Abstract
Magnetically recoverable Fe3O4/BiOCl nanocomposite photocatalysts were fabricated by a simple chemical coprecipitation method at room temperature. The amount of Fe3O4 incorporated into BiOCl was varied from 0 to 20 wt%. The as-synthesized samples were characterized by X-ray diffraction, transmission electron microscopy, energy dispersive spectroscopy, UV–Vis diffuse reflectance spectroscopy, and vibrating sample magnetometer. The obtained results show that the as-synthesized samples mainly contain both crystalline phases (Fe3O4 and BiOCl) and are composed of flower-like nanostructures. Compared to UV light-responsive BiOCl, all the nanocomposite photocatalysts show a strong light absorbance in the range of 250–800 nm, demonstrating that the Fe3O4/BiOCl nanocomposites can respond to visible as well as UV light. Moreover, visible light absorbance was increased with the increase in the Fe3O4 amount in the composite. The photocatalytic activity of nanocomposite photocatalysts was evaluated by the photodegradation of Rhodamine B (RhB) over the samples under visible light irradiation. The 10 wt% Fe3O4/BiOCl nanocomposite photocatalyst shows the highest photocatalytic efficiency among the samples. The Fe3O4/BiOCl nanocomposite photocatalyst was stable under visible light irradiation to efficiently degrade RhB molecules after five cycles and could be easily recovered with a magnet after each cycle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A rapid technological development, in turn, has led to the environmental pollution with various chemical compounds. The photocatalysts, regarded as one of the most prospective technologies to degrade the extremely toxic, carcinogenic and stable organic molecules in nature, have attracted increasing research interest. So far, a variety of photocatalysts, in the form of nanopowder, with enhanced adsorption and photocatalytic activity have been developed [1–3]. However, using nanophotocatalysts may generate another issue related to the complete recovery of photocatalyst nanoparticles suspended in the solution. A magnetic separation beneficially provides a convenient way to remove magnetically active species in the solution by applying an appropriate magnetic field [4].
Bismuth oxychloride (BiOCl), as one of the most important bismuth oxyhalides, has been used as a selective oxidation catalyst, ionic conductor, ferroelectric material, and pigment for many years [5, 6]. BiOCl is known to have a tetragonal layered structure consisting of [Cl–Bi–O–Bi–Cl] sheets stacked together by the non-bonding interaction through the Cl atoms along the c-axis. The strong internal static electric fields perpendicular to the Cl layer and the bismuth oxide-based fluorite-like layer enable the effective separation of the photoinduced electron-hole pairs, and result in an enhanced photocatalytic activity for the photodegradation of organic pollutants in wastewater under visible light irradiation [7]. As Zhang et al. [8] described, the BiOCl exhibits excellent photocatalytic activity for the degradation of Rhodamine B (RhB) dye under UV and visible light irradiation. Ma et al. [9] reported that the BiOCl nanoplates can be used to adsorb heavy metal ions from contaminated water/wastewater. The submicron-sized crystals of BiOCl with tunable morphologies from nanoflakes to hollow microspheres were synthesized, and their highly efficient photocatalytic activity under UV light was observed [10]. Rhodamine B and methylene blue (MO) molecule can also be photodegraded effectively by single-phase BiOCl nanoplate under visible light illumination [11, 12]. However, it is still challenging to recover BiOCl nanopowders completely after photodegradation process. Moreover, the photocatalytic efficiency of BiOCl under visible light is not high. To overcome these challenges, the combination of BiOCl with other visible light-responsive semiconductor would be one of the promising approaches.
As a traditional magnetic material, iron ferrite (Fe3O4) can be used in many different technological applications, such as photocatalysis [13], electronic devices [14], magnetic storage media [15], biomedicine [16, 17], etc. In the Fe3O4-based photocatalysis system, a magnetic separation provides an effective way for removing and recycling magnetically active nano- and microparticles of photocatalysts by applying an appropriate magnetic filed. Also, it is a convenient strategy for recovering photocatalyst particles after the photodegradation reaction and preventing water pollution with semiconductor nanoparticles. Furthermore, magnetically inactive species in the solution can also be recovered with a magnet by combining magnetically active and inactive species in one composite photocatalyst. For instance, Xu et al. [18] synthesized the Bi2WO6/Fe3O4 nanocomposite with enhanced photocatalytic efficiency and fast magnetic separability. Yu et al. [19] fabricated nanostructured TiO2/Fe3O4 coatings on transparent glasses by atmospheric plasma spraying. Other research groups have also produced Fe3O4-coupled composites, such as Fe3O4/SiO2/TiO2 [20], SiO2/Fe3O4 [21], Fe3O4/ZnCr-LDH [22], etc.
