Abstract
Photocatalytic behavior was investigated for TiO2–graphene nanocomposite in the degradation of acid orange 7 (AO7) as a model pollutant under ultraviolet light in aqueous solution. XRD, SEM, TEM, DRS, FT-IR and EDX techniques were used for the characterization of the prepared nanocomposite. The effect of synthesis variables such as weight ratio of TiO2 to graphene and operational key factors such as initial dye concentration, irradiation time, catalyst dosage and solution distance from UV lamp were studied in the photocatalytic degradation of AO7. This excellent catalytic ability is mainly attributed to the synergic effect of photocatalyst and adsorbent. The effect of operational variables was optimized for the photocatalytic degradation of AO7 as a pollutant model using the RSM technique. In this case, the amount of the determination coefficient (R2 = 0.97) shows that 97% of the variability in the response could be described by the model. The maximum degradation efficiency (96%) was achieved at the optimum operational conditions: catalyst dosage of 0.5 g L−1, the irradiation time of 50 min and distance the solution from UV lamp of 0.3 cm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is known, that the textile industry with its manufacturing process is characterized by the high consumption of resources like water, fuel and a large variety of chemicals [1]. Reactive dyes, including azo dyes, are widely used due to their higher performance. But many of them are environmentally extremely hazardous and are proven to be carcinogenic [2,3,4]. Hence, removal or degradation of organic pollutants is an important research subject. Considerable efforts have been devoted to developing a suitable purification method that can easily destroy these organic contaminants [5]. Nowadays it is well confirmed that advanced oxidation processes (AOPs) are the best-recommended technologies for the removal of different pollutants from water [6]. Heterogeneous photocatalysis oxidation, as the most popular AOP method, has been widely used for removal of water pollutants because of its advantages, especially capability of carrying out under ambient conditions [7,8,9,10,11].
Hereby a considerable attention was shown over the past years for TiO2 as a photocatalyst with high activity, low cost, non-toxicity, high stability in aqueous solutions and a possible use of solar irradiation [12, 13]. TiO2 with energy band gap about 3.2 eV, which implies an excitation at a wavelength <400 nm, is also able to reduce metallic ions besides the degradation of toxic organic compounds and its basic efficiency can be enhanced by coupling process [14, 15]. The quick recombination of photogenerated electron–hole pairs decreases the efficiency of TiO2 as a photocatalyst in wastewater treatment process [16, 17].
However, practical applications of TiO2 as an adsorbent or a photocatalyst in aqueous solutions are limited because of the recovery problems of fine TiO2 particles [18]. Recently, attempts have been made to immobilize the TiO2 particles on different supports, such as activated carbon [19], clay [20] and zeolites [21], to improve their separation from bulk water [22].
Graphene (GR) as a new carbon nanomaterial has many exceptional properties, such as high electron mobility, high transparency, flexible structure, and large theoretical specific surface area [23, 24]. It is worthy to mention that the nanoparticles are directly decorated on the graphene sheets and no molecular linkers are needed to bridge the nanoparticles and the graphene which may prevent additional trap states along the sheets. Therefore, many types of the second phase can be deposited on graphene sheets in the form of nanoparticles to impart new functionality to graphene aiming at catalytic, energy storage, photocatalytic, and optoelectronics applications [25]. Many different types of synthesis methods have been developed for preparing graphene–nanoparticles composites, includes three main strategies; pre-graphenization, post-graphenization and syn-graphenization [26, 27].
Considering its superior electron mobility and high specific surface area, graphene can be expected to improve the photocatalytic performance of semiconductor photocatalysts such as TiO2, where graphene can act as an efficient electron acceptor to enhance the photoinduced charge transfer and to inhibit the recombination of the photogenerated electron–holes [28]. Thus, the combination of TiO2 and graphene is promising to improve the photocatalytic performance of TiO2. Most recently, Lightcap et al. [29] demonstrated that TiO2–graphene composite shows an enhancement of photocatalytic activity for the degradation of methylene blue. Zhang et al. [30] pointed out that the TiO2–graphene composite was a highly efficient photocatalyst for the degradation of gas-phase benzene. Zhang et al. [31] reported that a TiO2–graphene composite prepared by growing TiO2 nanocrystals on graphene oxide (GO) through hydrolysis of Ti(BuO)4 has improved the photocatalytic activity of TiO2 for the degradation of rhodamine B.
