Abstract
Effect of triethanolamine, n-octylamine, starch, polyethylene glycol and hexadecyltrimethylammonium bromide as template have been investigated on the formation of Bi2O3 prepared via sol gel method. The prepared catalysts were characterized using X-ray diffraction, scanning electron microscopy, energy dispersive X-ray spectroscopy, Fourier transform infrared, UV–visible diffuse reflectance and Brunauer–Emmett–Teller techniques and subsequently used as photodegradation catalyst to purify contaminated water by Congo red and methylene blue as phenolic dyes. It was found that whereas Bi2O3-starch, Bi2O3-CTAB and Bi2O3-PEG have the most photodegradation effect on methylene blue under visible irradiation, Bi2O3-starch and Bi2O3-CTAB are the most active catalysts for Congo red under UV irradiation. In other word, the type of organic dyes, photocatalyst morphologies and templates are important factors on the photodegradation and adsorption phenomena.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the past decades, many research works were focused on the metal and mixed metal oxides as photodegradation catalysts for organic dyes under visible or UV irradiation. Since synthesis of azo dyes as a class of colored organic compounds and their extensive application in several industries such as textiles, leathers, paper, and additives [1] contaminate water, a large quantity of waste water containing dyestuffs with intensive color and toxicity are introduced into the aquatic systems [2]. As such, removing of this contamination from waste water to less than 1 ppm is an important task since if not, it is clearly visible and influences the environment considerably [3]. Recall that whereas catalysts such as TiO2, ZnO, Fe2O3, ZnS, WO3, CdS and Bi2O3 have been used in this regard, amongst which bismuth oxide has many peculiar properties including significant band gap (from 2 to 3.96 eV), dielectric permittivity, high refractive index, and marked photoconductivity and photoluminescence [4, 5]. These properties have led to intense investigation of this material for applications in many areas such as microelectronics [6], sensor technology [7], optical coatings [8], and transparent ceramic glass manufacturing [9]. Since bulk Bi2O3 is a good optoelectronic material [10] with high photoconductivity [11], it is widely used in third order nonlinear optical glasses [12]. Bi2O3 is known with six polymorphic forms of monoclinic α-phase, tetragonal β-phase, body-centered cubic ϒ-phase, face centered cubic δ-phase, tetragonal ε-phase and triclinic ω-phase. Among these, whereas the α-phase and δ-phase are the most stable at room and high temperatures (730–825 °C) respectively, the other four are metastable phases [13]. It was found that photocatalysts with different crystal structures show quite different photocatalytic performances [14]. The previous study showed that α-Bi2O3, β-Bi2O3 and δ-Bi2O3 exhibit good photocatalytic activities in the degradation of pollutants [15]. Up to now, α-Bi2O3 was mostly studied because of its thermal stability and easy preparation [16,17,18,19].
In this study, a number of Bi2O3 with different templates, morphologies and adsorptions were initially synthesized by sol–gel method and subsequently used as photodegradation catalyst to purify contaminated water by Congo red and methylene blue as phenolic dyes.
Experimental
Materials and Methods
Bismuth(III) nitrate pentahydrate Bi(NO3)3·5H2O, starch, polyethylene glycol (PEG), triethanolamine (TEA), n-octylamine (OA), hexadecyltrimethylammonium bromide (CTAB), nitric acid (HNO3, 60%), NaOH (5N), HCl (5N), Congo red (CR) and methylene blue (MB) were purchased from Merck Chemical Company and used without further purification.
The prepared catalysts were characterized by X-Ray powder diffraction (XRD) from a KEFA Company, PW3050/60 (Theta/Theta) and with Cu-Kα radiation (λ = 0.154178 nm). Scanning electron microscopy (SEM) images were carried out on a Hitachi S-4800 microscope. UV–Vis diffuse-reflectance spectra were obtained with a Shimadzu-UV-2550-8030 with slit width 5.0 nm and light source change wavelength 360.0 nm spectrophotometer at room temperature. FT-IR spectra were obtained on a FT-IR spectrometer using Bruker, Tensor 27 DTGS, 500–4000 nm using KBr disks. Nitrogen adsorption–desorption measurements were obtained at 77 K with BElSORP Mini model from Microtrac Bel Corp, using Barrett–Emmett–Teller (BET) calculations for the surface area.
