Alkaloids were analyzed using the FTIR technique and they have proved the role of these phytochemicals in the bio fabrication of the NPs as well as their biological applications [44]. Specific phytochemicals used by researchers to synthesize the NPs showed metabolites like alkaloids, tannin, flavonoids, and saponin from, Euphorbia hirta, Wrightia tinctoria Cassia alata, and Thespesia populnea showed the production of Ag NPs having rod and spherical shapes with 17 and 30 nm size employing good antimicrobial activity against Pseudomonas aeruginosa, and Bacillus subtilis [59]. The practice of using pure phytochemicals for the green synthesis of NPs may probably regulate the drawbacks of the use of crude extracts such as varying morphologies of synthesizing NPs and can improve the application of the synthesized NPs.
X-ray Diffraction Analysis (XRD)
XRD is a powerful tool for the characterization of NPs as it is employed for the investigation of the crystalline structure and phase identification of the NPs. It is considered one of the crucial nondestructive methods for the characterization of nanomaterials [98]. XRD analysis delivers crucial pieces of information about the synthesized NPs, such as their structure, phase, and preferred crystal orientations. The Average crystallite size (Dhkl) is estimated using Scherrer’s formula: mean crystallite size = (0.9 × λ)/(β cos θ), where θ is the diffraction angle of the highest intensity peak, β is the full width at half maximum (FWHM) of the highest-intensity peak, k is the Scherrer constant (0.9), and λ is the wavelength of the incident X-rays [79].
XRD was utilized to determine the crystal structure of eco-friendly green synthesized stable Ag NPs using Salmalia malabarica. The study analyzed the crystal angles and the FCC lattice of the synthesized NPs and found them to have an average crystallite size of 8.04 nm which was confirmed using TEM analysis [99]. The majority of the reviewed studies have utilized XRD analysis as one of the crucial characterization methods in their studies. In another study, based on the green synthesis of FeO nanocatalyst, XRD analysis was carried out to substantiate the formulation of nano-catalyst and investigate its structural characteristics. XRD spectrum of iron oxide nano-catalyst reported predominant peaks at angles of 18.97°, 30.09°, 35.42°, 37.02°, 43.05°, 53.09°, 57.07°, and 64.98° confirming the presence of FeO NPs [70
Microscopic Analysis
AFM, SEM, and TEM are the major microscopic analytic techniques used for the characterization of NPs. SEM and TEM investigate the physiological prospects of the NPs, including the size distribution and morphology at the nanometer to micrometer scale [100]. Compared to SEM, TEM gives 1000-fold higher resolution images. AFM measures the individual particle size and other physical properties of the NPs [101].
In both SEM and TEM, an electron source and electromagnetic lenses are employed to produce and focus electrons on the specimen which triggers the emission of high-energy backscattered electrons and low-energy secondary electrons from the analyzed sample surface. These emitted electrons visualize the surface morphology of the NPs. However, unlike TEM, SEM analysis is more economical as it doesn’t need any elaborate specimen preparation techniques. At the same time, it can accommodate large and bulky specimens for analysis [102]. It is visible from the table that the majority of the reviewed research has used SEM analysis to investigate the topology of the formulated NPs. During TEM analysis, the electron beam is passed through the ultra-thin section of samples. It visualizes the internal structure of the sample to get a two-dimensional image of the particles analyzed. It needs a complicated sample preparation to create an ultra-thin section of the sample. Even though TEM is costlier than SEM, as it gives the detailed 2D structure of the NPs, most researchers prefer TEM for the characterization of the synthesized NPs.
AFM gives both qualitative and quantitative information such as morphology, size, roughness, and surface texture. It also provides statistical information, such as size, volume distributions, and surface area. It can portray a broad range of particle sizes in the same scan (1–8 μm). It provides visualization in 3D images with high resolution. In addition to this, it can identify nanomaterials in multiple mediums, including controlled environments, ambient air, and even liquid dispersions [103]. In the current study, many researchers have used AFM to characterize the synthesized NPs [96, 104, 105, 106]. In a recent study, optical microscopy was utilized to confirm the development of NPs by observing the concentric rings of the Ag NPs [57].
Pure NPs such as metal NPs usually show small size ranges of less than 100 nm and NPs in a combined form such as bimetallic, metal oxides show a relatively bigger size. Avicennia alba leaf-mediated Ag NPs have reported the formulation of spherical and cuboidal shapes with 18.3 nm size [45]. At the same time, larger-sized Ag NPs have also been reported in the Azadirachta indica leaf-mediated Ag NPs shown at 200 nm with spherical, triangular, and cuboidal shapes [107]. Similarly green synthesized Au NPs having 4–8 nm with spherical, ovals, heart, or polyhedral shapes as well as NPs with 100 nm size ranges have also been reported [65, 108]. Interestingly small sized plant-mediated ZnO NPs also reported. ZnO NPs synthesized from Cassia fistula have been reported to have 3–68 nm and 5–15 nm sizes in two different studies [68, 109]. The larger sizes of the NPs may be due to the agglomeration of the synthesized NPs.
Applications of the Green Synthesized NPs
Green synthesized NPs are nontoxic, their biological activities can be efficiently utilized in living environments. Furthermore, the properties of the plant used for green synthesis are also considered an influential factor in the biological activities of the synthesized NPs. NPs synthesized from Indian medicinal plants have shown significant biological properties.
Antimicrobial Activity
The antimicrobial activity of silver is known from time immemorial. Generally, silver is used in its nitrate form to promote antimicrobial effects. Whereas, silver in the form of NPs results in a considerable increase in the surface area and provides increased scope for interactions with microbial cells [72]. The antimicrobial activity of Ag NPs is used in various industries like the health sector, food industry, textile industry, and environmental applications.