We employed response surface methodology (RSM) for statistically optimizing the operational and synthesis variables of TiO2–AgI by using a minimum number of experiments. The RSM is a mathematical and statistical technique that is widely employed in optimizing and modeling process. RSM technique is capable of analyzing the interactions of possible influencing factors and determining the optimum region of the factors level just by using a minimum number of designed experiments.
In the present study, TiO2–graphene composite was prepared via a combination of the ultrasonic and hydrothermal method. The photocatalytic performance of this composite was investigated for photodegradation of acid orange 7 (AO7) as a pollutant model. The effect of synthesis and operational variables, such as TiO2 content, irradiation time, catalyst dosage and solution distance from UV lamp were studied in the photocatalytic degradation of AO7. On the other hands, the effect of operational variables were optimized for the photocatalytic degradation of AO7 as a pollutant model using the RSM technique. In this case, the amount of the determination coefficient (R2 = 0.97) shows that 97% of the variability in the response could be described by the model. XRD, SEM, TEM, DRS, FT-IR and EDX techniques were used for the characterization of the prepared nanocomposite.
Experimental
Materials
All the reagents for the synthesis of TiO2–graphene such as potassium iodide, graphite, HNO3, H2SO4, KMnO4, H2O2 and titanium dioxide were commercially available from Merck and employed without further purification.
The synthesis was carried out at atmospheric pressure and ambient temperature with an ultrasonic irradiation provided by a probe sonicator (Qsonica Q700, Newtown, CT, US). FT-IR spectra was recorded a 400–4000 cm−1 region on Rayleigh WQF-510a. Powder X-ray diffraction (XRD) patterns were collected from a diffractometer of Philips Company with X’pertpro monochromatized Cu Kα radiation (λ = 1.54 Å). Microscopic morphology of products was visualized by SEM (MIRA3 TESCAN). TEM images were obtained on a CM30 transmission electron microscope with an accelerating voltage of 300 kV. The energy dispersive spectrometry (EDS) analysis was studied by XL30, Philips microscope. DRS of samples was obtained using AvaSpec 2048 TEC spectrometer for determination of the optical band gap (Eg) of pure TiO2, pure AgI, TiO2/AgI coupled nanoparticles and the ternary composite of TiO2–AgI–graphene. For determination of the Eg Eq. (1) was used:
where α is optical absorption coefficient, B is a constant dependent on the transition probability, h is the Plank’s constant and υ is the frequency of the radiation. The Eg values were calculated by plotting (αhυ)2 versus hυ, followed by extrapolation of the linear part of the spectra to the energy axis.
Preparation of Graphene Oxide
In this method, the improved Hummer method was applied to oxidize graphite for the synthesis of graphene oxide (GO) [32] from H2SO4, HNO3, KMnO4, H2O2 and distilled water.
Preparation of TiO2–Graphene Nanocomposite
A typical procedure for the synthesis of TiO2–graphene nanocomposite is as follows: 0.2 g graphite oxide was dispersed in 10 mL water for 15 min under ultrasound irradiation. Then a solution containing 0.02 g TiO2 in 10 mL of water was added dropwise into above mixture. After hydrothermal reaction at 80 °C for 5 h, graphene oxide was reduced to graphene nanosheets and TiO2 nanoparticles were fixed on its surface to generate a TiO2–graphene composite. Finally, the temperature of the mixture was brought down to room temperature and the mixture was filtered. The solid obtained was collected and washed with distilled water. After drying at 60 °C for 8 h, the TiO2–graphene composite was obtained. In order to investigate the effect of different weight ratios of TiO2 in as synthesized composite, seven different weight ratios of TiO2 to the graphene were prepared.
