Abstract
The present study was aimed to investigate the novel approach for the promotion of leather finishing properties through the incorporation of copper nanoparticles (Cu nanoparticles). Cu nanoparticles were synthesized by chemical reduction method, and particle sizes were in the range of 25–50 nm. X-ray diffraction, transmission electron microscope, ultra violet-vis spectrometry and scanning electron microscopy were used to characterize the Cu nanoparticles. The ascorbic acid was used as a protective agent to prevent the oxidation. Polyvinylpyrrolidone used as a stabilizing and dispersing agent, whereas sodium borohydride was used as a reducing agent. Spray coatings were carried out with Cu nanoparticles on both base and top coat formulations to evaluate their performance properties. Interestingly, the Cu nanoparticles coated leather samples showed the improved wet and rub fastness, color fastness to water and adhesion strength.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The leather is a nature material with excellent physical properties and its intrinsic values of texture visco-elastic, gentle, stretch, adaptability, feel, stretch, breathability, and strength, etc., that enables it to be used in several diverse applications. The leather finishing is an important process that determines the aesthetic and physical properties. It also confers high-performance properties such as fastness to dry and wet rubbing, adhesion, abrasion resistance, water and oil repellency. The distinct level of fastness property is an obvious requirement for the classic leather. Application of nanotechnology has been successful in various sectors such as information technology, biomedical applications, energy, coatings, textiles, ceramics, membranes, composite materials, glass products, prosthetic implants, anti-static packaging, cutting tools, industrial catalysts, displays and batteries [1, 2]. Currently, nanotechnology being used in the leather industry for tanning operations due to its size and high surface area. Nanomaterials can penetrate into fibers to improve the properties of leather [3]. Nanomaterials such as gold, zinc, copper, titanium could be assembled into many different shapes [4].
In recent years, the metal nanoparticles have gained more interest due to their wide range of potential applications [5–7]. Copper nanoparticles have been extensively studied for their unusual physical and chemical properties, and their potential applications in various fields such as optical, catalytic, electronic, antibacterial, antimicrobial and antifouling applications [8–11]. Cu nanoparticles have been synthesized by various methods such as thermal reduction [12], sonochemical reduction [13] metal vapor synthesis [14], chemical reduction [15–18], vacuum vapor deposition [19], radiation methods [20], and microemulsion techniques [21]. The chemical reduction method is preferred due to its feasibility and low cost. Moreover, the size and shape of the nanoparticles were controlled by optimizing the experimental parameters. The size of Cu nanoparticles obtained through chemical reduction method is present in the range of 10–100 nm [22]. The ascorbic acid is widely used for Cu nanoparticles synthesis and storage [16].
The present study was aimed to synthesize Cu nanoparticles by chemical reduction method and their application in the leather formulation.
Materials and Methods
Copper sulfate pentahydrate (CuSO4·5H2O), sodium borohydride (NaBH4) (assay 95 %, Merck), NaOH (assay 98 %, Himedia), polyvinylpyrrolidone (PVP) (SD Fine Chemicals Limited), ascorbic acid (assay 99 %, Merck), and spray gun bullows 630 were commercially purchased. All the leather finishing chemicals were procured from Stahl India Pvt. Ltd, (Chrompet, Chennai, India). The crust leather for coating was collected from Tannery Division, Council of Scientific and Industrial Research-Central Leather Research Institute (CSIR-CLRI), Chennai, India.
Synthesis of Cu Nanoparticles
A solution of CuSO4.5H2O (0.01 M) in deionized water was prepared separately. To the above solution, the ascorbic acid solution was added (52.8 mg in 15 mL of water). Then, a pinch of PVP was added for controlling the particle size. The pH was adjusted to 12 by dropwise addition of 1 M NaOH under stirring for 1 h. Finally, 0.1 M NaBH4 was added to the solution and allowed to stir for 10 min to complete the reaction. The solution was turned yellow to light red that confirms the formation of Cu nanoparticles. It was then centrifuged at 12,000 rpm for 15 min and washed with ethanol and water to remove the impurities, and it was collected and dried under vacuum for 3–4 h and subjected to characterization.