The size of the NPs acts as a vital part of their increased antimicrobial effect. NPs are reported to influence the cell permeability of microbial cells, thereby causing cell death. Furthermore, these NPs adhere to the bacterial cell membrane by developing bonds between the thiol groups of enzymes and cause the inactivation of enzymes in the cell membrane that is responsible for the trans-membrane energy generation and ion transport. Additionally, these NPs entering the bacterial cell form interactions with the amino acids and enzymes through the -SH groups, generating ROS, leading to the disturbance in the cell function and thereby resulting in cell death [32]. Bacterial studies have suggested the presence of phosphorus, and sulfur, which build the delicate bases of the DNA. NPs can bind to these weak bases and damage their DNA which would finally result in cell death. This way of cell lysis could be the leading cause of its antibacterial property [31
The green synthesized NPs are proven to have great antimicrobial potential against various Gram-positive, and Gram-negative bacteria as well as some fungal strains. Ag NPs synthesized from Triphala (Emblica officinalis, Terminalia belerica, and T. chebula) have shown significant antimicrobial activity on Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Bacillus subtilis [18]. Some studies have proved the combined action of NPs synthesized from various plant extracts against diverse groups of microbes [14, 107].
Green synthesized Au NPs from Terminalia arjuna fruit have been reported to have inhibitory effects on S. aureus B. subtilis, P. vulgaris, and K. pneumoniae [110]. In a comparable study, Zn NPs synthesized using Justicia procumbense have shown broad-spectrum antimicrobial activity against Pseudomonas aeruginosa, Escherichia coli, Aspergillus niger, Staphylococcus aureus, A. fumigatous, and A. flavus [44]. Biosynthesized CuO NPs from the mint leaf have shown synergistic antibacterial activity against Bacillus subtilis and Escherichia coli strains that have shown 35–38 nm inhibition zones in the analysis. The study has concluded the potentiality of CuO NPs to fight against microbes [47].
A detailed mechanism of antifungal activities of the NPs has not been studied extensively. Few researchers have reported antifungal activity against various fungal strains by green synthesized NPs [36, 111, 112]. NPs synthesized using Azadirachta indica have shown good antimicrobial activity against different bacterial and fungal strains [56, 107, 111, 113]. Various spices, including Piper nigrum, Zingiber officinale, Coriandrum sativum, Murraya koneigii, and Rosmarinus officinalis, have also been utilized for green synthesis of NPs as well as in antimicrobial applications [39, 114, 115, 116, 117]. It is evident from the reports that the green synthesized NPs have been less explored against Gram-positive bacteria in comparison with gram-negative strains (Table 3). It may be attributed to the thick peptidoglycan layer in the cell membrane of gram-positive microbes, which interferes with the easy entry of NPs into the bacterial cell. Studies need to be done to overcome this limiting factor in the journey of nontoxic green synthesized NPs in the medical field.
Antioxidant Activity
Antioxidants inhibit the oxidative processes of the cell by scavenging or chelating free catalytic metals and thereby acting as electron donors. These antioxidants can be classified as natural and synthetic, which are further divided into primary and secondary antioxidants. Phytochemical studies done on various plants have proved that plants are the source of natural antioxidants like carotenoid, ascorbic acid, and tocopherol. Studies done on synthetic antioxidants have revealed their negative health effects, so it is advised to use naturally occurring antioxidants as substitutes [118]. Modern scientific research is now focusing more on natural antioxidants that originated from plants. These safe antioxidant agents can prevent the human body from many degenerative and chronic diseases [119].
Among metal NPs like Au, Ag, Ce, Pt, Pd, and Zn, Ag NPs are popular for their potential anti-oxidative activities [120]. Ag NPs synthesized using aqueous extract of ginger, garlic, and cayenne pepper reduced 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid), and 2,2-diphenyl-1-picrylhydrazyl radicals [81]. Numerous research has been done to explore the antioxidant activity of green synthesized NPs from various medicinal plants. For example, NPs synthesized from Atropa acuminata have shown potential antioxidant properties [60]. Green synthesized Au NPs from Nigella arvensis leaf extract have been reported to have good antioxidant activity. During the study plant extract and the synthesized Au NPs recorded potential antioxidant activity in terms of DPPH scavenging activity and were found to be 32% and 12%, respectively [121]. The antioxidant properties of the green synthesized NPs may be associated with the phytochemicals that aid in the reduction of NPs as well as their nano-characteristics (Table 2).
Anticancer Activity
Despite extensive research on cancer biology, cancer persists as an aggressive killer worldwide. The scientific world needs novel anticancer agents to withstand this condition. In traditional medicine, elements obtained from plants have been used to cure diseases. Nowadays, treatment options derived from plants and natural products have received increasing consideration as novel cancer therapeutic agents [122]. Green synthesized NPs are one of the crucial milestones in the evolution of novel, effective cancer treatment.
Numerous studies have proved the anticancer capability of green-synthesized NPs. Ag NPs synthesized using Cleome viscosa were examined for their anticancer activity against human A549 and PA1 cell lines. The study has reported significant anticancer activities on lung and ovarian cancer cell lines with IC50 values at 28 and 30 mg/mL [41]. Atropa acuminata-mediated green synthesized Ag NPs have shown potential anticancer activity [60]. In a recent study, the anticancer effect of green synthesized NPs using Phyllanthus emblica leaf extract was investigated against diethyl nitrosamine-induced hepatocellular carcinoma (HCC) in Wistar rats. And the results of the study have proved the chemoprotective property of the bioengineered Ag NPs against HCC. The green synthesized NPs knocked down the production of free radicals and restore all the biochemical parameters in the DEN-induced group. The study has also reported that the Ag NPs modulated the pro-inflammatory cytokines and inflammatory mediators in a dose-dependent manner in Hepatic cancer [121]. Green synthesized Ag NPs from Alpinia officinarum were reported to have the potential to be used as an effective nephroprotective against cisplatin-induced nephrotoxicity via the down-regulating apoptotic pathway [122]. Recently researchers have also concentrated on the use of nanocomposites as anticancer agents. Green synthesized CuO NPs decorated with graphene oxide were reported to have 70% cytotoxic activity against HCT-116 Human colon cancer cell lines at 100 μg/ml. The study also reported that the GO-CuO nanocomposites have appreciable activity toward cancer cell lines in comparison to NPs as such. Numerous studies have proved the anticancer potential of green synthesized NPs [64, 73, 121, 123, 124] (Table 4).