Photocatalytic Degradation Experiment
The photocatalytic degradation processes were carried out at room temperature in a batch quartz reactor. Artificial irradiation was provided by an 8 W (UV-C) mercury lamp (Philips, Holland) emitting around 254 nm, positioned in the top of the batch quartz reactor. In each run, 40 mg of catalyst was dispersed in 100 mL water, then desired concentration of AO7 (20 mg L−1) and photocatalyst (400 mg L−1) were transferred into the batch quartz reactor and was stirred for 30 min to reach the adsorption equilibration in the dark before irradiation. The photocatalytic reaction was initiated by turning on the light source. At given irradiation time intervals, the samples (5 mL) were retrieved, centrifuged (sigma 2-16p) and then the AO7 concentration was analyzed by UV–vis spectrophotometer (Rayleigh UV-1600) at λmax = 485 nm. All experiments were performed in initial pH of dye solution (pH 6.1). The degradation efficiency is calculated by Eq. (2):
where C0 and C are the AO7 concentration (mg L−1) at the time 0 and t.
Experimental Design
Optimization of degradation percentage versus operational variables was considered via statistical analysis. Catalyst dosage (mg/L) (A) and distance of solution from a lamp (cm) (B) were selected as effective factors. Analysis was used in order to study the relationship between these variables and the optimum levels of them. For this purpose, ten experimental runs were required as per five-level central composite design (CCD). The results and predicted responses are offered (Table 1). Data from CCD were exposed to a regression analysis to describe the behavior of the system using the least squares regression procedure to obtain the factor estimators of the mathematical typical.
Results and Discussion
Based on our previous papers that were published in 2014 and 2015 graphene oxide was synthesized and characterized by using SEM, XRD and FT-IR [23, 24]. According to XRD pattern, FT-IR and SEM images of graphite and graphene oxide (GO), the as prepared GO was properly synthesized.
Characterization and Photocatalytic Performance of TiO2–G
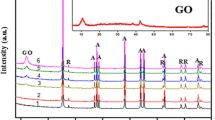
Figure 1a–c shows the XRD patterns of TiO2–G, TiO2 and graphene oxide. The diffractogram of graphene oxide exhibited the typical reflections at 2θ = 26.48° and 43.17° corresponding to the (002) and (100) reflections (JCPDS 01-0646) (Fig. 1c). According to Fig. 1b For the TiO2 compound, the XRD diffraction reflections at 2θ of 25.8°, 38°, 39.5°, 48°, 55°, 62.6°, 69.7° and 75.7° can be indexed to the characteristic reflections of the (101), (004), (112), (200), (211), (213), (220) and (215) plane reflections of anatase crystal structure TiO2. However, for the TiO2–graphene composite, only the reflections from TiO2 were detected. The (002) and (100) reflection of graphene overlapped the anatase (200) and (113) reflection of TiO2. However, for the TiO2–graphene composite, only the reflections from TiO2 were detected. The (002) reflection of graphene oxide overlapped the anatase (200) reflection of TiO2. Therefore, the intensity of the reflection at 2θ = 25.8° from the TiO2–graphene composite was stronger than that from the TiO2 compound.
Figure 2a–c show the SEM images of graphene, TiO2, and TiO2–G. As shown in Fig. 2a, graphene nanosheets are individually exfoliated. According to Fig. 2b spherical morphology of TiO2 is shown. Figure 2c shows the SEM images of the TiO2–graphene composite. According to this image, spherical TiO2 compounds were observed on the graphene sheets and particles were coated on the surface of the graphene. SEM images of graphene (Fig. 2a) and TiO2–graphene composite (Fig. 2c) show the best distribution of TiO2 particles on the graphene sheets.
TEM images of graphene and TiO2–G composite are shown in Fig. 3a, b respectively. According to TEM images, TiO2 particles are mounted on the graphene sheet surfaces and some particles intercalate between the graphene interlayer.
To get information on the elements, the TiO2–G composite was examined by EDX analysis. Figure 4 show the EDX analysis of the TiO2–G composite. In the EDX spectrum of TiO2–G composite, the main elements such as Ti, O and C were presented. According to EDX analysis, results confirmed the existence of TiO2 nanoparticles on the surface of graphene. TiO2 being present on the graphene surface emerged to trap the migrated electron (e−), leaving the holes (h+) free to react with water molecules to form hydroxyl radicals, which in turn could have degraded pollutant molecules and prevented the rapid recombination with electrons.