PVP structure consists of a polyvinyl skeleton with nitrogen and oxygen polar groups. The polar group will donate its lone pair of electrons to copper ions to form a coordinative interaction which creates the copper-PVP complex compound in the solution [23, 24]. PVP acts as a polymeric capping agent and to prevent agglomeration. It hinders the nuclei from aggregation through the polar groups, which strongly absorb the copper particles on the surface with coordination bonds [25]. PVP also serves as a dispersant and stabilizing agent to synthesis Cu nanoparticles. The concentration of PVP determines the size and shape of Cu nanoparticles during synthesis [26, 27].
Characterization
The synthesized Cu nanoparticles were subjected for evaluating the surface plasmon resonance spectrum recorded with use of a UV–visible spectrophotometer (Shimadzu UV-1800). The crystallographic studies and diffraction peaks were obtained by PANalytical X’Pert Powder X-Ray Diffraction (Model No: 9430 030 40601). The scanning rate was about 0.30, and 20 was about 10–80. Scanning Electron Microscopy (SEM) reveals the surface morphology (VEGA3 TESCAN). The average particle size and lattice patterns were examined by Transmission Electron Microscopy (TEM) (JEOL 3010).
Methodology of Leather Nanocoatings
Cu nanoparticles were synthesized by the chemical reduction method, and these particles were characterized by SEM, UV, and TEM for the confirmation of particle size and morphology. Furthermore, the Cu nanoparticles were mixed with conventional leather ingredients, and the surface coating was applied.
Preparation of Leather for Coating
Standard cow upper crust leather from Indian origin was selected for the evaluation. A clearing coat (a mixture of ammonia, isopropyl alcohol (IPA) and water) was applied to increase the season adhesion with leather. Furthermore, there was two cross coat formulation was sprayed on the leather by High Volume Low Pressure (HVLP) spray gun bullows 630 (Table 1).
Determination of Applied Physical Properties of Cu Nanoparticles Coated Leather
Measurement of Finish Adhesion
Measurement of leather finish adhesion carried out by International Organization for Standardization (ISO) 11644:2009 method (Table 2).
Wet and Dry Rub Analysis
Measurement of wet and dry rub fastness test was carried out according to IUF 450 by veslic C-4500 (Table 3).
Colorfastness to Water
Measurement of colorfastness to water was carried out by SATRA TM 335-1 method (Table 4).
Results and discussion
UV–Vis Spectroscopy
The UV–Visible absorption spectrum recorded for Cu nanoparticles (Fig. 1), exhibits maximum absorption at 568 nm indicates the existence of Cu nanoparticles, which agrees with the values reported in the literature in the range of ∼560 to 570 nm [28]. This absorption band can be attributed to the surface plasma resonance of Cu nanoparticles, and it confirms the formation of pure Cu nanoparticles without any oxide layers. The value of surface plasmon resonance was located around 568 nm in water. Mallick et al. [29], have reported that the blue shift of surface plasmon resonance occurs with the decreasing size. It is well known that the position and shape of plasmon resonance of metal nanoparticles depend on particle size, dielectric medium, and surface adsorbed species [30, 31]. Moreover, Cu nanoparticles with size around 50 nm typically exhibit a surface plasmon peak in the range of 560–570 nm [32]. Also, there was no increased background absorption at 800 nm that indicates the colloidal particles were reduced [33–35]. Sasmal et al. [36], have reported the fabrication of superhydrophobic copper surface on various substrates for roll-off, self-cleaning, and water/oil separation. Jana et al. [37], have reported the seed-mediated growth method to prepare cubic copper nanoparticles.
X-ray Diffraction (X-RD)
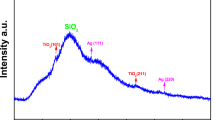
X-RD pattern of Cu nanoparticles was taken (Fig. 2). The diffraction peaks at 2θ = 43.5°, 50.6°, and 74.38° can be assigned to be [111], [200], and [220] planes and are in agreement with the values of fcc structure of pure Cu nanoparticles (JCPDS No: 85-1326). These results were agreed with results of previously reported metallic Cu nanoparticles [38–40]. The d-spacing values of the diffraction planes (d 111 = 0.2101, d 200 = 0.1819, d 220 = 0.1285) were in good agreement with the d-spacing values of the copper. X-RD results confirm that the synthesized nanoparticles were in pure copper form. The particle size was calculated from the primary diffraction plane at 43.5 (d 111), and it was found to be 50 nm. Therefore, it is a feasible method to synthesize pure Cu nanoparticles.