Other Applications of the Green Synthesized NPs
The green synthesized NPs are widely studied for applications such as photocatalytic and larvicidal activities. These eco-friendly NPs can effectively be used in environmental remediation, including the degradation of toxic chemicals. Ag NPs synthesized from Astragalus gummifer (gum tragacanth) are used for the degradation of Congo red and methylene blue [127]. The catalytic potential of the Salmalia malabarica/gum-capped Ag NPs to trigger the reduction of 4-nitrophenol (4-NP) in the presence of NaBH4 was reported [99]. Several studies have proved the potential of the green synthesized NPs to be used as potential catalytic agents in the degradation of toxic chemicals, including synthetic dyes used in industries [128, 129]. Metal oxide NPs such as ZnO NPs, SnO2 NPs, CuO NPs, FeO NPs and TiO2 NPs have good photocatalytic potentials due to their band gap properties [17, 19, 30, 69, 79, 130] (Table 4).
Green synthesized NPs are also reported to have larvicidal activities against many vector mosquitoes. Green synthesized Ag NPs from Sida acuta plant extract have shown significant activity against Culex quinquefasciatus, Anopheles stephensi, and Aedes aegypti mosquitoes larvae [131]. In a concurrent study, the efficient inhibitory potential of Ag NPs produced from Heliotropium indicum leaf extract against adult mosquitoes of Anopheles stephensi, Aedes aegypti, Culex quinquefasciatus, and Aedes aegypti was determined [132]. Bio-synthesized NPs from Ventilago maderaspatana have been reported to have intensive toxicity on Filariasis, Malaria, and Zika Virus Mosquito Vectors [106]. Also, NPs synthesized using Terminalia arjuna, Leptadenia reticulata, Azadirachta indica, and Annona muricata leaf extracts are proved to have the potentials to be used as potential mosquito vector control agents [22, 73, 93, 133] (Table 4).
Various reports have proved the potentiality of green synthesized Ag, ZnO, and Au NPs for enhancing seed germination activity in the agricultural field. Green synthesized NPs using Calotropis gigantea, Terminalia arjuna, and Syzygium cumini are proven to have seed germination enhancement properties [130, 134].
Toxicity of the Green Synthesized NPs
Compared to the NPs synthesized through physical and chemical methods, green synthesized NPs are less toxic as there are no toxic chemicals taking part in the synthesis process. Low concentrations of NPs are nontoxic or less toxic compared to high concentrations of NPs. Green synthesized NPs can be used as an alternative to physical and chemical synthesized NPs. Toxicity analysis of the synthesized NPs is an important aspect of its biological applications. Even though green synthesized NPs are less toxic due to the reagents used, the toxicity of the NPs in the environment should also be studied. The exposure of NPs to the environment can happen at the stage of synthesis, storage, application, or improper disposal of the NPs after their use. And the NPs that are there in the environment can be accumulated in the human body through inhalation, ingestion, or through absorption. As inhalation is the main route of NP exposure to the human body, the effects on the respiratory system as well as skin problems are prominent in humans [135].
Long-term exposure of NPs to the human body can cause various adverse effects on the different parts of the body such as Alzheimer's and Parkinson’s disease on exposure to the brain, respiratory issues such as Asthma, Bronchitis, Lung cancer, gastrointestinal disorders such as Colon cancer, Crohn’s disease, skin problems such as Dermatitis and autoimmune diseases [135, 136]. NPs also show toxic effects on Plants and other living organisms in the environment. The exposure of NPs to the aquatic ecosystem can affect the survival of the aquatic flora and fauna at different levels [137]. The phytotoxic and cytotoxic studies done during the green synthesis studies have revealed the level of toxicity of the synthesized NPs.Several studies have reported the potential of certain green synthesized NPs for enhancement of seed germination. It is also reported that the same NPs have toxic effects at higher concentrations [107]. NPs also show adverse effects on certain disease vectors such as mosquitoes that can be effectively utilized in pest control applications [138]. Metal oxide NPs and doped NPs show higher toxic effects as compared to the NPs in their pure form. Studies have reported the harmful effects of AlO2 and TiO2 NPs in the human body system as well on other living organisms [139, 140].
Conclusion
The current review is an attempt to explore the available data on green synthesized NPs using Indian medicinal plants. This review examines the influencing parameters of green synthesis, and common characterization techniques of green nanotechnology as well as their potential biological applications. Various research has been performed to explore the opportunities of traditional medicinal plants for the green synthesis of NPs as a substitute for many toxic chemicals. Despite such huge research to explore the advanced biological properties of the green synthesized NPs, the demand for practical usage of these results in huge amounts is persisting. The plant phytochemicals that are utilized in the green synthesis of NPs are an important reason for their biological properties. The biomolecules that act as reducing and capping agents in the plant extracts are an essential part of the green synthesis mechanism. It is noted that the green synthesized NPs are varying in size and shape. Therefore, the use of specific phytochemicals may improve the results. Moreover, to meet the high demand for NPs for various applications in different fields of science, medicine, industries as well as in agriculture, research needs to focus on the unexplored traditional plants and also scale up the process to an industrial or large scale.
References
L. D. Chandra (2016). Adv. Plants Agric. Res. 5, 00186.
S. Kumar, G. J. Dobos, and T. Rampp (2017). Evid-Based Complementary Altern. Med 22, 494.
P. Navaneethan, S. Nautiyal, T. Kalaivani, and C. Rajasekaran (2011). Medicinal Plants. Int. J Phytomed. 3, 27.
M. Saxena, J. Saxena, R. Nema, D. Singh, and A. Gupta (2013). J. Pharmacogn. Phytochem. 1, 168-182.
K. V. Kumar, M. Ashok, and J. V. Reddy (2011). Int. J. Future Eng. Technol. 6, 18–24.
R. Kaewmeesri, J. Nonkumwong, T. Witoon, N. Laosiripojana, and K. Faungnawakij (2020). J. Nanomater. 10, 2548.
A. Barhoum and A. S. H. Makhlouf, (Elsevier, Amsterdam, Netherlands, 2018).
L. Karthik, A. V. Kirthi, S. Ranjan, and V. M. Srinivasan, (CRC Press, 2019).
D. K. Patra, C. Pradhan, and H. K. Patra (2020). Environ. Technol. Innov. 18, 100672.
N. Patil, R. Bhaskar, V. Vyavhare, R. Dhadge, V. Khaire, and Y. Patil (2021). Int. J. Curr. Pharm. Sci. 13, 11.
J. Singh, T. Dutta, K.-H. Kim, M. Rawat, P. Samddar, and P. Kumar (2018). J. Nanobiotechnol. 16, 1.
P. G. Jamkhande, N. W. Ghule, A. H. Bamer, and M. G. Kalaskar (2019). J. Drug. Deliv. Sci. Technol. 53, 101174.
S. Iravani (2011). Green Chem. 13, 2638.
T. A. Abalkhil, S. A. Alharbi, S. H. Salmen, and M. Wainwright (2017). Biotechnol. Biotechnol. Equip. 31, 411.
T. Jayaramudu, K. Varaprasad, G. M. Raghavendra, E. R. Sadiku, K. Mohana Raju, and J. Amalraj (2017). J. Biomater. Sci. Polym. Ed. 28, 1588.