Since the synthesis procedure of TiO2–G was post-graphenization, so we used graphene oxide for synthesized the TiO2–G composite. Because graphene oxide is a hydrophilic compound due to presence of oxygen functional group on its surface, so, this combination is well dispersible in water. On the other hands, TiO2 is a hydrophilic compound that can connect to graphene oxide surface via hydrogen-bond to oxygen groups. Since there is no thermal stage in the preparation method of TiO2–G nanocomposite, so, after being on the graphene surface, not change is shown in the TiO2 phase. We suspected that, since there has been no change in the TiO2 phase, so TiO2’s chemical surrounding have not any change after combining with rGO. Finally, as shown in SEM and TEM images, spherical TiO2 nanoparticles placed on the surface of graphene oxide.
UV–visible diffused reflectance spectra of TiO2 and TiO2–G were shown in Fig. 5. Compared with absorption edge of TiO2 which was detected at around 399 nm, a red shift to the higher wavelength at 425 nm in the absorption edge of TiO2–G sample could be observed. It means that the TiO2–G catalyst underwent a red shift of about 26 nm. This result indicated that the narrowing of the band gap of TiO2 occurred with the graphene introduction and on the other hand, this narrowing should be attributed to the Ti–O–C bonds. In the case of TiO2–G nanocomposite, a heterojunction forms at the interface, where there is a space-charge separation region. Electrons have a tendency to flow from the higher to lower Fermi level to adjust the Fermi energy levels. The calculated work function of graphene is 4.42 eV and the conduction band position of anatase TiO2 is about −4.21 eV with a band gap of about 3.2 eV, graphene can accept the photoexcited electrons from TiO2. So, the photoinduced electron–hole pairs are effectively separated and the probability of electron–hole recombination is reduced.
The valence band (VB) and conduction band (CB) potentials of semiconductors are two important factors for the effective separation of photogenerated electron–hole pairs to generate OH radicals and superoxide anions. The VB and CB potential edges were calculated using the following empirical formula [32, 33].
where EVB and ECB are the valence and conduction band edge potentials of a semiconductor, respectively, X is the electronegativity value of the semiconductor, which is the geometric mean of the electronegativities of constituent atoms, Ee is the energy of free electrons on the hydrogen scale (~4.5 eV), Eg is the band gap energy of the semiconductor.
The photocatalytic performance of the prepared catalysts was evaluated using aqueous AO7 dye as a model compound under UV light irradiation. It is well known that the adsorption of dye molecules on catalyst surface is one of the key factors for the degradation of organic pollutants [31]. Especially carbon based materials have shown excellent adsorption capacity. Compared to TiO2, graphene loaded composite shows higher adsorption of dye molecules. This increase in dye adsorption on catalytic surfaces plays a significant role for achieving higher photocatalytic activity.
Figure 6 displays UV–vis absorption spectra of the TiO2–G composite, pure TiO2 and rGO. The enhanced absorption of the TiO2–G composites compared to TiO2 can be attributed to the presence of graphene. However, the obvious redshift of absorption edge in comparison with the pure TiO2 may suggest a rearrangement of the energy level of TiO2. This variation of the absorption property of TiO2 is expected to enhance the utilizing efficiency of solar energy in photocatalysis. The absorption peak at 235 nm in the UV–vis spectrum of rGO correspond to π–π* transition of the remaining sp2 C=C bonds.
