Abstract
Cancer nanotechnology has emerged as the promising cutting edge of researches to combat cancer. Cancer nanotechnology can be described as the science of synthesizing and utilizing nanoscale materials for cancer theranostics. Colorectal cancer is a common type of cancers worldwide and a leading cause of cancer-related death. Up to know, a number of laboratory studies represented the anticancer potential of biogenic silver nanoparticles (AgNPs) against colorectal cancers. This study was aimed to systematically review the published articles to assess the cytotoxicity of biogenic AgNPs against colorectal cancer cells through the laboratory in vitro investigations. The international electronic databases involving Cochrane, PubMed, Scopus, Web of Science, ProQuest, Science Direct, and Embase were searched to identify the records. The results revealed different biological resources for the synthesis of AgNPs such as plants, bacteria, fungi, and algae. Most of the AgNPs were synthesized with spherical morphology and particle size of less than 100 nm. Most of the studies showed considerable anticancer effects of AgNPs with the half maximal inhibitory concentration (IC50) against colorectal cancer cells. The findings of this study promise the bright future of biogenic AgNPs for colorectal cancer therapy.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer remains the third most common cancer-related leading cause of death with a total estimated 51,020 deaths in 2019 in the United States (US) comprising 27,640 deaths in men and 23,380 deaths in women. According to the American Cancer Society, an estimated 78,500 and 67,100 cases of colorectal cancer will be diagnosed in 2019 in the US among males and females, respectively [1]. From the histopathological aspect, adenocarcinomas include above 90% of colorectal cancers and the rare types include adenosquamous, squamous cell, spindle cell, neuroendocrine, and undifferentiated carcinomas [2]. Surgery is the standard treatment for colorectal cancer to remove the tumour. However, chemotherapy and radiation therapy may also be required in some cases. The current approaches for colorectal cancer therapy are not satisfactory due to their adverse effects. Surgery can cause pain, fatigue, and bowel consistency alteration. Chemotherapy may also cause fatigue, neuropathy, hair loss, and gastrointestinal problems. Besides, radiation therapy may cause rectal irritation, nausea, diarrhea as well as sexual problems. Significantly, these approaches not only cause side effects, but also impose a large cost to the patients [3, 4]. Hence, to explore novel strategies for colorectal therapy, it would be highly valuable to choose the optimal theranostic regimens to prevent from unnecessary side effects and costs.
Nanotechnology is an emerging field that deals with design, characterization, production, and application of materials at nanoscale with considerable potentials in different fields including pharmaceutics, medicine, biology, physics, electronics, etc. [5,6,7]. The unique physicochemical properties of nanomaterials have made them attractive for various applications in different fields of science. The metallic nanoparticles (MNPs) have gained highlighted interest for cancer theranostics [6, 8]. These MNPs can be prepared through physical, chemical and biological approaches. Among the mentioned approaches, the biological approach appears to have much more superiorities over physical and/or chemical approaches owing to their eco-friendliness, simplicity, and cost-effectiveness [9]. In biological approaches for MNPs preparation, different biological resources such as plants, microorganisms and even algae have been reported in the literature that can bio-fabricate the MNPs with different sizes and shapes [10,11,12,13,14,15,16,17,18]. Among MNPs, silver nanoparticles (AgNPs) have emerged significant attention for cancer theranostics during recent years [6, 8]. Recently, a 2019 study, systematically reviewed the published papers to evaluate the anticancer potential of biologically synthesized AgNPs against breast cancer cells through in vitro investigations and represented significant breast anticancer activity of eco-friendly synthesized AgNPs [6]. Likewise, another 2019 study, systematically reviewed the recent developments in the prostate anticancer activity of biogenic AgNPs and gold nanoparticles (AuNPs) among laboratory studies and showed a high potential of biogenic AgNPs and AuNPs to combat prostate cancer cells through in vitro model [8]. Up to now, a number of studies evaluated the cytotoxicity of biogenic AgNPs against colorectal cancer cells. A study reported the nontoxicity of plant-mediated synthesized AgNPs in the size distribution of 25–40 nm with spherical morphology against HCT-116 human colon cancer cells at the concentration of 350 µg/mL using the MTT assay [19]. In contrast, a study reported the dose-dependent and time-dependent cytotoxicity of plant-mediated synthesized spherical shaped AgNPs with the average size of 30 nm against HCT-116 cells with the IC50 of 175 and 45.6 µg/mL after 48 and 72 h of treatment, respectively [20]. Moreover, a study reported that phytosynthesized AgNPs were more cytotoxic against SW620 colorectal cancer cells compared to HCT-8 colorectal cancer cells [21]. Surprisingly, a study reported that algal-mediated synthesized AgNPs with an average of 31 nm and spherical morphology were significantly cytotoxic against HT-29 colorectal cancer cells, while the synthesized AgNPs were found nontoxic against Caco-2 colorectal cells [22]. Here, a question arises that the biogenic AgNPs are effective against colorectal cancer or not? The literature suffers from the lack of comprehensive review to represent the efficacy of biogenic AgNPs against colorectal cancer cells. To the best of our knowledge, the current systematic review is the first comprehensive investigation representing the anticancer potential of bio-mediated synthesized AgNPs against colorectal cancer cells through laboratory studies.