Synthesis of Photocatalysts
Synthesis of Bi2O3 Without Template
Bi2O3 was synthesized via the sol–gel method using Bi(NO3)3·5H2O as precursor of Bi3+. The synthesis procedure involved the homogenous dissolution of Bi(NO3)3·5H2O (2 g, 4 mmol) in deionized water (20 mL) and HNO3 (20 mL, 60%). The resultant mixture was kept under vigorous stirring at 90 °C until the solvent of the formed gel completely evaporated. The residue was then washed with ethanol to remove the impurities and dried at 80 °C for 24 h. The resultant solid was finally calcinated at 600 °C for 4 h.
Synthesis of Bi2O3 with TEA, OA, and Starch Templates
Bi(NO3)3·5H2O (2 g, 4 mmol) was dissolved in deionized water (20 mL) and nitric acid solution (20 mL, 60%). After formation of a clear solution, the desired amount of templates such as TEA (1.1 mL, 8 mmol), OA (1.4 mL, 8 mmol), starch (1.33 g, 8 mmol) were added and the mixture stirred in 90 °C for 1 h on a water bath. The resultant mixtures were kept under vigorous stirring at 90 °C until the solvent of the formed gels completely evaporated. The residues were then washed with ethanol to remove the impurities and dried at 80 °C for 24 h. The resultant solids were finally calcinated at 600 °C for 4 h.
Synthesis of Bi2O3 with CTAB Template
Bi(NO3)3·5H2O (2 g, 4 mmol) was dissolved in deionized water (20 mL) and nitric acid solution (20 mL, 60%). After formation of a clear solution, sodium hydroxide (3.5 mL, 5N) and CTAB (0.75 g, 2 mmol) were added and the mixture stirred in 90 °C for 1 h on a water bath. The resultant mixture was kept under vigorous stirring at 90 °C until the solvent of the formed gel completely evaporated. The residue was then washed with ethanol to remove the impurities and dried at 80 °C for 24 h. The resultant solid was finally calcinated at 600 °C for 4 h.
Synthesis of Bi2O3 with PEG Template
Bi(NO3)3·5H2O (0.61 g, 1.2 mmol) was dissolved in deionized water (20 mL) and nitric acid solution (5 mL, 60%). After formation of a clear solution, distilled water (20 mL) and PEG (0.75 g, 2 mmol) were added and the mixture stirred in 90 °C for 1 h on a water bath. The resultant mixture was kept under vigorous stirring at 90 °C until the solvent of the formed gel completely evaporated. The residue was then washed with ethanol to remove the impurities and dried at 80 °C for 24 h. The resultant solid was finally calcinated at 600 °C for 4 h.
Catalytic Photodegradation of Organic Dyes
Photodegradations of Congo red and methylene blue as phenolic dyes were carried out under visible and ultraviolet light irradiations. As a general experiment, 0.05 g of the synthesized bismuth oxide either without or with template was poured into a crystallizer containing distilled water (100 mL) and dye (5 ppm). A 55 W UV or 60 W tungsten lamp was used as UV and visible light source respectively, using 8 cm distance between the incandescent light bulb and crystallizer. During the reaction, the air was always injecting into the solution through an oxygen pump to provide a constant amount of dissolved oxygen. Since Congo red is anionic, the pH of the solution was initially adjusted to 3 with HCl (5N). Prior to irradiation, the complex was stirred in the dark for 15 min in order to become balanced for adsorption/desorption between the catalyst particles, dye and air oxygen. During irradiation, the solution color was sampled at specified intervals (every 15 min) and the catalyst was separated from the solution using centrifugation. The amount of degraded color was evaluated by measuring the rate of absorption changes by UV–Vis spectrophotometer.
Results and Discussion
To investigate the effect of different templates on Bi2O3 morphology, Bi2O3 with different templates such as TEA, starch, OA, CTAB and PEG was initially synthesized by sol–gel method (Scheme 1). In fact in this study attempts has been made to see the effect of different template on Bi2O3 morphology. Therefore the total procedure is the same but the type of template is different.
XRD Patterns
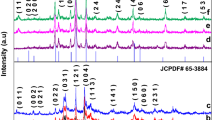
The XRD patterns of all calcined Bi2O3 associated with various templates such as TEA, OA, Starch, CTAB, and PEG shown in Fig. S1 (See Supplementary) are similar with the intensive and sharp peaks in the region of 2θ = 27° and 2θ = 33° related to the planes 120 and 200, respectively. These are consistent with the JCPDS card no. 2274-71 represent α-Bi2O3 with monoclinic crystalline phase [20].