N. Nagar and V. Devra (2019). J. Inorg. Organomet. Polym. Mater. 29, 1645.
K. Ganesan, V. K. Jothi, A. Natarajan, A. Rajaram, S. Ravichandran, and S. Ramalingam (2020). Arab. J. Chem 13, 6802.
C. Krishnaraj, E. G. Jagan, S. Rajasekar, P. Selvakumar, P. T. Kalaichelvan, and N. Mohan (2010). Colloids Surf. B 76, 50.
P. S. Sundara Selvam, D. Ganesan, V. Rajangam, A. Raji, and V. Kandan (2020). Iran. J. Sci. Technol. Trans. A: Sci. 44, 661.
C. Karthik, S. Suresh, S. Mirulalini, and S. Kavitha (2020). Inorg. Nano-Met. Chem 50, 606.
K. M. Ezealisiji, X. S. Noundou, and S. E. Ukwueze (2017). Appl Nanosci 7, 905.
S. B. Santhosh, R. Yuvarajan, and D. Natarajan (2015). Parasitol. Res 114, 3087.
V. Sharma, S. Kaushik, P. Pandit, D. Dhull, J. P. Yadav, and S. Kaushik (2019). Appl. Microbiol. Biotechnol 103, 881.
S. A. M. Ealia and M. P. Saravanakumar, (IOP Publishing, 2017), p. 032019.
V. Alagesan and S. Venugopal (2019). BioNanoSci. 9, 105.
M. Ismail, M. I. Khan, S. B. Khan, M. A. Khan, K. Akhtar, and A. M. Asiri (2018). J. Mol. Liq 260, 78.
N. D. Dung, C. T. Son, P. V. Loc, N. H. Cuong, P. T. Kien, P. T. Huy, and N. N. Ha (2016). J. Alloys Compd. 668, 87.
F. Dong, W. Zhao, Z. Wu, and S. Guo (2009). J. Hazard. Mater. 162, 763.
D. H. Kim, D.-K. Choi, S.-J. Kim, and K. S. Lee (2008). Catal. Commun. 9, 654.
A. M. Shaik, M. David Raju, and D. Rama Sekhara Reddy (2020). Inorg. Nano-Met. Chem. 50, 569.
M. Chatterjee and A. Mukherjee, ed. By S. C. Mandal, R. Chakraborty, and S. Sen (Springer Singapore, Singapore, 2021), pp. 799–815.
R. H. Ahmed and D. E. Mustafa (2020). Int. Nano Lett. 10, 1.
A. Gupta, M. Naraniwal, and V. Kothari (2012). Int. J. Nat. Sci. 1, 8.
A. Nostro, M. P. Germano, V. D’angelo, A. Marino, and M. A. Cannatelli (2000). Lett. Appl. Microbiol. 30, 379.
P. S. Ramesh, T. Kokila, and D. Geetha (2015). Spectrochim. Acta. A Mol. Biomol. Spectrosc. 142, 339.
N. H. Rao, N. Lakshmidevi, S. V. N. Pammi, P. Kollu, S. Ganapaty, and P. Lakshmi (2016). Mater. Sci. Eng. C 62, 553.
C. Anupama, A. Kaphle, Udayabhanu, and G. Nagaraju (2018). J. Mater. Sci: Mater. Electron. 29, 4238.
N. Atale, S. Saxena, J. G. Nirmala, R. Narendhirakannan, S. Mohanty, and V. Rani (2017). Appl. Biochem. Biotechnol. 181, 1140.
R. Augustine, N. Kalarikkal, and S. Thomas (2014). Appl. Nanosci. 4, 809.
R. H. Babu, P. Yugandhar, and N. Savithramma (2018). Bull. Mater. Sci. 41, 65.
G. Lakshmanan, A. Sathiyaseelan, P.T. Kalaichelvan, and K. Murugesan (2018). Karbala Int. J. Mod. Sci. 4, 61-68.
V. Kumar, S. C. Yadav, and S. K. Yadav (2010). J. Chem. Technol. Biotechnol. 85, 1301.
L. Soon, P. Q. Ng, J. Chellian, T. Madheswaran, J. Panneerselvam, A. Hsu, P. M. Hansbro, K. Dua, T. Collet, and D. K. Chellappan (2020). JIF 16, 9.
S. Umavathi, M. Ramya, C. Padmapriya, and K. Gopinath (2020). J. Biol. Act. Prod. Nat. 10, 153.
M. Bakshi, S. Ghosh, and P. Chaudhuri (2015). BioNanoSci. 5, 162.
M. Asariha, A. Chahardoli, N. Karimi, M. Gholamhosseinpour, A. Khoshroo, H. Nemati, Y. Shokoohinia, and A. Fattahi (2020). Bull. Mater. Sci. 43, 57.
W. J. Aziz, M. A. Abid, and E. H. Hussein (2020). Mater. Technol. 35, 447.
A. K. Das, A. Marwal, D. Sain, and V. Pareek (2015). Int. Nano Lett. 5, 125.
K. M. Ezealisiji, X. Siwe-Noundou, B. Maduelosi, N. Nwachukwu, and R. W. M. Krause (2019). Int. Nano Lett. 9, 99.
B. Kumar, K. Smita, A. Debut, and L. Cumbal (2020). Bioengineering 7, 54.
D. Mahendiran, G. Subash, D. Arumai Selvan, D. Rehana, R. Senthil Kumar, and A. Kalilur Rahiman (2017). BioNanoScience 7, 530.