Optimization of Synthesis Variables
Effect of TiO2 Content
As shown in Fig. 7, when experiments were carried out without catalyst, no degradation of AO7 was observed indicating that AO7 was not degraded when exposed to UV light. This observation clearly attributes that degradation of AO7 depends on the role of the photocatalyst. In order to evaluate the effect of TiO2 loading, the different weight ratio of TiO2 (1, 2, 3, 4, 10, 12.5 and 25wt%) were loaded to graphene and their photocatalytic performance was also tested under identical experimental conditions. The composite with 4wt% TiO2 showed enhanced photocatalytic degradation compared to that of pure graphene and bare TiO2 nanoparticle, whereas 12.5 and 25wt% TiO2 loaded graphene photocatalyst decreased the photocatalytic activity. As shown in Fig. 7, with increasing the weight ratio of TiO2 to graphene, the photodegradation performance of composite for AO7 was decreased, these results arise from increased the recombination rate instead of Impressive carrier separation, decreased the specific surface area and adsorptivity of graphene for adsorb dye molecules, thereby affecting the photodegradation and also cause disruption in the delivery of light to the photocatalyst. It can be seen in Fig. 7 that graphene with 4wt% TiO2 exhibits 84.26% AO7 degradation within 50 min but only 61.14 and 36.03% of degradation occurred by bare TiO2 and graphene, respectively. This enhancement in degradation was achieved due to the synergic effect in the TiO2–graphene composite. Overlap the conduction bands of TiO2 and graphene resulting in an amazing separation of charge carrier between graphene and the TiO2 interface.
Increasing adsorption and specific surface area those are related to loading graphene, the formation of ∏–∏ conjugations between dye molecules and aromatic rings of graphene and finally, the formation of ionic interactions between dye molecules and oxygen-containing functional groups of graphene are important factors that play a crucial role in the degradation of dyes. Here, graphene prevented the rapid recombination of electrons and holes because it plays the roles of adsorbent and electron acceptor.
As shown in Fig. 8, when only AO7 dye was exposed to UV light, no degradation of AO7 was observed. According to this observation degradation of AO7 is only due to the presence of photocatalyst. As shown in Fig. 8, photocatalytic performance of TiO2–G (84%) composite for photodegradation of AO7 enhanced compared to pure TiO2 (61%) and rGO (36%).
Optimization of Operational Variables with RSM Method
The aim of conducting experimental design is to optimize degradation amount. The experimental design protocol and the corresponding results are obtained (Table 1). The experimental results of the CCD were fitted with a second order equation. A number of regression coefficients were considered and the fitted equation (in terms of coded values) for prediction of kLa (Y) was as follows (R2 = 0.97)
where A and B are catalyst dosage (mg/L) and distance of solution from lamp (cm), respectively. The coefficients of the regression model (Eq. 1) that seems as one constant, two linear and two quadratics are listed in Table 1 The importation of each coefficient, determined by p values, is also given in this table.
The first order of catalyst dosage and distance of solution from the lamp are taken to be important, also. Consequently, they can act as effective parameters since differences values of them will change degradation amount to a considerable extent. It is essential to test the important and the adequacy of the model through analysis of the variance. The F value or Fisher variance ratio is a statistically valid measure of how well the factors describe the variation in the data about its mean. The greater the F value, the more certain it is that the parameters explain adequately the variation in the data around its mean, and the estimated factor effects are real. The analysis of variance of the regression model demonstrates that the model is important, as is evident from the Fisher’s F test. The goodness of fit with the quadratic model was established by the determination coefficient (R2). In this case, the amount of the determination coefficient (R2 = 0.97) shows that 97% of the variability in the response could be described by the model. The optimum values of the variables; obtained by the differentiation of the quadratic model, for achieving maximum photo-degradation of organic dye. The predicted optimal degradation value corresponding to these values is about 92%. To confirm the model precision for predicting the maximum value of photo-degradation, additional experiments in triplicates using the optimized key factors were performed. As shown in Table 1, the experimentally determined photo-degradation amount at optimum conditions (A: 0.27 and B: 1) was 92%, which agreed relatively well with model prediction. The 2D contour plots are the graphical representation of the regression equation and are plotted to recognize the interaction of the variables. The non-elliptical contour plots show that the interaction is not important for the development of optimal photo-degradation. The whole relationships between factors and response can be better understood by examining the planned series of contour plots (Fig. 9) generated from the predicted models (Eq. 1). Figure 9 represents contour plot between the responses i.e. photo-degradation value (%) and the combined effect of initial dye and catalyst dosage on the photo-degradation (%). The figure shows that the amount of photo-degradation (%) enhanced in the middle amount of catalyst dosage and also increased after decreasing from maximum amount (Table 2).