SEM Images
Based on the SEM image results, whereas the morphologies of Bi2O3-TEA before calcination and Bi2O3 using no template have cubic and worm structures respectively (Fig. 1a, b), the former is transformed to the worm like morphology with 300–500 nm thickness after calcination (Fig. 1c, d). On the other hand, the morphology of the Bi2O3-OA is porous with worm shape and diameter of about 250 nm with uniform surface and identical holes (Fig. 1e–f). Similarly, Bi2O3starch is recognized with worm like morphology with cubic packing and the same size with a length of about 8 μm, consisting of particles with a diameter of about 500 nm and pore size of about 100–300 nm (Fig. 1 g, h). Finally, Bi2O3-CTAB and Bi2O3-PEG have morphologies of a bone structure with a diameter of about 1.5 to 2 μm with uniform surfaces (Fig. 2i–j) and porous cubic portions consisting of rods with diameter of about 500 nm with uniform distribution (Fig. 1k–l), respectively.
The SEM images of the as-prepared Bi2O3 catalysts with various templates a hybride Bi2O3 and TEA template before calcined, b Bi2O3 with no template, c, d Bi2O3 with TEA template, e, f Bi2O3 with OA template, g, h Bi2O3 with starch template, i, j Bi2O3 with CTAB template, k, l Bi2O3 with PEG template
The results of the EDX analysis of bismuth oxide after calcination with different templates shown in Fig. S2a–f indicate the presence of bismuth, oxygen elements and trace amount of carbon.
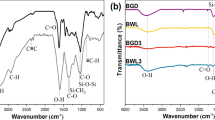
The FTIR spectra of PEG, Bi2O3-PEG hybrid before and after calcination at 600 °C and reusing as catalyst are shown in Fig. S3a–d, respectively. Whereas the peaks centered at 1705 and 1695 cm−1 are attributed to the R–O vibrations of PEG (see supplementary, Fig. 3Sa) [21], the two peaks observed at 1000 and 1320 cm−1 are due to R–O–R as ester groups. The appearance of two rather medium peaks at 400–700 and 1400 cm−1 after calcination are attributed to the Bi–O and Bi–O–Bi vibrations, respectively (Fig. 3Sc) [22, 23]. Particularly significant is the similarity observed in the FTIR spectra of Bi2O3 before and after using as catalyst (Fig. S3d). The remaining FTIR spectra of other templates are shown in the supplementary Figs. S4–8. The band gap, surface area and pore diameter of the prepared Bi2O3 with different morphologies are given in Table 1 and Fig. 2.
Photocatalytic Degradation of Bi2O3 with Different Morphologies
The results obtained for the degradation rate of CR and MB in the presence of Bi2O3 either without or with template as catalyst under visible and ultraviolet light irradiation are presented in Tables 2 and 3 and Fig. S9–12 (see supplementary). The model of kinetic destruction shown in Figs. S13 and S14 (see supplementary) are similar to that of Langmuir–Hinshelwood process (Eq. 1) [24]:
where r, k and K are reaction rate (mg/L min), limiting rate constant at maximum coverage under the given the experimental conditions and equilibrium constant for adsorption of the substrate, respectively. If the initial concentration C0 is small, the Eq. 1 can be simplified to a first order equation which on integrating within the limits of concentration (C0 to C) and time (0 to t) gives Eq. 2.
Then, a plot of Ln (C0/C) versus time gives a straight line with the slope of kapp and regression constant R2 [25, 26] (Tables S2 and S3). Recall that the linear curves presented in Figs. S13 and S14 indicate that the photodegradation is approximately consistent with a first order kinetic, but for starch and cetyl shows some deviation from first order. This mechanism has also been used for the heterogeneous optical catalysts on the basis of cavity and electron production by optical excitation. The adsorbing color on the catalyst surface is degraded by the in situ generated active radical via the interaction with cavity. Subsequently, the catalyst is then re-produced.
A decrease in the intensity of the Congo red absorbance at 497, 347 and 235 nm is observed in the presence of bismuth oxide as catalyst on exposure of the solution to UV–visible light, perhaps due to the destruction of the azo chromophore band, naphthalene and benzoic rings, respectively. This may has occurred due to the direct or indirect oxidation through hydroxyl radicals generated by air oxygen and water present in the dye solution [27]. In methylene blue solution, the highest absorbance is observed in the 660 nm region. In the optical degradation of methylene blue, N-dealkylation of the alkylamine group exhibits the key feature of the photocatalytic degradation. The hypochromic shift occurs concurrently with the degradation of methyl groups [28, 29].