Materials and Methods
This study is a systematic review of published articles to assess the cytotoxicity of biogenic AgNPs against colorectal cancer cells through in vitro studies.
Search Strategy
The search strategy was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [23]. The international online databases including Cochrane, PubMed, Scopus, Web of Science, Science Direct, Embase, and ProQuest were searched for the articles published up to 19 June 2019 with no restriction on publication date using the keywords of “silver”, Ag”, and “synthesis”, “fabrication”, “biosynthesis”, “biofabrication”, “green”, “bioreduction”, “myco*”, “biogenic”, “biomimetic”, “plant*”, “phyto*”, “herbal”, “fungal”, “bacterial”, “algal”, “microbial”, “biological”, and “nanoparticle*”, “colloidal”, “nanomaterial*”, “nanostructure*”, “nano-silver”, and “antitumor*”, “anticancer*”, “antineoplastic”, “cell line*”, “cancer*”, “tumor*”, “cytotoxicity”, “cytotoxic”, “colon”, “colorectal”. Moreover, to avoid missing matching articles, the references list of selected articles were reviewed.
Study Selection
To assess the eligibility of the identified records, the first screening was performed based on the reviewing of the titles and abstracts of the articles and consequently, the secondary screening was conducted based on the reviewing of their full texts by two independent researchers.
Inclusion Criteria
The articles with the following characteristics were included: (i) articles obtained from the aforementioned key search; (ii) articles contain sufficient information; (iii) original articles; (iv) English language articles; (v) published and/or in press articles; (vi) the articles that evaluated the cytotoxicity of biogenic AgNPs through in vitro models against colorectal cancer cells.
Exclusion Criteria
The articles with the following characteristics were excluded: (i) duplicated articles; (ii) congress posters; (iii) non-related articles; (iv) editorials; (v) review articles; (vi) letters to the editor; (vii) case reports; (viii) articles are written in any language except English; (ix) articles without full text; (x) articles that studied the cytotoxicity of biogenic AgNPs against any cancer cells except colorectal cancer cells; (xi) articles that studied the cytotoxicity of chemical and/or physical-mediated fabricated AgNPs against colorectal cancer cells; (xii) articles that studied the cytotoxicity of other metallic NPs except AgNPs against colorectal cancer cells.
Data Collection
Data extraction was done from the selected articles using an extraction form (Table 1) comprising first author, year of publication, a biological source with scientific name, characterization techniques, size (nm), morphology, colorectal cancer cell line, dose, exposure time, cytotoxicity method, and major outcome. It is highlighted that two researchers not only assessed the eligibility of the above-mentioned inclusion and exclusion criteria to select the relevant articles, but also checked the data extraction from the selected articles to limit the bias.
Results
Search Results
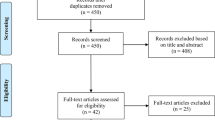
The flow diagram of the current literature search was shown in Fig. 1. Of 1650 identified records, 1117 duplicated records, and 464 irrelevant articles (during the first screening of titles or abstracts) were excluded. Consequently, the secondary screening was performed on 69 articles’ full text for eligibility assessment. Eventually, 39 articles were identified eligible to enter into the present systematic review which reported the in vitro anticancer activity of biogenic AgNPs against colorectal cancer cells.
