Abstract
Recently, the green synthesis of silver nanoparticles gained increasing attention due to interesting properties for optical, antimicrobial and pest control applications. However, their toxicity against micro-crustaceans and fishes has been scarcely explored, while most of the research efforts focused on mosquito control with the green-synthesized nanocomposites. In this study, we investigated the toxic effects of AgNO3, Cissus quadrangularis (Cq)-synthesized AgNPs and Cq extract in two different study models, the larvae of Poecilia reticulata fishes and adults of the micro-crustacean Ceriodaphnia cornuta. In both species, AgNO3 and Cq-AgNPs showed high mortality rates even if tested at very low doses. Molecular analysis revealed high DNA damages induced by Cq-AgNPs on both aquatic organisms. Furthermore, light microscopy studies evidenced lesions in the gills and vacuolization in the gills and in the abdomen of P. reticulata larvae. Overall, our research pointed out that the exposure of aquatic organisms to AgNO3 or green-fabricated AgNPs can damage fishes and crustaceans, posing noteworthy risks to the aquatic ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, the green synthesis of silver nanoparticles gained increasing attention due to interesting properties for optical, antimicrobial and pest control applications. However, their toxicity against micro crustaceans and fishes has been scarcely explored, while most of the research efforts focused on mosquito control with the green-synthesized nanocomposites [1–3]. Notably, several studies have shown the toxic effect of silver nanoparticles (AgNPs) to bacteria, plants and vertebrates [4–6]. One of the widely known mechanisms for this toxicity could be mainly due to their metallic nature, causing detrimental effects on normal cellular proliferation and protein functions [7, 8]. Despite the number of reports on AgNO3 toxicity, there is lack of information on how the metal nanoparticles trigger toxicity at molecular level. On the other hand, the sulfidation of AgNPs on adult zebra fishes reduces biological toxicity [9]. Levard et al. [10] showed the feasibility of sulfidation of AgNPs in reducing their toxicity against four diverse organisms, Danio rerio, Fundulus heteroclitus, Caenorhabditis elegans and the aquatic plant Lemna minuta demonstrating that sulfidation could definitely be a potential mechanism for mitigating nanoparticle toxicity.
Cissus quadrangularis (Cq) is a common species in Southeast Asian countries, including India and Sri Lanka. It has been used as an Ayurveda medicine for many decades. Recently, we have shown that by using the green synthesis approach, it is possible to produce stable AgNPs from Cq-extract and the nanocomposite exhibits excellent antimicrobial activity [11]. However, information on the non-target effects of this nanoformulation in the aquatic environment is scarce. In this study, we have investigated the toxicity on two different model systems (both vertebrate and invertebrate aquatic animal models, i.e. Poecilia reticulata fishes and Ceriodaphnia cornuta crustaceans), testing three different products, AgNO3, Cq-AgNPs and Cq-extract, in order to shed light on the toxicity of these nanoparticles on non-target aquatic organisms, using acute toxicity assays, molecular analyses and microscopy investigations.
Materials and Methods
Plant Extract
Fresh stems of C. quadrangularis (Cq) were collected and washed with tap water as well as with distilled water three times each. Five grams of air dried stems were cut into fine pieces and boiled for 10 min in microwave oven to get leaves extract. The extract was cooled at room temperature and then filtered by using Whatman filter paper No. 1.
Synthesis of Silver Nanoparticles
A 10 ml of stem extract was added into the 90 ml of aqueous solution of 1 mM of silver nitrate and kept at room temperature. The color change of the solution indicates the formation of silver nanoparticles.
Collection and Maintenance of Sample
P. reticulata and C. cornuta were maintained in the aquatic animal rearing lab of Department of Animal Health and Management. For P. reticulata, the fourth-instar larvae were taken and acclimatized to the laboratory environment for 5 days. They were fed with commercial food pellets. For, C. cornuta, the culture was maintained under constant light (16:8 light:dark cycle), temperature (20–22 °C) and culture medium conditions.