SEM and SEM
SEM analysis of the Cu nanoparticles indicates that the particles were agglomerated, and hence, the morphology of the particle was not clearly visible. The size distribution calculation was complicated due to the agglomeration. However, the further observation with high magnification TEM analysis reveals that the morphology and the size distribution of the Cu nanoparticles (Fig. 3). The particle size of the Cu nanoparticles was calculated from the TEM images. The particle size was varied from 20 to 50 nm and size was in good agreement with the particle size calculated from the X-RD data. Nanoparticles were found to be cubic and aggregated. The d-spacing of the lattice fringes was found to be 0.2083 nm, which was matched with the d-value of the (111) plane of the fcc copper (Fig. 4).
Preparation of Coat Formulation with Cu Nanoparticles for Cow Upper Crust
The synthesized Cu nanoparticles were sonicated for 10 min and incorporated in cow-upper base coat leather finishing formulations (Table 5). The leather finish formulation was sprayed on cow upper leather crust by HVLP gun. Furthermore, two cross coating was done for intermediate drying.
Preparation of Cow Upper Crust Leather Finishing in Top Coat Formulation with Cu Nanoparticles
Cu nanoparticles were sonicated for 10 min and then it was incorporated into the top coat formulation with various concentrations for optimum results (Table 6). The above formulation was sprayed by HVLP spray gun bullows 630. Furthermore, one cross coat of NC lacquer formulation was applied on the top coat. Then, the leather was kept on the clean hot plate.
Conclusion
The synthesis of Cu nanoparticles by chemical reduction method is simple, cost-effective, non-toxic and suitable for large-scale production. SEM analysis shows that the particles are spherical and size in the range of 20–50 nm. Surface plasmon band of the UV–vis spectrum at 568 nm indicates the existence of Cu nanoparticles. Cu nanoparticles increased the dry and wet rub fastness, finish film adhesion and color fastness to water.
References
L. Reijnders (2006). J. Clean. Prod. 14, 124.
P. Krajnik, R. Amir, P. Franci, R. Maja, Y. Akinori, N. Nader, and S. T. Muhammet (2015). J. Clean. Prod.. doi:10.1016/j.jclepro.2015.08.064.
M. Jianzhong, L. Xiujuan, G. Dangge, L. Yun, L. Bin, and Z. Jing (2014). J. Clean. Prod. 72, 120.
A. R. Zulfiqar, R. Asma, M. Muhammad, Z. B. Sadia, A. Faiza, N. Abdul, and A. Niaz (2015). J. Clean. Prod. 101, 377.
J. Zheng, P. R. Nicovich, and R. M. Dickson (2007). Annu. Rev. Phys. Chem. 58, 409.
H. Wang, J. G. Wang, Z. R. Shen, Y. P. Liu, D. T. Ding, and T. H. Chen (2010). J. Catal. 275, 140.
J. Perelaer, P. J. Smith, D. Mager, D. Soltman, S. K. Volkman, V. Subramanian, J. G. Korvink, and U. S. Schubert (2010). J. Mater. Chem. 20, 8446.
X. F. Tang, G. Zhen, Z. G. Yang, and W. J. Wang (2010). Colloid Surf. A 8, 99.
M. Vaseem, K. M. Lee, D. Y. Kim, and Y. B. Hahn (2011). Mater. Chem. Phys. 125, 334.
J. Ryu, H. S. Kim, and H. T. Hahn (2010). J. Electron. Mater. 40, 42.
N. Cioffi, L. Torsi, N. Ditaranto, G. Tantillo, L. Ghibelli, L. Sabbatini, T. Bleve-Zacheo, M. D’Alessio, P. G. Zambonin, and E. Traversa (2005). Chem. Mater. 17, 5255.
M. Salavati-Niasari and F. Davar (2009). Mater. Lett. 63, 441.
R. V. Kumar, Y. Mastai, Y. Diamant, and A. Gedanken (2001). J. Mater. Chem. 11, 1209.
G. Vitulli, M. Bernini, S. Bertozzi, E. Pitzalis, P. Salvadori, S. Coluccia, and G. Martra (2002). J. Chem. Mater. 14, 1183.