Characteristics of Included Studies
Table 1 represented the extracted data from the selected articles. According to Table 1, phytosynthesis was the most prevailing approach for fabrication of AgNPs (n = 30). Besides, the bacterium (n = 5), fungus (n = 3), and alga (n = 2) were other biological sources that were used for fabrication of AgNPs. Significantly, most of the studies reported spherical shaped AgNPs (n = 38), while only one study (n = 1) reported the cubic morphology for prepared AgNPs. Besides, the AgNPs were synthesized below 100 nm in most of the studies (n = 35), while few studies (n = 4) reported the size of AgNPs above 100 nm. Moreover, among all studies, eleven colorectal cancer cell lines were evaluated for anticancer activity of biogenic AgNPs. Among these cell lines, HT-29 and HCT-116 colorectal cell lines were the first (34.09% of studies) and second (22.72% of studies) most dominated cancer cells through all studies, respectively. Additionally, the cytotoxicity of biogenic AgNPs was investigated using MTT (n = 34), WST-1 (n = 2), Alamar Blue (n = 2), and MTS (n = 1) assays. Impressively, most of the studies demonstrated significant anticancer activity of biogenic AgNPs with the half maximal inhibitory concentration (IC50) against colorectal cancer cells.
Discussion
Nanomedicine can be described as the application of nanotechnology for diagnosis, prevention and treatment of diseases. Nanomedicines not only can solve the difficulties related to pharmacokinetics, bioavailability and solubility of the drugs, but also can overcome the off-target side effects as well as dose-dependent toxicities of drugs [59]. Nanomedicines have attracted significant interest as novel strategies to combat colorectal cancer. Up to now, several nanoparticulate drug delivery systems have been successfully applied for drug [60, 61], gene [62], and vaccine [63] delivery to colorectal cancer cells. MNPs and particularly AgNPs have attracted significant attention in different field of studies including pharmaceutics due to their unique optical, electrical and mechanical properties which are attributed to their high surface area to volume ratio. The studies confirmed the beneficial of AgNPs for drug delivery formulations, tissue scaffold, wound dressing, antimicrobial agents, and diagnosis platforms [64]. Recently, a number of studies investigated the cytotoxicity of biogenic AgNPs against many cancer and normal cell lines. Interestingly, it was shown that the odds of cytotoxicity of microbial-mediated synthesized AgNPs in cancer cells were found 15,973 times more than normal cells through in vitro investigations (OR = 15.973, P < 0.001), which represents much more AgNPs-induced cytotoxicity chance in cancer cells [6]. On the other hand, the promising anticancer activity of biogenic AgNPs against breast and prostate cancer provides preliminary evidence for the next generation of anticancer drugs [6, 8]. Due to the lack of comprehensive information to show the efficacy of biogenic AgNPs against colorectal cancers, we decided to conduct a global systematic review to evaluate the anticancer potential of bio-mediated synthesized AgNPs against colorectal cancer cells from the original published articles up to 19 June 2019. Among all studies, the cytotoxicity of biogenic AgNPs was evaluated against different colorectal cancer cells including HCT-116 (human colon carcinoma), HT-29 (human colorectal adenocarcinoma), Caco-2 (human epithelial colorectal adenocarcinoma), SW480 (colon carcinoma), HCT-15 (human colorectal adenocarcinoma), COLO205 (human colon adenocarcinoma), LoVo (human colon adenocarcinoma), HCT-8 (human colorectal adenocarcinoma), SW620 (human colorectal adenocarcinoma), C26 (murine colon carcinoma), and HT-115 (human colon carcinoma). The current systematic review revealed the different anticancer potential of biogenic AgNPs against different colorectal cell lines. In a study, algal-mediated synthesized AgNPs exhibited much more cytotoxicity against HT-29 cells (IC50 = 13 µM) compared to Caco-2 cells (IC50 = 170 µM) [22]. Likewise, SW480 cell line showed more sensitivity to phytosynthesized AgNPs compared to HCT-116 cell