M. Raghavendra, K. V. Yatish, and H. S. Lalithamba (2017). Eur. Pys. J. Plus 132, 1.
M. Ahamed, M. A. Majeed Khan, M. K. J. Siddiqui, M. S. AlSalhi, and S. A. Alrokayan (2011). Phys. E Low-Dimens Syst. Nanostructures 43, 1266.
J. Saha, A. Begum, A. Mukherjee, and S. Kumar (2017). Sustain. Environ. Res 27, 245.
T. Santhoshkumar, A. A. Rahuman, C. Jayaseelan, G. Rajakumar, S. Marimuthu, A. V. Kirthi, K. Velayutham, J. Thomas, J. Venkatesan, and S.-K. Kim (2014). Asian Pac. J. Trop Med. 7, 968.
A. Verma and M. S. Mehata (2016). J. Radiat. Res. Appl. Sci 9, 109.
S. Ranjani, K. Tamanna, and S. Hemalatha (2020). Appl Nanosci 10, 1269.
K. N. Ravindra, K. S. Krishna, K. P. Akhilesh, and B. Vaishali, in (Mangalore, India, 2020), p. 070026
P. Raji, A. V. Samrot, D. Keerthana, and S. Karishma (2019). J Clust Sci 30, 881.
S. Rajput, D. Kumar, and V. Agrawal (2020). Plant Cell Rep 39, 921.
K. Singh, M. Panghal, S. Kadyan, U. Chaudhary, and J. P. Yadav (2014). J. Nanobiotechnology 12, 1.
P. Velmurugan, J. Shim, H. Kim, J.-M. Lim, S. A. Kim, Y.-S. Seo, J.-W. Kim, K. Kim, and B.-T. Oh (2020). Res Chem Intermed 46, 999.
P. K. Singh and S. Kundu, in (Coimbatore, India, 2019), p. 020009.
M. Qasim Nasar, T. Zohra, A. T. Khalil, S. Saqib, M. Ayaz, A. Ahmad, and Z. K. Shinwari (2019). Green Chem. Lett. Rev. 12, 310.
R.-D. Pasca, A. Mocanu, S.-C. Cobzac, I. Petean, O. Horovitz, and M. Tomoaia-Cotisel (2014). Part. Sci. Technol. 32, 131.
S. Das, B. G. Bag, and R. Basu (2015). Appl. Nanosci. 5, 867.
P. N. V. K. Pallela, S. Ummey, L. K. Ruddaraju, P. Kollu, S. Khan, and S. V. N. Pammi (2019). SN Appl. Sci. 1, 421.
M. Naseer, U. Aslam, B. Khalid, and B. Chen (2020). Sci. Rep. 10, 9055.
V. Kamath, P. Chandra, and G. P. Jeppu (2020). Int. J. Phytoremed. 22, 1278.
S. Meenachi, S. Kandasamy (2019). Int. J. Environ. Anal. Chem. 99, 1286-1297.
M. Rai and C. Posten, (CABI Digital library, 2013).
S. Ahmed, Saifullah, M. Ahmad, B. L. Swami, and S. Ikram (2016). J. Radiat. Res. Appl. Sci. 9, 1.
M. Kumara Swamy, K. M. Sudipta, K. Jayanta, and S. Balasubramanya (2015). Appl. Nanosci. 5, 73.
C. Dipankar and S. Murugan (2012). Colloids Surf. B 98, 112.
N. B. Romes, R. Abdul Wahab, M. Abdul Hamid, H. A. Oyewusi, N. Huda, and R. Kobun (2021). Sci. Rep. 11, 20851.
N. Krithiga, A. Rajalakshmi, and A. Jayachitra (2015). J. Nanosci. 2015, 1.
B. Bhattarai, Y. Zaker, and T. P. Bigioni (2018). Curr. Opin. Green Sustain. Chem. 12, 91.
M. Ramezani, S. Siami, M. Rezaei, S. Khazaei, and M. Sadeghi (2020). BioMedicine 10, 21.
P. C. Nagajyothi, S. V. Prabhakar Vattikuti, K. C. Devarayapalli, K. Yoo, J. Shim, and T. V. M. Sreekanth (2020). Crit. Rev. Environ. Sci. Technol. 50, 2617.
K. Kon and M. Rai, (Elsevier Academic Press 2017).
G. Otunola, A. Afolayan, E. Ajayi, and S. Odeyemi (2017). Phcog Mag 13, 201.
R. H. Ahmed and D. E. Mustafa (2020), Int Nano Lett 10, 1
M. Naushad, S. Rajendran, and F. Gracia, editors, 1st ed. (Springer International Publishing: Imprint: Springer, Cham, 2019).
B. Kumar, K. Smita, L. Cumbal, and A. Debut (2017). Inorg. Nano-Met. Chem. 47, 138.
A. Tripathi, S. Kumari, and A. Kumar (2016). Appl. Nanosci. 6, 61.
S. W. Balogun, O. O. James, Y. K. Sanusi, O. H. Olayinka, and S. N. Appl (2020). Sci. 2, 504.
S. Biswas, S. Chakraborty, and A. F. Mulaba-Bafubiandi (2017). Afr. J. Sci. Technol. Innov. Dev. 9, 131.
A. A. Attia, H. S. Ramdan, R. A. Al-Eisa, B. O. A. Adle Fadle, and N. S. El-Shenawy (2021). Molecules 26, 3045.
M. D. C. Dragan J. Cvetkovic and Goran S. Nikolic, (IntechOpen, 2017).
C. Neelu, in Silver nanoparticles: Fabrication, Characterization and Applications, ed. By K. Maaz, (IntechOpen, 2018) pp. 21-58.
M. Parveen, F. Ahmad, A. M. Malla, and S. Azaz (2016). Appl. Nanosci. 6, 267.
S. Poopathi, L. J. De Britto, V. L. Praba, C. Mani, and M. Praveen (2015). Environ. Sci. Pollut. Res. 22, 2956.
A. Chaudhary, N. Kumar, R. Kumar, and R. K. Salar (2019). SN Appl. Sci. 1, 136.
K. D. Arunachalam, S. K. Annamalai, A. M. Arunachalam, and S. Kennedy (2013). Asian J. Chem. 25, S311–S314.
A. K. Mishra, K. N. Tiwari, R. Saini, P. Kumar, S. K. Mishra, V. B. Yadav, and G. Nath (2020). J. Inorg. Organomet Polym. 30, 2266.