Toxicity Assays
For the mortality test, there were three treatments (i) AgNO3, (ii) Cq-AgNPs and (iii) Cq-plant extract at different concentrations in both C. cornuta and P. reticulata. For crustacean assays, 10 neonates (6–24 h old) of C. cornuta were placed in a 50 ml glass beaker containing 30 ml test solution. Four test concentrations (10, 20, 30 and 40 µg/ml) and a blank control (0 µg/ml) were used. Beakers were incubated at 20 °C in the dark and mortality was noted for each beaker at the end of the test. For fish assays, 10 randomly selected P. reticulata larvae were placed in 200 ml of water. For experiments, the following concentrations were tested, (i) AgNO3 0.2, 0.4, 0.6, 0.8 and 1 µg/ml, (ii) Cq-AgNPs 4, 5, 10, 15 and 20 µg/ml, and (iii) Cq-plant extract 5, 10, 20, 30 and 40 µg/ml. The control was set-up with dechlorinated tap water with 10 P. reticulata larvae without any treatment. No food or supplements were added during the exposure period. Immobilization and behavioral abnormalities were assessed after 24 h of exposure. Mortality of each organism was carefully recorded for calculating LC50 values based on the method by Karber [12]. All experiments were conducted in triplicates.
Histopathology Studies on P. reticulata
P. reticulata larvae treated with AgNO3 and Cq-AgNPs were fixed in 10% formalin, dehydrated and embedded in paraffin blocks according to the routine processing protocol. The sections were stained with haematoxylin and eosin for histopathalogical examination using bright field light microscope.
Light and Confocal Laser Scanning Microscopic Studies on C. cornuta
To understand the effect of physical adsorption and internalization of Cq-AgNPs on the survival of C. cornuta, studies on the uptake of nanoparticles was carried out using a Nikon inverted light microscope ECLIPSE ×40 magnification. A Carl Zeiss LSM 710 confocal laser scanning microscope (CLSM) at PMT mode using a 488-nm argon laser and BP 500–640 band pass emission filter and running Zen, 2009 software (Carl Zeiss, Germany) was also used to image the accumulation/internalization in the exoskeletons and alimentary canal of C. cornuta.
DNA Ladder Assay
Total genomic DNA was isolated from the tissue of P. reticulata and C. cornuta following the method by Aswathi et al. [13] with slight modifications. Nanoparticle-treated cells were incubated at 37 °C for 24 h, then centrifuged at 500 rpm for 5 min and suspended in 10 ml of PBS. Cells were lysed by the addition of 100 ml of lysing solution having 0.25 M NaCl, 0.1% sodium lauroyl sarcosine, 10 mM EDTA, 1 mM Tris (pH 7.5) at room temperature for 30 min. Samples in microfuge tubes were centrifuged at 13,000 rpm for 15 min to pellet cellular debris. Supernatants, which consisted mainly of lysed apoptotic cells, were incubated first with 10 mg/ml of RNAse A for 30 min at 37 °C, and then with 1 mg/ml of proteinase K for 30 min at 37 °C. They were then mixed with loading dye and electrophoresed on 1% Agarose gel (in Tris borate buffer (pH 8) for 4 h at 100 V). Gels were stained in 0.35 mg/ml ethidium bromide in Tris borate buffer.
Statistical Analysis
Data were expressed as mean ± standard deviation (SD) of three independent experiments. Wherever appropriate, the data were subjected to statistical analysis by one-way analysis of variance followed by Tukey’s honest significant difference. A value of p < 0.05 was considered significant and p < 0.01 level was set as highly significant.
Results and Discussion
Green Synthesis of Silver Nanoparticles
Silver nanoparticles were synthesized using aqueous extract of C. quadrangularis, within 30 min of incubation. It was observed that upon addition of the C. quadrangularis extract into the flask containing the aqueous silver nitrate solution, the color of the medium changed to yellowish brown within 30 min, which was due to the excitation of surface plasmon vibrations within the synthesized AgNPa [1, 3]. This indicates the formation of AgNPs [11].