line with the IC50 of around 50 and 75 µg/mL, respectively [27]. Besides, herbal-mediated synthesized AgNPs showed more cytotoxicity against HT-29 cells (IC50 = 6 µg/mL) compared to HCT-15 (IC50 = 8 µg/mL) cells after 24 h of treatment using MTT assay [57]. Remarkably, the studies showed that the AgNPs obtained from different plants had different cytotoxicity against the same colorectal cancer cells. A study reported the phytosynthesis of AgNPs using the plant extracts of Ferula asafetida, Acacia nilotica, and Phoenix dactylifera, separately and then evaluated the anticancer activity of these green AgNPs against LoVo colorectal cancer cells. The results showed different anticancer potential of these AgNPs against LoVo cells with the IC50 values of 46.15 ± 2.0, 58.02 ± 2.1, and 69.73 ± 2.02 µg/mL, respectively [30]. Similarly, a study reported the bacterial-mediated synthesis of AgNPs against, Bacillus thuringiensis, Staphylococcus aureus, Escherichia coli, Salmonella typhimurium, separately and consequently evaluated the anticancer activity of AgNPs against HCT-116 colorectal cancer cells. The results revealed various anticancer potential of AgNPs with the IC50 of < 200, ~ 100, ~ 75, and ~ 70 µg/mL, respectively [34]. Here, a question arises that why different biologically synthesized AgNPs represented different cytotoxicity against the same colorectal cancer cell lines in the same situation? To answer this question, it is of high importance to mention that different features of AgNPs influence their cytotoxicity. These features include morphology, size distribution, surface charge, surface area, particle aggregation, and capping agent of AgNPs [6]. In the biological-mediated synthesis of AgNPs, different biomolecules are responsible for bioreduction of silver cations to convert them to AgNPs. Moreover, these biomolecules remain attached to the surface of these AgNPs and act as capping and stabilizing agents. In the chemical synthesis of AgNPs, the stabilizing agents should be added externally, but in biological approach no external stabilizers are required [9]. The probable mechanisms for AgNPs internalization through the mammalian cells were suggested as fluid phase endocytosis, PI3/Akt-mediated endocytosis, and macropinocytosis, as well as pinocytosis, caveolae- and clathrin-dependent mediated endocytosis, and phagocytosis [65]. The AgNPs can cause a variety of cellular changes such as conformational alteration, mutations, signaling pathways alteration, enzyme failure, and membrane permeability disorders [66]. Oxidative stress and overproduction of Reactive Oxygen Species (ROS) are the most probable mechanism for AgNPs-induced cytotoxicity that can cause cellular damage to proteins, lipids and DNA [65]. It was reported that the AgNPs-induced apoptosis is mitochondria-dependent because AgNPs cause apoptosis through the cytochrome c release into the cytosol and Bax translocation to the mitochondria [67]. In addition to the role of ROS in AgNPs-induced apoptosis, it was reported that AgNPs can induce the inflammatory cytokines (TNF-α, IL-1β, IL-1, IL-6) release in cells which can cause DNA damage [65]. Huang et al. [25] reported the significant anticancer activity of plant-mediated synthesized spherical shaped AgNPs with average diameter size of 50 nm against HT-29 colorectal cells with IC50 of 254 µg/mL after 24 h of treatment using MTT assay. The authors believed that the anticancer activity of phytosynthesized AgNPs is significantly attributed to the plant extract chemical compositions in addition to physicochemical characteristics of AgNPs. Wang et al. 2018 evaluated the anticancer activity of Fungus-mediated synthesized AgNPs using Aspergillus niger against HT-29 colorectal cancer cells through MTT assay. The spherical shaped AgNPs in the range of 20–25 nm exhibited major cytotoxic effects against HT-29 cells with the IC50 of around 120 µg/mL. The ROS generation assay and Caspase-3 activity assay revealed an elevated level of ROS and caspase-3 expression in the HT-29 cells group that were treated with mycogenic AgNPs. The authors reported that this activation of caspase-3 indicated the AgNPs-induced programmed cell