R. Molaei, K. Farhadi, M. Forough, and A. Pourhossein (2015). Synthe. React. Inorg. Metal-Organic, Nano-Metal Chem. 45, 1489.
M. Maqbool and T. Khan (2006). Int. J. Mod. Phys. B 20, 217.
I. Murali Krishna, G. Bhagavanth Reddy, G. Veerabhadram, and A. Madhusudhan (2016). Appl. Nanosci. 6, 681.
B. Schaffer, U. Hohenester, A. Trügler, and F. Hofer (2009). Phys. Rev. B 79, 041401.
H. S. Ho Soonmin (2016). Orient. J. Chem. 32, 1515.
M. Russo, in Metallography in Failure analysis, ed. By J. L. McCall and P. M. French (Springer US, Boston, MA, 1978), pp. 65–95.
M. Aliofkhazraei and N. Ali, in Comprehensive Materials Processing, ed. By Saleem Hashmi, Gilmar Ferreira Batalha, Chester J. Van tyne, and Bekir Yilbas, (Elsevier, 2014), pp. 191–241.
S. Muthukrishnan, S. Bhakya, T. Senthil Kumar, and M. V. Rao (2015). Ind. Crops. Prod. 63, 119.
M. Z. Siddiqui, A. R. Chowdhury, B. R. Singh, S. Maurya, and N. Prasad (2021). Natl. Acad. Sci. Lett. 44, 203.
R. M. S. Thameem Azarudeen, M. Govindarajan, M. M. AlShebly, F. S. AlQahtani, A. Amsath, and G. Benelli (2017). J. Clust. Sci. 28, 369.
P. Banerjee, M. Satapathy, A. Mukhopahayay, and P. Das (2014). Bioresour. Bioprocess 1, 1.
U. K. Parida, B. K. Bindhani, and P. Nayak (2011). World J. Nano Sci. Eng. 1, 93.
D. Suresh, P. C. Nethravathi, and R. Udayabhanu, Mater. Sci. Semicond. Process 31, 446 (n.d.)
K. Gopinath, S. Gowri, V. Karthika, and A. Arumugam (2014). J. Nanostruct. Chem. 4, 115.
M. Haroon, A. Zaidi, B. Ahmed, A. Rizvi, M. S. Khan, and J. Musarrat (2019). Indian J. Microb. 59, 273.
G. Kuppurangan, B. Karuppasamy, K. Nagarajan, R. Krishnasamy Sekar, N. Viswaprakash, and T. Ramasamy (2016). Appl. Nanosci. 6, 973.
P. Roy, B. Das, A. Mohanty, and S. Mohapatra (2017). Appl. Nanosci. 7, 843.
R. K. Chahande, B. A. Mehere, P. K. Pantawane, P. B. Chouke, and S. R. Murai (2020). Mater. Today: Proc 29, 923.
M. Ghaedi, M. Yousefinejad, M. Safarpoor, H. Z. Khafri, and M. K. Purkait (2015). J. Ind. Eng. Chem. 31, 167.
K. Kalantari, E. Mostafavi, B. Saleh, P. Soltantabar, and T. J. Webster (2020). Eur. Polym. J. 134, 109853.
G. M. Nazeruddin, N. R. Prasad, S. R. Prasad, Y. I. Shaikh, S. R. Waghmare, and P. Adhyapak (2014). Ind. Crops. Prod. 60, 212.
N. K. Dubey, A. K. A. Kedia, B. P. B. Prakash, N. K. N. Kishore, in Plants as a source of natural antioxidants, ed. By N. K. Dubey, (CABI, Wallingford, Oxfordshire, UK; Boston, MA, USA, 2015) pp. 1–14.
S. S. Azimova, Natural compounds: plant sources, structure and properties, 1st edn. (Springer, New York, 2013).
S. Kishen, A. Mehta, and R. Gupta, in Green nanomaterials: Processing, Properties, and Applications, ed By S. Ahmed and W. Ali, (Springer, Singapore, 2020), pp. 139–157.
A. Chahardoli, N. Karimi, F. Sadeghi, and A. Fattahi (2018). Artif Cells Nanomed. Biotechnol. 46, 579.
E. Solowey, M. Lichtenstein, S. Sallon, H. Paavilainen, E. Solowey, and H. Lorberboum-Galski (2014). Sci. World J. 2014, 1.
D. Singh, E. Yadav, N. Falls, V. Kumar, M. Singh, and A. Verma (2019). Inflammopharmacology 27, 1037.
Z. Zhang, G. Xin, G. Zhou, Q. Li, V. P. Veeraraghavan, S. Krishna Mohan, D. Wang, and F. Liu (2019). Artif Cells Nanomed. Biotechnol. 47, 3212.
C. Kamaraj, G. Balasubramani, C. Siva, M. Raja, V. Balasubramanian, R. K. Raja, S. Tamilselvan, G. Benelli, and P. Perumal (2017). J. Clust. Sci. 28, 1667.
S. P. Vinay, Udayabhanu, G. Nagaraju, C. P. Chandrappa, and N. Chandrasekhar (2019). J. Clust. Sci. 30, 1545.
M. K. Indana, B. R. Gangapuram, R. Dadigala, R. Bandi, and V. Guttena (2016). J. Anal. Sci. Technol. 7, 1.
J. S. Pawar and R. H. Patil (2020). SN Appl. Sci 2, 1.
M. Sivaramakrishnan, V. Jagadeesan Sharavanan, D. Karaiyagowder Govindarajan, Y. Meganathan, B. S. Devaraj, S. Natesan, R. Kothandan, and K. Kandaswamy (2019). SN Appl. Sci. 1, 1.
M. Rafique, R. Tahir, S. S. A. Gillani, M. B. Tahir, M. Shakil, T. Iqbal, and M. O. Abdellahi (2020). Int. J. Environ. Anal. Chem. 13, 1662.