The photocatalytic properties of the prepared bismuth oxides as semiconductors were also investigated. As reported, the absorbing radiation is equal or higher than their energy band gap, metal oxide semiconductors can produce electrons in conduction band and holes in valence band, which have redox activities [30].
\({\text{e}}^{ - }_{\text{cb}}\) are electrons in the conduction band and \({\text{h}}^{ + }_{\text{vb}}\) is the cavities in the capacity layer respectively and can reduce the oxidation reactions with the catalysts. In most cases, \({\text{h}}^{ + }_{\text{vb}}\) reacts with H2O attached to the catalyst surface and produces OH. and \({\text{e}}^{ - }_{\text{cb}}\) reacts with O2 to produce radical anion of oxygen superoxide.
These reactions will prevent the combination of cavities and electrons that created in the first phase. OH. and O2− can react with the colors and destroy them [31]. Dye is also converted to the cationic dye radicals (Dye.+) that undergoes degradation to yield products as given in Eqs. (6–8). The cationic dye radicals readily reacts with hydroxyl ions undergoing oxidation via Reactions 10 and 11 or interacts effectively with O .−2 , HO2. or HO.− species to generate intermediates that ultimately lead to CO2 and H2O [32, 33].
The Effect of Photocatalyst Amount on Photodegradation of Organic Pollutants Dyes
The effect of photocatalyst amount on photodegradation rate of Congo red is shown in Fig. S15a, b (see supplementary). As seen, 0.09 g of catalyst for visible light and 0.05 g of catalyst under UV irradiation show 100% optical degradation of dye which represent the optimal amount of catalyst for purification of 100 mL color solution with 5 ppm concentration in 30 min.
The Effect of Bi2O3 Morphology as Catalyst on Photodegradation
Based on the obtained results, whereas Bi2O3Starch is active for photodegradation of Congo red with 82% and 100% under Vis and UV irradiation, respectively (Table 2), Bi2O3Starch or Bi2O3CTAB are the most active for photodegradation of methylene blue under visible irradiation (Table 3). On the other hand, it was found that Bi2O3TEA show the least catalytic activity for photodegredatoion of Congo red and methylene blue under Vis or UV irradiation (Tables 2, 3).
Finally, our results obtained for photocatalytic activity of Bi2O3 with various structures or morphologies has been compared with those of previously reported (Table 4) [34,35,36,37]. As seen in this Table, the effect of using metal free Bi2O3-starch or Bi2O3-CTAB and Bi2O3-starch on the photocatalytic degradation of methylene blue and Congo red respectively under visible and visible or UV irradiations seem promising.
Conclusions
In conclusion, Bi2O3 with 5 different templates of TEA, OA, CTAB, Starch and PEG were prepared by sol gel method followed by calcination at 600 °C. The prepared samples were used as photodegradation catalyst for Congo red and methylene blue as organic colors under Vis and UV irradiation. It was found that whereas Bi2O3-starch, Bi2O3-CTAB and Bi2O3-PEG show the most photodegradation effect on methylene blue, Bi2O3-starch and Bi2O3-CTAB are the most active catalyst for photodegradation on Congo red under UV irradiation. In other word, the type of organic dyes, the morphology of photocatalysts and the templates are important factors on the photodegradation and adsorption phenomena.
References
K. Kaur, R. Badru, P.-P. Singh, and S. Kaushal (2020). J. Environ. Chem. Eng. 8, 103666.
C. Chen, W. Ma, and J. Zhao (2010). Chem. Soc. Rev. 39, 4206.
N. Sobana, M. Muruganadham, and M. Swaminathan (2006). J. Mol. Catal. A: Chem. 258, 124.
L. Leontie, M. Caraman, M. Alexe, and C. Harnagea (2002). Surf. Sci. 507–510, 480.
W. Li (2006). Mater. Chem. Phys. 99, 174.
G. Bandoli, D. Barecca, E. Brescacin, G. A. Rizzi, and E. Tondello (1996). Chem. Vap. Depos. 2, 238.
H. Woo Kim (2008). Thin Solid Films 516, 3665.
M. Schuisky and A. Harsta (1996). Chem. Vap. Depos. 2, 235.
P. M. V. Almeida, C. B. Gozzo, E. H. N. S. Thaines, A. J. M. Sales, R. G. Freitas, A. J. Terezo, A. S. B. Sombra, and M. M. Costa (2017). Mater. Chem. Phys. 10, 1.