death through apoptosis. Because the caspase-3 enzyme is a protease that acts as a mediator and has a major role in apoptosis [26]. Likewise, the overexpression of caspase-3, caspase-8, and caspase-9 was reported in a study that evaluated the anticancer activity of phytosynthesized AgNPs against HCT-116 colorectal cancer cells. The activation of the caspase enzymes is a hallmark feature of apoptosis [20]. Additionally, Kim et al. [32] evaluated the cytotoxicity of plant-mediated synthesized AgNPs against HT-29 colorectal cancer cells and reported the considerable cytotoxic influence of AgNPs on HT-29 cells with IC50 of less than 20 µg/mL. This study also confirmed the activation of the caspase-3 enzyme in the AgNPs treated group. The authors showed that the AgNPs-induced apoptosis in HT-29 cells was attributed to excessive production of ROS by activation of the Caspase-3/p38 MAPK pathway. In this global systematic review, we provided valuable information indicating the significant anticancer potential of biogenic AgNPs against colorectal cancer cells through in vitro studies. We also discussed the proposed anticancer mechanisms of AgNPs. Further studies are required to show the anticancer efficacy of biogenic AgNPs through in vivo studies. Moreover, although many studies have shown the significant potential of biologically synthesized MNPs [68,69,70,71,72,73,74], it does not mean that the biosynthesis of MNPs prevents from the MNPs-induced adverse effects [75]. Hence, to translate these laboratory investigations to clinical trials, many challenges should be addressed in the future studies such as genotoxicity, immunogenicity, AgNPs adverse effects on noncancerous cells, etc. Besides, the information about protein corona and their role on the cellular uptake and biogenic AgNPs cytotoxicity is not still understood well and should be considered in the future studies. Moreover, as a new strategy to combat cancer, it is suggested to evaluate the synergistic effects of FDA approved anticancer drugs in conjugation to biogenic AgNPs against different cancers in further studies.
Conclusion
The current study systematically reviewed the anticancer potential of biologically synthesized AgNPs against colorectal cancer cells through in vitro models. This study not only provided valuable information indicating the strong in vitro cytotoxicity of biogenic AgNPs, but also discussed the proposed mechanisms of AgNPs-induced cytotoxicity. Most of the studies showed dose and/or time-dependent cytotoxicity against colorectal cancer cells. Besides, these AgNPs have the potential to be conjugated to other colorectal drugs. Moreover, as the size, morphology, surface chemistry and surface charge of AgNPs are the significant factors that can influence the cytotoxicity, hence, the biological procedure of AgNPs preparation can be designed to achieve the AgNPs with optimal characteristics. Further studies should address many challenges and concerns about the safety of these AgNPs for the environment and human. Future anticancer studies should focus on the animal models and provide enough information about the biogenic AgNPs pharmacokinetics, and pharmacodynamics.
References
R. L. Siegel, K. D. Miller, and A. Jemal (2019). CA Cancer J. Clin.69, 7.
M. Fleming, S. Ravula, S. F. Tatishchev, and H. L. Wang (2012). J. Gastrointest. Oncol.3, 153.
S. Sridhar Chapter 81—Colorectal cancer. in D. Rakel (ed.), Integrative Medicine, 4th ed (Elsevier, Philadelphia, 2018), p. 800.
M. Beheshti, A. Rezaee, K. Herrmann, M. Raderer, and W. Langsteger Chapter 6—Colorectal cancer. in M. Beheshti, W. Langsteger, and A. Rezaee (eds.), PET/CT in Cancer: An Interdisciplinary Approach to Individualized Imaging (Elsevier, Philadelphia, 2018), p. 111.
R. Balachandar, P. Gurumoorthy, N. Karmegam, H. Barabadi, R. Subbaiya, K. Anand, P. Boomi, and M. Saravanan (2019). J. Clust. Sci.. https://doi.org/10.1007/s10876-019-01591-y.
H. Barabadi, M. A. Mahjoub, B. Tajani, A. Ahmadi, Y. Junejo, and M. Saravanan (2019). J. Clust. Sci.30, 259.
M. Saravanan, T. Asmalash, A. Gebrekidan, D. Gebreegziabiher, T. Araya, H. Hilekiros, H. Barabadi, and K. Ramanathan (2018). Pharm. Nanotechnol.6, 17.