Effect of Initial Dye Concentration and Irradiation Time on Degradation Efficiency
Figure 10 shows the initial dye concentration and irradiation time effect on the photodegradation of AO7, while rest of the parameters kept constant (10 cm distance of the solution from UV lamp and catalyst dosage of 0.4 g L−1). As shown in the Fig. 10 the increase in the initial concentration of AO7 from 10 to 30 mg L−1, decreases the photodegradation efficiency from 94 to 65%. When the initial concentration of the AO7 increases, the photons scattered before they can reach the TiO2–G composite surface [34, 35]. Therefore, the absorption of photons by the TiO2–G catalysts decreased and subsequently, the photodegradation efficiency decreased. On the other hand, with increasing of AO7 concentration more organic substances (AO7 and intermediates) are adsorbed on the surface of TiO2–G catalysts, therefore the generation of active species such as hydroxyl radicals will be reduced [36].
According to results, irradiation time has the impressive effect on photodegradation of AO7. For all initial dye concentration highest photocatalytic removal efficiency was obtained after an irradiation time of 50 min as shown in Figs. 8, 9 and 10 the photocatalytic removal efficiency of AO7 and UV irradiation time have a direct relationship.
Effect of Catalyst Dosage and Irradiation Time on Degradation Efficiency
As can be understood from RSM results the increase in the TiO2–G dosage from 0.2 to 0.5 g L−1 improves the removal efficiency of AO7 from 70 to 96%. These observations are the result of increasing catalytic and adsorption sites on the surface of TiO2–G which are affected the photocatalytic activity. However, further increasing of the TiO2–G dosage have no effect on removal efficiency of AO7. Increasing of the catalyst dosage from 0.5 to 0.8 g L−1 can slightly decrease the photodegradation of AO7 from 96 to 64%. Why so, at high TiO2–G catalyst loading scattering effect and turbidity of aqueous solution increases which causes a decrease in UV light penetration to the solution [37]. So, the absorption of photons by the catalyst surface decreased and subsequently, the photocatalytic removal efficiency of AO7 decreased.
Effect of Solution Distance from UV Lamp and Irradiation Time on Degradation Efficiency
Figure 9 shows the effect of the solution distance from UV lamp on the photocatalytic activity of TiO2–G nanocomposite, while two other variables kept at their respective zero level (catalyst dosage of 0.5 g L−1 and the irradiation time of 50 min). According to results, the removal efficiency increased from 51 to 96% with the decrease of the distance between the solution and UV lamp from 10 to 2 cm. Since, in lower distance to the lamp, the UV light irradiation produces the photons needed for the transferring of electron from the valence band to the conduction band of the TiO2–G catalyst. Therefore, when the UV light intensity is low, faster recombination of electrons and holes occurs and decreases the formation of hydroxyl radicals and because hydroxyl radicals have a direct relation with photocatalytic removal efficiency, subsequently the photodegradation of AO7 decreased [38]. But, when more UV radiations reach to the surface of TiO2–G catalyst, more hydroxyl radicals are produced and the photodegradation rate of AO7 dye increased [39]. The highest photocatalytic removal efficiency has direct and indirect relationship with irradiation time and distance between UV lamp and the solution, respectively.
Photocatalytic Mechanism
TiO2–G photocatalyst can absorb UV light to produce photogenerated electron–hole pairs due to the appearance of impurity energy levels. Normally in pure TiO2 photocatalyst, the photogenerated electrons will be quickly recombined and only a small fraction of photogenerated electrons and holes may participate in the photocatalytic reaction, thus lead to the low photocatalytic activity, whereas the photogenerated electrons can be trapped by carbon atoms on the surface and O2 molecules adsorbed on the surface of TiO2–G to form superoxide anion radicals. This could effectively restrain the recombination of photogenerated electrons and holes. Subsequently, the generated O2 on the surface of TiO2–G photocatalysts will further react with electrons and h+ in succession to form active OH radicals. The produced OH radicals are the main oxidizing.