X. Cheng, X. Zhang, H. Yin, A. Wang, and Y. Xu (2006). Appl. Surf. Sci. 253, 2727.
W. Yu, H. Q. Xie, L. F. Chen, Y. Li, and C. Zhang (2009). Nanoscale Res. Lett. 4, 465.
C. Wu, B. P. Mosher, and T. Zeng (2006). J. Nanopart. Res. 8, 965.
P. Pulkkinen, J. Shan, K. Leppanen, A. Kansakoshi, A. Laiho, M. Jarn, and H. Tenhu (2009). Appl. Mater. Interfaces. 2, 519.
Z. Liu and Y. Bando (2003). Adv. Mater. 15, 303.
S. S. Joshi, S. F. Patil, V. Iyer, and S. Mahumuni (1998). Nanostr. Mater. 10, 1135.
J. N. Solanki, R. Sengupta, and Z. V. P. Murthy (2010). Solid State Sci. 12, 1560.
D. V. Goia (2004). J. Mater. Chem. 14, 451.
S. Giuffrida, L. L. Costanzo, G. Ventimiglia, and C. Bongiorno (2008). J. Nanopart. Res. 10, 1183.
I. Haas, S. Shanmugam, and A. Gedanken (2006). J. Phys. Chem. 110, 16947.
Y. Wei, X. Huaqing, C. Lifei, and L. Yang (2009). Nanoscale Res. Lett. 4, 465.
L. Huang, F. Peng, H. Yu, and H. J. Wang (2008). Mater. Res. Bull. 43, 3047.
A. Gniewek, A. M. Trzeciak, J. J. Zio´łkowski, L. Kepi’nski, J. Wrzyszcz, and W. Tylus (2005). J. Catal. 229, 332.
T. M. D. Dang, T. T. T. Le, E. Fribourg-Blanc, and M. C. Dang (2011). Adv. Nat. Sci. 2, 2.
K. Mallick, M. J. Witcomb, and M. S. Scurrell (2006). Eur. Polym. J. 42, 670.
A. Moores and F. Goettmann (2006). New J. Chem. 30, 1121.
U. Kreibig and M. Vollmer Optical Properties of Metal Clusters (Springer, Berlin, 1995).
P. Mulvaney (1996). Langmuir 12, 788.
J. A. Creighton and D. G. Eadon (1991). J. Chem. Soc. Faraday Trans. 87, 3881.
I. Lisiecki and M. P. Pileni (1993). J. Am. Chem. Soc. 115, 3887.
A. Yanase and H. Komiyama (1991). Surf. Sci. 248, 11.
A. K. Sasmal, C. Mondal, A. K. Sinha, S. S. Gauri, J. Pal, T. Aditya, M. Ganguly, S. Dey, and T. Pal (2014). ACS Appl. Mater. Interfaces. 6, 22034.
N. R. Jana, Z. L. Wang, T. K. Sau, and T. Pal (2000). Curr. Sci. 79, 1367.
I. Lisiecki, F. Billoudet, and M. P. Pileni (2006). J. Phys. Chem. 100, 4160.
K. Cheirmadurai, B. Soma, R. Murali, and P. Thanikaivelan (2014). RSC Adv. 4, 19507.
S. Panigrahi, S. Kundu, S. K. Ghosh, S. Nath, S. Praharaj, S. Basu, and T. Pal (2006). Polyhedron. 25, 1263.
Acknowledgments
The authors would like to thank Anna University for supporting financially under the Anna Centenary Research Fellow scheme (ACRF) and STRAIT project from CLRI. This work was supported by Centre for Nanoscience and Technology (Anna University). It made use of Shared Experimental Facilities at the Central Leather Research Institute (CSIR-CLRI) and Centre for Nanoscience and Technology (Anna University).
This paper was supported by the KU Research Professor Program, Konkuk University, Seoul, South Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kothandam, R., Pandurangan, M., Jayavel, R. et al. A Novel Nano-finish Formulations for Enhancing Performance Properties in Leather Finishing Applications. J Clust Sci 27, 1263–1272 (2016). https://doi.org/10.1007/s10876-016-0997-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-016-0997-8