H. Barabadi, K. Damavandi Kamali, F. Jazayeri Shoushtari, B. Tajani, M. A. Mahjoub, A. Alizadeh, and M. Saravanan (2019). J. Clust. Sci.. https://doi.org/10.1007/s10876-019-01588-7.
H. Barabadi, S. Honary, P. Ebrahimi, A. Alizadeh, F. Naghibi, and M. Saravanan (2019). Inorg. Nano-Met Chem.49, 33.
S. Salari, S. E. Bahabadi, A. Samzadeh-Kermani, and F. Yousefzaei (2019). Iran. J. Pharm. Res.18, 430.
Z. R. Amin, Z. Khashyarmanesh, B. S. F. Bazzaz, and Z. S. Noghabi (2019). Iran. J. Pharm. Res.18, 210.
T. Ramezani, M. Nabiuni, J. Baharara, K. Parivar, and F. Namvar (2019). Iran. J. Pharm. Res.18, 222.
H. Barabadi, F. Kobarfard, and H. Vahidi (2018). Iran. J. Pharm. Res.17, 87.
M. Maham and R. Karami-Osboo (2017). Iran. J. Pharm. Res.16, 462.
N. Karimi, A. Chardoli, and A. Fattahi (2017). Iran. J. Pharm. Res.16, 1167.
R. Dobrucka (2017). Iran. J. Pharm. Res.16, 753.
Q. Abbas, M. Saleem, A. R. Phull, M. Rafiq, M. Hassan, K.-H. Lee, and S.-Y. Seo (2017). Iran. J. Pharm. Res.16, 760.
M. M. O. Rashid, M. S. Islam, M. A. Haque, M. A. Rahman, M. T. Hossain, and M. A. Hamid (2016). Iran. J. Pharm. Res.15, 591.
S. Naraginti and Y. Li (2017). J. Photochem. Photobiol. B.170, 225.
H. Padinjarathil, M. M. Joseph, B. S. Unnikrishnan, G. U. Preethi, R. Shiji, M. G. Archana, S. Maya, H. P. Syama, and T. T. Sreelekha (2018). Int. J. Biol. Macromol.118, 1174.
S. Devanesan, M. S. AlSalhi, R. Vishnubalaji, A. A. Alfuraydi, N. M. Alajez, M. Alfayez, K. Murugan, S. R. M. Sayed, M. Nicoletti, and G. Benelli (2017). J. Clust. Sci.28, 595.
N. Gonzalez-Ballesteros, M. C. Rodriguez-Arguelles, S. Prado-Lopez, M. Lastra, M. Grimaldi, A. Cavazza, L. Nasi, G. Salviati, and F. Bigi (2019). Mater. Sci. Eng. C Mater. Biol. Appl.97, 498.
D. Moher, A. Liberati, J. Tetzlaff, and D. G. Altman (2009). PLoS Med.6, e1000097.
S. Majeed, F. H. B. Aripin, N. S. B. Shoeb, M. Danish, M. N. M. Ibrahim, and R. Hashim (2019). Mater. Sci. Eng. C102, 254.
F. Huang, Y. Long, Q. Liang, B. Purushotham, M. K. Swamy, and Y. Duan (2019). J. Nanomater.2019, 8.
C. Z. Wang, J. Z. Wen, S. J. Chen, M. K. Swamy, U. R. Sinniah, M. S. Akhtar, and A. Umar (2018). J. Nanosci. Nanotechnol.18, 3673.
F. Samari, H. Salehipoor, E. Eftekhar, and S. Yousefinejad (2018). New J. Chem.42, 15905.
S. V. Nampoothiri, B. Suresh Kumar, T. Esakkidurai, and K. Pitchumani (2018). J. Biol. Act. Prod. Nat.8, 352.
J. R. Nakkala, R. Mata, K. Raja, V. K. Chandra, and S. R. Sadras (2018). Mater. Sci. Eng. C Biomim. Mater. Sens. Syst.91, 372.
A. E. Mohammed, A. Al-Qahtani, A. Al-Mutairi, B. Al-Shamri, and K. Aabed (2018). Nanomaterials8, 382.
A. B. Leila, A. Parinaz, L. Zarei, and K. Mehri (2018). Med. Sci.22, 99.
C. G. Kim, V. Castro-Aceituno, R. Abbai, H. A. Lee, S. Y. Simu, Y. Han, J. Hurh, Y. J. Kim, and D. C. Yang (2018). Biomed. Pharmacother.99, 128.