Species responsible for the subsequent degradation of pollutants as confirmed by the above active species trapping experiments shown in Fig. 11. Meanwhile, the photogenerated holes may directly oxidize organic dyes or react with OH− to form OH radicals, which is subsequently involved in the degradation reaction.
The possible proposed photocatalytic reaction pathway mechanism of AO7 degradation over TiO2–G nanocomposite is illustrated in Fig. 11. When TiO2–G was irradiated with UV light, electrons from the valence band (VB) of TiO2 are excited to the conduction band (CB) of TiO2 and so, leaving positively charged holes in the valence bond. Furthermore, interfacial carrier separations take place with the aid of graphene. Since the CB work function of TiO2 matches with CB work function of graphene, electrons can be easily transferred from CB of TiO2 to graphene [40].
As a result, the oxygen sites can readily accept the electrons and undergo reduction reaction to generate more \({\text{O}}_{2}^{ - \cdot }\)radicals. Thus, the presence of graphene in photocatalysts can produce an excess amount of reactive \({\text{O}}_{2}^{ - \cdot }\) radicals due to good electron acceptor and transporter behavior of graphene. Therefore, the ultrafast transportation of photogenerated electrons over graphene sheet can directly reduce O2 to produce \({\text{O}}_{2}^{ - \cdot }\) radicals which lead to enhanced AO7 degradation.
Conclusion
TiO2–G nanocomposite was synthesized by using ultrasound irradiation and then it was used for degradation of AO7 as a model pollutant. XRD, SEM, TEM, DRS, FT-IR and EDX techniques were used for the characterization of the prepared nanocomposite. Several operational and synthetic parameters were investigated in degradation of AO7 by TiO2–G nanocomposite. The effect of synthesis variables such as weight ratio of TiO2 to graphene and operational key factors such as initial dye concentration, irradiation time, catalyst dosage and solution distance from UV lamp were studied in the photocatalytic degradation of AO7. The optimum amount of all factors were obtained. According to our results, the maximum degradation efficiency (94%) was achieved at the optimum operational conditions: initial AO7 concentration of 10 mg L−1, catalyst dosage of 0.4 g L−1, the irradiation time of 50 min and distance the solution from UV lamp of 2.5 cm.
References
T. Robinson, G. McMullan, R. Marchant, and P. Nigam (2001). Bioresour Technol 77, 247.
B. Y. Chen, M. M. Zhang, C. T. Chang, Y. Ding, K. L. Lin, C. S. Chiou, C. C. Hsueh, and H. Xu (2010). Bioresour Technol 101, 4737.
I. Arslan Alaton and J. L. Ferry (2002). Dyes Pigm 54, 25.
K. Golka, S. Kopps, and Z. W. Myslak (2004). Toxicol. Lett 151, 203.
F. Saadati, N. Keramati, and M. Mehdipour Ghazi (2016). Environmental Science and Technology 46, 757.
X. Li, F. Chen, C. Lian, S. Zheng, Q. Hu, S. Duo, W. Li, and C. Hu (2016). Journal of Cluster Science 27, 1877.
A. R. Nezamzadeh-Ejhieh and A. Shirzadi (2014). Chemosphere 107, 136.
L. Yue, Sh Wang, G. Shan, W. Wu, L. Qiang, and L. Zhu (2015). Applied Catalysis B: Environmental 176, 11.
Z. Xian, R. Liu, H. Li, S. Zhang, Z. Yang, W. Zheng, and C. Chen (2016). Journal of Cluster Science 27, 241.
Z. Yaping, J. Chengguang, P. Ran, M. A. Feng, and O. U. Guangnan (2014). Journal of Central South University 21, 310.
A. Azarian (2015). Journal of Cluster Science 26, 1607.
J. Zhang, G. F. Huang, D. Li, B. X. Zhou, S. Chang, A. Pan, and W. Q. Huang (2016). Appl. Phys. A 122, 994.
X. Wang, Y. Sang, X. Yu, B. Liu, and H. Liu (2016). Appl. Phys. A 122, 884.
A. Alinsafi, F. Evenou, E. M. Abdulkarim, M. N. Pons, O. Zahraa, A. Benhammou, A. Yaacoubi, and A. Nejmeddine (2007). Dyes Pigm 74, 439.