Acute Toxicity and Microscopic Analysis on C. cornuta
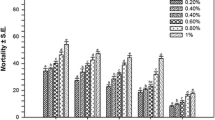
In our experiments, treating with three combinations of AgNO3, Cq-AgNPs and Cq extract, the mortality rate increases as shown in Fig. 1a. Briefly, the mortality rate of C. cornuta was 100% at 40 µg/ml of AgNO3 whereas in Cq-AgNPs, the mortality rate was 65% at 40 µg/ml. In case of Cq plant extract, the mortality rate was 46% at 40 µg/ml. The mortality rate and concentration (LC50) of three combinations (AgNO3, Cq-derived AgNPs and Cq-extract) tested on C. cornuta differed among the treatments, as shown in Fig. 1b. Thus, AgNO3 would seem to be more toxic to aquatic organisms than Cq-AgNPs in freshwater media. Recent studies shown that the dissolved Ag+ released from AgNPs plays a critical role in the aquatic toxicity of these nanomaterials [14–16].
Mortality of Ceriodaphnia cornuta caused by a C. quadrangularis extract (Cq), AgNO3 and Cissus quadrangularis-synthesized silver nanoparticles (Cq-AgNPs) (from left to right), b lethal concentration achieved by Cq on Ceriodaphnia cornuta, AgNO3 and Cq-AgNPs. T-bars represent standard errors. Different alphabets in each bar indicate significant differences among values (mean ± SE, p = 0.05)
In addition, the uptake and accumulation of Cq-AgNPs, the C. cornuta were visualized through light microscopy and CLSM, as shown in Fig. 2. We observed that large amounts of AgNPs aggregates were found in the gastrointestinal tract of C. cornuta after Cq-AgNPs exposure (indicated by arrow marks, Fig. 2), resulting in the accumulation and damage in the organs of C. cornuta from head to antenna. In the control groups, 100% of the live C. cornuta exhibited completely normal swimming ability. Thus, the results suggest that the exposure of aquatic organisms to such nanoparticles may pose a risk of bioaccumulation, especially for filter-feeding copepods such as C. cornuta. In this regard, Zhao [17] showed that Daphnia can retain a large amount of AgNPs in their guts after ingestion. Other studies have also indicated that D. magna can uptake nanomaterials from test solutions [18, 19]. Since Daphnia spp. are part of the diet of other organisms, including fishes, there is a potential for uptake and the subsequent transfer of nanoparticles to higher organisms.
Light (×40) and confocal laser scanning microscope (PMT view) microscopic observation about the uptake and adsorption of Cissus quadrangularis-synthesized silver nanoparticles by Ceriodaphnia cornuta after 24 h of exposure to 40 µg/ml. The arrow marks indicates that the presence of nanoparticles in the gastrointestinal tract
Toxicity on P. reticulata Larvae and Histopathology Studies
In P. reticulata assays, the mortality was 100% at 1 µg/ml of AgNO3 (Fig. 3a), whereas treatment with Cq-AgNPs, showed 100% mortality at 20 µg/ml (Fig. 3b). However, the Cq-extract showed 70% mortality at 40 µg/ml (Fig. 3c). The treatment of AgNO3, Cq-AgNPs and Cq-extract on P. reticulata was studied at different lethal concentrations (LC50) shown in the Fig. 3d. Gallo et al. [20] compared the acute toxicity of two carbamate pesticides, aldicarb and carbaryl and found that guppies are more sensitive (i.e. the toxicity of both carbamates was higher) if compared to zebra fishes. Later on, Dolezelova et al. [21] reported that the toxicity of Ag ions at 96 h LC50 to zebrafish was 17 µg/l. Histopathalogical observations (Fig. 4) highlighted that the fishes exposed to AgNPs showed signs of gill stimulation and increased mucus secretion when compared to control. Histopathalogical studies pointed out that the fish exposed to AgNPs had signs of gill stimulation and increased mucus secretion when compared with control fishes. The histological responses in the gills of fish were typically associated with circulatory disturbances and regressive and progressive changes [22]. In addition, the higher AgNPs concentrations (>1 mg/l) resulted in visible long strands of a mucus-nanosilver mixture on the surface of the gills. The earlier report of Rajkumar et al. [23] reported the similar toxic effect of AgNPs on the vital organs of the freshwater fish Labeo rohita, which is compatible with our results.