K. Veerakumar, M. Govindarajan, and M. Rajeswary (2013). Parasitol Res. 112, 4073.
K. Veerakumar, M. Govindarajan, and S. L. Hoti (2014). Parasitol Res. 113, 4567.
S. A. Saiqa Ikram (2015). J. Nanomed. Nanotechnol. 06, 4.
S. K. Chaudhuri and L. Malodia (2017). Appl. Nanosci. 7, 501.
R. Garg, P. Rani, R. Garg, and N. O. Eddy (2021). Turk. J. Chem. 45, 1690.
A. Rana, K. Yadav, and S. Jagadevan (2020). J. Clean. Prod 272, 122880.
T. A. J. de Souza, L. R. R. Souza, and L. P. Franchi (2019). Ecotoxicol. Environ. Saf. 171, 691.
G. Benelli and C. M. Lukehart (2017). J. Clust. Sci. 28, 1.
E. Baranowska-Wójcik, D. Szwajgier, P. Oleszczuk, and A. Winiarska-Mieczan (2020). Biol. Trace Elem. Res. 193, 118.
M. I. Yousef, T. F. Mutar, and M.A.E.-N. Kamel (2019). Toxicol. Rep. 6, 336.
D. Badma Priya and I. V. Asharani (2017). J. Clust. Sci. 28, 1837.
M. Moyo, M. Gomba, and T. Nharingo (2015). Int. J. Ind. Chem. 6, 329.
V. Vinmathi and S. J. Packia Jacob (2015). Bull. Mater. Sci. 38, 625.
G. Mamatha, A. Varada Rajulu, and K. Madhukar (2020). J. Nat. Fibers 17, 1121.
K. S. Venkatesh, S. R. Krishnamoorthi, N. S. Palani, V. Thirumal, S. P. Jose, F.-M. Wang, and R. Ilangovan (2015). Indian J. Phys 89, 445.
M. Kasithevar, M. Saravanan, P. Prakash, H. Kumar, M. Ovais, H. Barabadi, and Z. K. Shinwari (2017). J. Interdiscip. Nanomed. 2, 131.
A. B. Alayande, M. Obaid, and I. S. Kim (2020). Mater. Sci. Eng. C 109, 110596.
A. K. Singh and O. N. Srivastava (2015). Nanoscale Res. Lett. 10, 353.
G. G. Naik, M. Alam, V. Pandey, D. Mohapatra, P. K. Dubey, A. S. Parmar, and A. N. Sahu (2020). J. Fluoresc. 30, 407.
A. J. Kora, S. R. Beedu, and A. Jayaraman (2012). Org. Med. Chem. Lett. 2, 1.
U. B. Jagtap and V. A. Bapat (2013). Ind. Crops Prod. 46, 132.
S. S. Shankar, A. Rai, A. Ahmad, and M. Sastry (2004). J. Colloid Interface Sci. 275, 496.
A. K. Jha and K. Prasad (2010). Internat. J. Green Nanotechnol.: Phys. Chem. 1, P110.
N. Nagar, S. Jain, P. Kachhawah, and V. Devra (2016). Korean J. Chem. Eng. 33, 2990.
M. F. Sohail, M. Rehman, S. Z. Hussain, Z.-E. Huma, G. Shahnaz, O. S. Qureshi, Q. Khalid, S. Mirza, I. Hussain, and T. J. Webster (2020). J. Drug. Deliv. Sci. Technol. 59, 101911.
S. Kunjiappan, R. Chowdhury, and C. Bhattacharjee (2014). Front. Mater. Sci. 8, 123.
S. Lakshminarayanan, M. F. Shereen, K. L. Niraimathi, P. Brindha, and A. Arumugam (2021). Sci. Rep. 11, 1.
B. Ginting, I. Maulana and I. Karnila (2020). Surf. Interfaces. 21, 100799.
P. V. Kumar, S. V. N. Pammi, P. Kollu, K. V. V. Satyanarayana, and U. Shameem (2014). Ind. Crops Prod. 52, 562.
J. Kadam, P. Dhawal, S. Barve, and S. Kakodkar (2020). SN Appl. Sci. 2, 1.
S. Swain, S. K. Barik, T. Behera, S. K. Nayak, S. K. Sahoo, S. S. Mishra, and P. Swain (2016). BioNanoScience 6, 205.
V. G. Kumar, S. D. Gokavarapu, A. Rajeswari, T. S. Dhas, V. Karthick, Z. Kapadia, T. Shrestha, I. A. Barathy, A. Roy, and S. Sinha (2011). Colloids Surf. B 87, 159.
S. C. Bankalgi, R. L. Londonkar, U. Madire, and N. K. Tukappa (2016). J. Clust. Sci. 27, 1485.
R. K. Das, B. B. Borthakur, and U. Bora (2010). Mater. Lett 64, 1445.
A. Rout, P. K. Jena, U. K. Parida, and B. K. Bindhani (2013). Int J Pharm Biol Sci 4, 661.
M. S. Aref and S. S. Salem (2020). Biocatal. Agric. Biotechnol. 27, 101689.
S. S. Dash, S. Samanta, S. Dey, B. Giri, and S. K. Dash (2020). Biol. Trace Elem. Res. 198, 681.
M. Vanaja, G. Gnanajobitha, K. Paulkumar, S. Rajeshkumar, C. Malarkodi, and G. Annadurai (2013). J. Nanostr. Chem. 3, 1.
P. Sowndarya, G. Ramkumar, and M. S. Shivakumar (2017). Artif Cells Nanomed. Biotechnol. 45, 1490.
M. Vanaja and G. Annadurai (2013). Appl. Nanosci. 3, 217.
I. Johnson and H. J. Prabu (2015). Int. Nano Lett. 5, 43.
O. Dlugosz, J. Chwastowski, and M. Banach (2020). Chem. Pap. 74, 239.
E. T. Enan, A. A. Ashour, S. Basha, and N. H. Felemban (2020). Nanotechnology. 32, 215101.
N. Saha, P. Trivedi, and S. Dutta Gupta (2016). J. Clust Sci. 27, 1893.
N. Muniyappan and N. S. Nagarajan (2014). Process Biochem. 49, 1054.
S. R. Guntur, N. S. Kumar, M. M. Hegde, and V. R. Dirisala (2018). Anal. Chem. Insights 13, 1177390118782877.
M. Krishnan, K. Ranganathan, P. Maadhu, P. Thangavelu, S. Kundan, and N. Arjunan (2020). Coatings 10, 626.
U. S. Senapati and D. Sarkar (2015). J. Mater. Sci. Mater. Electron 26, 5783.
B. Ankamwar, C. Damle, A. Ahmad, and M. Sastry (2005). J. Nanosci. Nanotechnol. 5, 1665.
S. Raj, S. C. Mali, and R. Trivedi (2018). Biochem. Biophys. Res. Commun. 503, 2814.
M. A. Alshehri, A. T. Aziz, S. Trivedi, N. A. Alanazi, C. Panneerselvam, R. Baeshen, and A. Alatawi (2020). J. Clust Sci. 31, 177.