C. Chankaew, S. Somsri, W. Tapala, S. Mahatheeranont, C. Saenjum, and A. Rujiwatra (2018). Particuology40, 160.
S. K. Verma, E. Jha, B. Sahoo, P. K. Panda, A. Thirumurugan, S. K. S. Parashar, and M. Suar (2017). RSC Adv.7, 40034.
S. Palanisamy, P. Rajasekar, G. Vijayaprasath, G. Ravi, R. Manikandan, and N. M. Prabhu (2017). Mater. Lett.189, 196.
H. Khalili, S. A. S. Shandiz, and F. Baghbani-Arani (2017). J. Clust. Sci.28, 1617.
E. Z. Gomaa (2017). J. Genet. Eng. Biotechnol.15, 49.
A. A. El-Hela, N. M. Abdelhady, M. H. Gonaid, and K. A. Badr (2017). Int. J. Pharm. Sci. Rev. Res.45, 223.
K. D. Datkhile, P. P. Durgavale, and M. N. Patil (2017). J. Bionanosci.11, 416.
A. Chahardoli, N. Karimi, and A. Fattahi (2017). Iran. J. Pharm. Res.16, 1169.
F. Baghbani-Arani, R. Movagharnia, A. Sharifian, S. Salehi, and S. A. S. Shandiz (2017). J. Photochem. Photobiol. B173, 640.
B. Varsha, R. Arvindganth, and G. Kathiravan (2016). Res. J. Pharm. Biol. Chem. Sci.7, 1578.
R. Sriranjani, B. Srinithya, V. Vellingiri, P. Brindha, S. P. Anthony, A. Sivasubramanian, and M. S. Muthuraman (2016). J. Mol. Liq.220, 926.
M. Sengani and V. D. Rajeswari (2016). Res. J. Biotechnol.11, 65.
A. Rajasekar, V. Janakiraman, and K. Govindarajan (2016). Asian J. Pharm. Clin. Res.9, 189.
S. Jena, R. K. Singh, B. Panigrahi, M. Suar, and D. Mandal (2016). J. Photochem. Photobiol. B164, 306.
M. S. Alsalhi, S. Devanesan, A. A. Alfuraydi, R. Vishnubalaji, M. A. Munusamy, K. Murugan, M. Nicoletti, and G. Benelli (2016). Int. J. Nanomed.11, 4439.
H. M. Abd-Elnaby, G. M. Abo-Elala, U. M. Abdel-Raouf, and M. M. Hamed (2016). Egypt J. Aquat. Res.42, 301.
M. Ramar, B. Manikandan, T. Raman, K. Arunagirinathan, N. M. Prabhu, M. J. Basu, M. Perumal, S. Palanisamy, and A. Munusamy (2015). Spectrochim. Acta A Mol. Biomol. Spectrosc.138, 120.
P. Premasudha, M. Venkataramana, M. Abirami, P. Vanathi, K. Krishna, and R. Rajendran (2015). Bull. Mater. Sci.38, 965.
M. Potara, M. Bawaskar, T. Simon, S. Gaikwad, E. Licarete, A. Ingle, M. Banciu, A. Vulpoi, S. Astilean, and M. Rai (2015). Colloids Surf. B.133, 296.
R. Nalavothula, J. Alwala, V. B. Nagati, and P. R. Manthurpadigya (2015). Int. J. Chemtech. Res.7, 2460.
R. Mata, J. R. Nakkala, and S. R. Sadras (2015). Colloids Surf. B128, 276.
S. Z. Ghozali, L. Vuanghao, and N. H. Ahmad (2015). J. Nanomed. Nanotechnol.6, 1.
G. Singh, P. K. Babele, S. K. Shahi, R. P. Sinha, M. B. Tyagi, and A. Kumar (2014). Int. J. Microbiol. Biotechnol.24, 1354.