D. Beydoun, R. Amal, G. Low, and S. McEvoy (1999). J. Nanopart. Res 1, 4394.
T. Yoshida, N. Yaghi, R. Nakagou, A. Sugimura, and I. Umezu (2014). Appl. Phys. A, DOI: 10.1007/s00339-014-8378-3
J. Chen, Y. Qian, and X. Wei (2010). J. Mater. Sci 45, 6018.
A. Abbasi, D. Ghanbari, M. Salavati-Niasari, and M. Hamadanin (2016). Journal of Materials Science: Materials in Electronics. doi:10.1007/s10854-016-4361-4.
J. W. Shi, J. T. Zheng, and X. J. Ji (2010). Environmental Engineering Science 27, 923.
B. Paul, W. N. Martens, and R. L. Frost (2012). Applied Clay Science 57, 49.
H. Wang, B. Yang, and W. J. Zhang (2010). Advanced Materials Research 129, 733.
S. Liu, M. Lim, and R. Amal (2014). Chemical Engineering Science 105, 46.
F. Tavakoli and M. Salavati Niasari (2014). J. Ind & Eng chem 20, 3170.
M. Salavati Niasari and F. Tavakoli (2015). J. Ind & Eng chem 21, 1208.
V. Singh, D. Joung, L. Zhai, S. Das, S. Khondaker, and S. Seal (2011). Materials Science 56, 1178.
V. Singh, D. Joung, L. Zhai, S. Das, S. I. Khondaker, and S. Seal (2011). Progress in Materials Science 56, 1178.
X. Zhang, X. Liu, W. Zheng, and J. Zhu (2012). Carbohydrate Polymers 88, 26.
S. Escobedo, B. Serrano, A. Calzada, J. Moreira, and H. D. Lasa (2016). Fuel 181, 438.
I. V. Lightcap, T. H. Kosel, and P. V. Kamat (2010). Nano Lett 10, 577.
H. Zhang, X. J. Lv, Y. M. Li, Y. Wang, and J. H. Li (2010). ACS Nano 4, 380.
Y. H. Zhang, Z. R. Tang, X. Z. Fu, and Y. J. Xu (2010). ACS Nano 4, 7303.
X. Lin, J. Xing, W. Wang, Z. Shan, F. Xu, and F. Huang (2007). J. Phys. Chem. C 111, 18288.
L. Chen, D. Jiang, T. He, Z. Wu, and M. Chen (2013). Cryst. Eng. Commun 15, 7556.
M. A. Behnajady, N. Modirshahla, M. Shokri, H. Elham, and A. Zeininezhad (2008). J. Environ. Sci. Health. Part A Toxic/Hazard. Subst. Environ. Eng 43, 460.
S. Chakrabarti and B. K. Dutta (2004). J. Hazard. Mater 112, 269.
M. A. Behnajady, N. Modirshahla, N. Daneshvar, and M. Rabbani (2007). Chem. Eng. J 127, 167.
L. A. Ghule, A. A. Patil, K. B. Sapnar, S. D. Dhole, and K. M. Garadkar (2011). Toxicol. Environ. Chem 93, 623.
M.A. Behnajady, H. Eskandarloo, Res. Chem. Intermed. http://dx.doi.org/10.1007/s11164-013-1327-5.
B. Neppolian, H. C. Choi, S. Sakthivel, B. Arabindoo, and V. Murugesan (2002). J. Hazard. Mater 89, 303.
S. D. Perera, R. G. Mariano, K. Vu, N. Nour, O. Seitz, Y. Chabal, and K. J. Balkus (2012). ACS Catal 2, 949.
Acknowledgements
Authors are grateful to Council of University of Tehran and Center for International Scientific Studies Collaboration for providing financial support to undertake this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tavakoli, F., Badiei, A., Yazdian, F. et al. Optimization of Influential Factors on the Photocatalytic Performance of TiO2–Graphene Composite in Degradation of an Organic Dye by RSM Methodology. J Clust Sci 28, 2979–2995 (2017). https://doi.org/10.1007/s10876-017-1250-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-017-1250-9