Histopathalogical analysis of Poecilia reticulata larvae exposed to AgNO3 and Cissus quadrangularis-synthesized silver nanoparticles (Cq-AgNPs). a Control (not exposed to AgNO3 or Cq-AgNPs), b exposed to AgNO3 and c exposed to Cq-AgNPs. The arrows indicate lesions in the gills (b), vacuole formation in the abdominal tissue (c), vacuolarization in the gills and vacuolarization in the abdomen (c)
Impact of Silver Nanoparticles on DNA of Non-target Organisms
In agreement with the DNA damage, specific DNA smearing is a characteristic feature of cell death. AgNPs injured cells have often been reported as cells with denatured DNA by disrupting the hydrogen bonds between purine and pyrimidine base pairs [24]. The presence of these materials in a suspension indicates cell damage at the membrane level. Agarose gel electrophoresis images revealed destructive effects of Cq-AgNPs on P. reticulata and C. cornuta. Results showed that 40 µg/ml of Cq-AgNPs led to remarkable DNA damage in C. cornuta when compared to control (Fig. 5a), whereas in P. reticulata, 20 µg/ml of Cq-AgNPs led to DNA damage (Fig. 5b). The Cq-AgNPs treated C. cornuta and P. reticulata DNA showed very light band whereas the control showed thick dark band, indicating DNA damage. Silver has a great affinity with sulfur and phosphorous containing biomolecules in the cell. Indeed, sulfur containing proteins in the cell membrane, inside the cells and phosphorous containing elements like DNA are likely to be the preferred sites for binding of AgNPs [25, 26].
a Agarose gel showing the DNA of Ceriodaphnia cornuta exposed or not to Cissus quadrangularis-synthesized silver nanoparticles (Cq-AgNPs). Lane 1 marker (1 Kb). Lane 2 control. Lane 3 treated with Cq-AgNPs. b Agarose gel showing the DNA of Poecilia reticulata larvae exposed or not with Cq-AgNPs. Lane 1 DNA marker (1 Kb). Lane 2 and 3 control. Lane 4 and 5 treated with 10 and 20 µg/ml of Cq-AgNPs respectively
Conclusions
Overall, based on the current findings, Cq-AgNPs would appear to have less toxic effect than AgNO3 on aquatic organisms, especially on P. reticulata and C. cornuta. Histopathology and microscopic observations clearly pointed out the toxicity of Ag, including metallic nanosilver, on both species. Furthermore, our results evidenced that P. reticulata was more sensitive than C. cornuta when exposed to AgNPs. Thus, the releases of nanoparticles waste to the environment pose noticeable risks to aquatic organisms and ecosystems.
References
G. Benelli (2016). Parasitol. Res. 115, 23–34.
G. Benelli (2016). Asia. Pacif. J. Trop. Biomed. 6, 353–354.
G. Benelli (2016). Enzyme. Microbial. Technol. doi:10.1016/j.enzmictec.2016.08.022.
P. V. AshaRani, G. L. K. Mun, M. P. Hande, and S. Valiyaveettil (2009). ACS Nano. 39, 279–290.
K. Kawata, M. Osawa, and S. Okabe (2009). Environ. Sci. Technol. 43, 6046–6051.
K. J. Lee, P. D. Nallathamby, L. M. Browning, C. J. Osgood, and X. H. N. Xu (2007). ACS. Nano. 2, 133–143.
N. Lewinski, V. Colvin, and R. Drezek (2008). Small 4, 24–49.
L. Braydich-Stolle, S. Hussain, J. Schlager, and M. C. Hofmann (2005). Toxicol. Sci. 88, 412–419.
G. P. Devi, K. B. A. Ahmed, M. K. N. S. Varsha, B. S. Shrijha, K. K. S. Lal, V. Anbazhagan, and R. Thiagarajan (2015). Aquac. Toxicol. 158, 149–156.