J. Deng and Z. Zhao (2018). Comput. Mater. Sci. 142, 312.
S. Schmidt, E. T. Kubaski, D. P. Volanti, T. Sequinel, V. D. N. Bezzon, and S. M. Tebcherani (2018). Part Sci. Technol. https://doi.org/10.1080/02726351.2018.1457108.
N. Sugimoto, H. Kanbara, S. Fujiwara, K. Tanaka, and Y. Shimizugawa (1999). J. Opt. Soc. Am. B 16, 1904.
M. Drache, P. Roussel, and J.-P. Wignacourt (2007). Chem. Rev. 107, 80–96.
J. Yu and A. Kudo (2006). Adv. Funct. Mater. 16, 2163.
H. Cheng, B. Huang, J. Lu, Z. Wang, B. Xu, X. Qin, X. Zhang, and Y. Dai (2010). Phys. Chem. Chem. Phys. 12, 15468.
Z. Ai, Y. Huang, S. Lee, and L. Zhanga (2011). J. Alloys. Compd. 509, 2044.
A. Hameed, T. Montini, V. Gombac, and P. Fornasiero (2008). J. Am. Chem. Soc. 130, 9658.
Z. Bian, J. Zhu, S. Wang, Y. Cao, X. Qian, and H. Li (2008). J. Phys. Chem. C 112, 6258.
H. Y. Jiang, J. Liu, K. Cheng, W. Sun, and J. Lin (2013). J. Phys. Chem. C 117, 20029.
S. Anandan, G. Lee, P. Chen, C. Fan, and J. J. Wu (2010). Ind. Eng. Chem. Res. 49, 9729.
V. Bekiari, M. Sotiropoulou, G. Bokias, and P. Lianos (2008). Colloid Surf. A 312, 214.
M. Mallahi, A. Shokuhfar, M. R. Vaezi, A. Esmaeilirad, and V. Mazinani (2014). Am. J. Eng. Res. 3, 162.
A. K. Hezama, Q. A. Namrathaa, Z. H. Drmoshb, Q. A. Yamanib, and K. Byrapaa (2017). Ceram. Int. 43, 1.
K. Vasanth- Kumar, K. Porkodi, and F. Roch (2008). Catal. Commun. 9, 82.
C. G. Da Silva and J. L. Faria (2003). J. Photochem. Photobiol. A 155, 133.
N. Serpone and A. Emeline (2002). IJP 2, 91.
M. Movahedi, A. R. Mahjoub, and S. Janitabar-Darzi (2009). JICS 6, 570.
R. S. Dariani, A. Esmaeili, A. Mortezaali, and S. Dehghanpour (2016). Optik 12, 7143.
J. Hong, N. Ta, S. Yang, Y. Liu, and C. Sun (2007). Desalination 214, 62.
T. Saison, N. Chemin, C. Chaneac, O. Durupthy, V. Ruaux, L. Mariey, F. Mauge, P. Beaunier, and J. P. Jolivet (2011). J. Phys. Chem. C 115, 5657.
I.K. Konstantinou and T.A. Albanis (2004). Appl. Catal. B: 491.
T. N. Soitah, Y. Chunhui, Y. Yong, N. Yinghua, and S. Liang (2010). Appl. Phys. 10, 1372.
N. M. Mahmoodi, M. Arami, N. Y. Limaee, and N. S. Tabrizi (2006). J. Colloid Interface Sci. 295, 159.
Y. C. Wu, Y. C. Chaing, C. Y. Huang, S. F. Wang, and H. Y. Yang (2013). Dyes Pigm. 98, 25.
M. Jalalah, M. Faisal, H. Bouzid, J. G. Park, S. A. Al-Sayari, and A. A. Ismail (2015). J. Ind. Eng. Chem. 30, 183.
L. Song and S. Zhang (2010). React. Kinet. Mech. Catal. 99, 235.
W. Raza, A. Khan, U. Alam, M. Muneer, and D. Bahnemann (2016). J. Mol. Struct. 1107, 39.
S. M. Yakout (2020). J. Environ. Chem. Eng. 8, 103644.
Acknowledgements
The financial support from Alzahra University is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moghadam, S.A., Farzaneh, F. Synthesis and Investigation of Template Effects on Nanoporous Bi2O3 Morphologies by Sol Gel Method as Photocatalysts for Degradation of Some Organic Dyes as Water Pollutants. J Clust Sci 33, 495–502 (2022). https://doi.org/10.1007/s10876-021-01994-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-021-01994-w