G. Rahimi, K. S. Mohammad, M. Zarei, M. Shokoohi, E. Oskoueian, M. R. M. Poorbagher, and E. Karimi (2022). Biol. Res. 55, 1.
M. Karuppiah and R. Rajmohan (2013). Mater. Lett. 97, 141.
M. K. Ghosh, S. Sahu, I. Gupta, and T. K. Ghorai (2020). RSC Adv. 10, 22027.
S. Joglekar, K. Kodam, M. Dhaygude, and M. Hudlikar (2011). Mater. Lett. 65, 3170.
H. P. Borase, C. D. Patil, R. B. Salunkhe, R. K. Suryawanshi, B. K. Salunke, and S. V. Patil (2014). Bioprocess Biosyst. Eng. 37, 1695.
D. Bose and S. Chatterjee (2015). Indian J. Microbiol. 55, 163.
M. M. Poojary, P. Passamonti, and A. V. Adhikari (2016). BioNanoSci. 6, 110.
T. Elavazhagan and T. Elavazhagan (2011). IJN 6, 1265.
J. Singh and A. S. Dhaliwal (2019). Anal. Lett. 52, 213.
R. K. Das, N. Gogoi, and U. Bora (2011). Bioprocess Biosyst. Eng. 34, 615.
S. Basu, P. Maji, and J. Ganguly (2016). Appl. Nanosci. 6, 1.
C. Karthik, S. Suresh, G. Sneha Mirulalini and S. Kavitha (2020). Inorg. Nano-Met Chem.. 50, 606.
N. A. Karim, N. J. Rubinsin, M. A. A. Burukan, and S. K. Kamarudin (2019). Intern. J. Green Energy 16, 1518.
Md. M. R. Mollick, B. Bhowmick, D. Maity, D. Mondal, M. K. Bain, K. Bankura, J. Sarkar, D. Rana, K. Acharya, and D. Chattopadhyay (2012). Int. J. Green Nanotechnol. 4, 230.
K. Sri Sindhura, T. N. V. K. V. Prasad, P. Panner Selvam, and O. M. Hussain (2014). Appl. Nanosci. 4, 819.
M. Azizi, S. Sedaghat, K. Tahvildari, P. Derakhshi, and A. Ghaemi (2017). Green Chem. Lett. Rev. 10, 420.
C. Shobana, B. Rangasamy, S. Surendran, R. K. Selvan, and M. Ramesh (2018). J. Clust Sci. 29, 267.
T. Sujin Jeba Kumar, C. K. Balavigneswaran, R. Moses Packiaraj, A. Veeraraj, S. Prakash, Y. Natheer Hassan, and K. P. Srinivasakumar (2013). BioNanoScience 3, 394.
M. Beg, A. Maji, A. K. Mandal, S. Das, M. N. Aktara, P. K. Jha, and M. Hossain (2017). J. Mol. Recognit 30, e2565.
H. Alam, N. Khatoon, M. Raza, P. C. Ghosh, and M. Sardar (2019). BioNanoScience 9, 96.
D. Dhamecha, S. Jalalpure, K. Jadhav, and D. Sajjan (2016). Part. Sci. Technol. 34, 156.
B. Şahin, A. Aygün, H. Gündüz, K. Şahin, E. Demir, S. Akocak, and F. Şen (2018). Colloids Surf. B 163, 119.
A. Kanchana, S. Devarajan, and S. R. Ayyappan (2010). Nano-Micro Lett. 2, 169.
S. Narendhran, M. Manikandan, and P. B. Shakila (2019). Bull. Mater. Sci. 42, 133.
S. Kagithoju, V. Godishala, and R. S. Nanna (2015). 3 Biotech 5, 709.
S. Yadav, M. Chauhan, D. Mathur, A. Jain, and P. Malhotra (2021). Environ. Dev. Sustain. 23, 2071.
A. Rautela, J. Rani, and M. Debnath (Das) (2019). J. Anal. Sci. Technol. 10, 5.
S. Priya and J. Appusamy (2020). J. Pharmacogn. Phytochem. 9, 1901.
Z. Noohpisheh, H. Amiri, S. Farhadi, and A. Mohammadi-gholami (2020). Spectrochim. Acta. A Mol. Biomol. Spectrosc 240, 118595.
F. A. Dawodu, C. U. Onuh, K. G. Akpomie, and E. I. Unuabonah (2019). SN Appl. Sci. 1, 346.
A. K. Khajuria, N. S. Bisht, R. K. Manhas, and G. Kumar (2019). SN Appl. Sci. 1, 455.
S. Veena, T. Devasena, S. S. M. Sathak, M. Yasasve, and L. A. Vishal (2019). J Clust Sci. 30, 1591.
M. Zargar, A. A. Hamid, F. A. Bakar, M. N. Shamsudin, K. Shameli, F. Jahanshiri, and F. Farahani (2011). Molecules 16, 6667.
H. Nadaroglu, A. Alayli, S. Ceker, H. Ogutcu, and G. Agar (2020). Int. J. Nano Dimens. 11, 158.
Funding
This work was supported by Christ University (Grant numbers [MRPDC-1934]. Joseph Kadanthottu Sebastian has received research support from Christ University.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by JTK, PC, and JKS. The first draft of the manuscript was written by JTK and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kurian, J.T., Chandran, P. & Sebastian, J.K. Synthesis of Inorganic Nanoparticles Using Traditionally Used Indian Medicinal Plants. J Clust Sci 34, 2229–2255 (2023). https://doi.org/10.1007/s10876-022-02403-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-022-02403-6