C. Levard, E. M. Hotze, B. P. Colman, A. L. Dale, L. Truong, X. Y. Yang, A. J. Bone, G. E. Brown, R. L. Tanguay, R. T. Di Giulio, E. S. Bernhardt, J. N. Meyer, M. R. Wiesner, and G. V. Lowry (2013). Environ. Sci. Technol. 47, 13440–13448.
J. Sivakamavalli and B. Vaseeharan (2012). Mater. Lett. 82, 171–173.
M. Yilmaz, A. Gul, and K. Erabasli (2004). Chemosphere 56, 381–385.
K. K. Awasthi, A. Awasthi, N. Kumar, P. Roy, K. Awasthi, and P. J. John (2013). J. Nanopart. Res. 15, 1898.
A. Kennedy, M. Hull, A. J. Bednar, J. Goss, J. Gunter, J. Bouldin, P. Vikesl, and J. Steevens (2010). Environ. Sci. Technol. 44, 9571–9577.
M. C. Artal, R. D. Holtz, F. Kummrow, O. L. Alves, and G. D. A. Umbuzeiro (2013). Environ. Toxicol. Chem. 32, 908–912.
K. M. Newton, H. L. Puppala, C. L. Kitchens, V. L. Colvin, and S. J. Klaine (2013). Environ. Toxicol. Chem. 32, 2356–2364.
C. M. Zhao and W. X. Wang (2011). Nanotoxicology 6, 361–370.
M. Heinlaan, A. Kahru, K. Kasemets, B. Arbeille, G. Prensier, and H. C. Dubourguier (2011). Water. Res. 45, 179–190.
X. Zhu, Y. Chang, and Y. Chen (2010). Chemosphere 78, 209–215.
D. Gallo, A. Merendino, J. Keizer, and L. Vittozzi (1995). Sci. Total. Environ. 171, 131–136.
P. Dolezelova, S. Macova, V. Pistekova, Z. Svobodova, I. Bedanova, and E. Voslarova (2008). Interdisc. Toxicol. 1, 200–202.
J. C. Van Dyk, M. J. Marchand, G. M. Pieterse, I. E. J. Barnhoorn, and M. S. Bornman (2009). Afr. J. Aquat. Sci. 34, 283–291.
K. S. Rajkumar, N. Kanipandian, and R. Thirumurugan (2016). Appl. Nanosci. 6, 19–29.
U. Klueh, V. Wagner, S. Kelly, A. Johnson, and J. D. Bryers (2000). J. Biomed. Mater. Res. 53, 621–631.
G. Donnell and A. D. Russell (1999). Microbiol. Rev. 12, 147–179.
N. Zhao, J. Gao, C. A. Enns, and M. D. Knutson (2010). J. Biol. Chem. 285, 32141–32150.
Acknowledgements
RI gratefully acknowledges the Alagappa University Research Fellowship [Ph.D/1075/AURF Fellowship/2015], Karaikudi, India. The corresponding author (BV) thanks the Department of Biotechnology (DBT), New Delhi, India, for research grant under the project code BT/PR7903/AAQ/3/638/2013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ishwarya, R., Vaseeharan, B., Shanthi, S. et al. Green Synthesized Silver Nanoparticles: Toxicity Against Poecilia reticulata Fishes and Ceriodaphnia cornuta Crustaceans. J Clust Sci 28, 519–527 (2017). https://doi.org/10.1007/s10876-016-1126-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-016-1126-4