R. Nalvolthula, R. Merugu, and M. P. Pratap Rudra (2014). Int. J. Chemtech. Res.7, 2374.
P. Durai, C. Arulvasu, B. Gajendran, M. Ramar, S. Pappu, G. Kasivelu, and A. Thirunavukkarasu (2014). Eur. J. Med. Chem.84, 90.
D. Prabhu, C. Arulvasu, G. Babu, R. Manikandan, and P. Srinivasan (2013). Process Biochem.48, 317.
A. I. Matos, B. Carreira, C. Peres, L. I. F. Moura, J. Conniot, T. Fourniols, A. Scomparin, A. Martinez-Barriocanal, D. Arango, J. P. Conde, V. Preat, R. Satchi-Fainaro, and H. F. Florindo (2019). J. Control Release.307, 108.
C. Yang, H. Z. Liu, Z. X. Fu, and W. D. Lu (2011). BMC Biotechnol.11, 21.
K. Liu, Z.-Q. Wang, S.-J. Wang, P. Liu, Y.-H. Qin, Y. Ma, X.-C. Li, and Z.-J. Huo (2015). Int. J. Nanomed.10, 6445.
L. Tan, S. Han, S. Ding, W. Xiao, Y. Ding, L. Qian, C. Wang, and W. Gong (2017). Int. J. Nanomed.12, 3095.
T. J. Goodwin and L. Huang (2017). Vaccine35, 2550.
A. C. Burdusel, O. Gherasim, A. M. Grumezescu, L. Mogoanta, A. Ficai, and E. Andronescu (2018). Nanomaterials8, 681.
X. F. Zhang, W. Shen, and S. Gurunathan (2016). Int. J. Mol. Sci.17, 1534.
T. Xia, N. Li, and A. E. Nel (2009). Annu. Rev. Public Health30, 137.
Y. H. Hsin, C. F. Chen, S. Huang, T. S. Shih, P. S. Lai, and P. J. Chueh (2008). Toxicol. Lett.179, 130.
M. Ovais, A. Khalil, M. Ayaz, I. Ahmad, S. Nethi, and S. Mukherjee (2018). Int. J. Mol. Sci.19, E4100.
M. Ovais, A. T. Khalil, A. Raza, N. U. Islam, M. Ayaz, M. Saravanan, M. Ali, I. Ahmad, M. Shahid, and Z. K. Shinwari (2018). Appl. Microbiol. Biotechnol.102, 4393.
M. Ovais, A. Nadhman, A. T. Khalil, A. Raza, F. Khuda, M. F. Sohail, N. U. Islam, H. S. Sarwar, G. Shahnaz, I. Ahmad, M. Saravanan, and Z. K. Shinwari (2017). Nanomedicine12, 2807.
M. Ovais, A. T. Khalil, N. U. Islam, I. Ahmad, M. Ayaz, M. Saravanan, Z. K. Shinwari, and S. Mukherjee (2018). Appl. Microbiol. Biotechnol.102, 6799.
M. Ovais, A. T. Khalil, A. Raza, M. A. Khan, I. Ahmad, N. U. Islam, M. Saravanan, M. F. Ubaid, M. Ali, and Z. K. Shinwari (2016). Nanomedicine11, 3157.
M. Ovais, A. Raza, S. Naz, N. U. Islam, A. T. Khalil, S. Ali, M. A. Khan, and Z. K. Shinwari (2017). Appl. Microbiol. Biotechnol.101, 3551.
M. Ovais, I. Ahmad, A. T. Khalil, S. Mukherjee, R. Javed, M. Ayaz, A. Raza, and Z. K. Shinwari (2018). Appl. Microbiol. Biotechnol.102, 4305.
H. Barabadi, M. Najafi, H. Samadian, A. Azarnezhad, H. Vahidi, M. A. Mahjoub, M. Koohiyan, and A. Ahmadi (2019). Medicina55, 439.
Acknowledgements
This work was financially supported by Shahid Beheshti University of Medical Sciences, Tehran, Iran [Grant Number: 19352].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Barabadi, H., Vahidi, H., Damavandi Kamali, K. et al. Emerging Theranostic Silver Nanomaterials to Combat Colorectal Cancer: A Systematic Review. J Clust Sci 31, 311–321 (2020). https://doi.org/10.1007/s10876-019-01668-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-019-01668-8