In this paper, we demonstrate a simple chemical co-precipitation method for the synthesis of Fe3O4/BiOCl nanocomposite photocatalysts at room temperature. The amount of Fe3O4 incorporated into BiOCl is varied from 0 to 20 wt%. The as-synthesized samples are characterized by X-ray diffraction (XRD), transmission electron microscopy (TEM), energy dispersive spectroscopy (EDS), UV–Vis diffuse reflectance spectroscopy, and vibrating sample magnetometer (VSM). It is found that the Fe3O4/BiOCl nanocomposite photocatalysts can be easily recovered and recycled after the photodegradation process because of the presence of magnetic Fe3O4.
Experimental
Synthesis of Magnetic Fe3O4 Nanoparticles
The magnetic Fe3O4 nanoparticles were fabricated by a chemical coprecipitation method at room temperature. First, FeCl2 and FeCl3 with the molar ratio of 1:2 were dissolved in deionized water under constant stirring for 15 min. To ensure complete precipitation, the pH of the yellow-colored solution obtained was adjusted to nine with the dropwise addition of aqueous NaOH solution. A reddish-brown-colored suspension was aged for 2 h at room temperature before centrifugation. The Fe3O4 nanoparticles precipitated were collected by centrifugation, washed with deionized water several times, and finally dried at 60 °C for 8 h.
Synthesis of Fe3O4/BiOCl Nanocomposite Photocatalysts
The Fe3O4-coupled BiOCl nanocomposite photocatalysts were also prepared by a chemical coprecipitation method at room-temperature. First, BiCl3 was dissolved in 1 M HCl, and then the as-prepared Fe3O4 nanoparticles were introduced into the clear solution of BiCl3 in different weights (0, 5, 10, and 20 wt%). The pH of the suspension was then adjusted to nine with the dropwise addition of aqueous ammonia under vigorous stirring, and the brown-colored precipitate was obtained after aging for 20 min. Finally, the precipitates were collected by centrifugation, washed with deionized water several times, and dried at 60 °C for 8 h. For comparison purposes, pure BiOCl was also prepared under the identical experimental conditions.
Characterizations
The crystalline phases of samples were identified by X-ray powder diffraction (XRD) using a D/Max 2550 diffractometer (Rigaku, Japan) with monochromated Cu Kα radiation (λ = 1.5405 Å) at 40 kV and 40 mA. The XRD patterns of the samples were measured at a scanning rate of 2°/min in the 2θ range of 10–70°. TEM observations were performed on samples using a JEM-2100 electron microscope (JEOL, Japan) with an acceleration voltage of 200 kV to distinguish the crystal structures and particle sizes. The UV–Vis diffuse reflectance spectra of samples were recorded using a λ 950 UV–Vis-NIR spectrophotometer (Perkin-Elmer, USA) in the wavelength range of 200–800 nm, using BaSO4 as a reference. The magnetic properties of samples were measured using a vibrating sample magnetometer (Lake Shore Cryotronics, Inc., USA) at room temperature with an applied field of 20 kOe.
Photodegradation Experiments
The photocatalytic activity of the as-prepared samples was evaluated by the degradation of Rhodamine B (RhB) over the samples under visible light irradiation using 400 W tungsten halogen lamps (with a λ < 420 nm cutoff filter). The reaction temperature was maintained at 25 °C by water in the cooling jacket of the reactor. The initial concentration of RhB was 20 mg/L and the catalyst was 1.0 g/L. The suspensions were stirred in the dark for 60 min to ensure adsorption/desorption equilibrium prior to visible light irradiation. During irradiation, 2 mL of suspension was taken out and filtered at a given time interval for subsequent RhB concentration analysis. The RhB concentration was analyzed by using a U-3010 UV–Vis spectrophotometer (Hitachi, Japan).
Result and Discussion
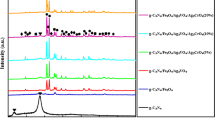
The XRD patterns of Fe3O4/BiOCl nanocomposite photocatalysts with different amounts of Fe3O4 are shown in Fig. 1. Figure 1a shows the XRD pattern of the BiOCl powders prepared at room temperature. All the diffraction peaks in Fig. 1a can be readily indexed to the tetragonal phase BiOCl (JCPDS card no. 06-0249). No diffraction peaks corresponding to the impurity phases were detected, indicating high purity of BiOCl. The intensive diffraction peaks suggest that the as-synthesized product is well-crystallized. Figure 1b–d show the XRD patterns of Fe3O4/BiOCl nanocomposite photocatalysts with 5, 10 and 20 wt% Fe3O4, prepared at room temperature. The most intensive diffraction peaks correspond to the tetragonal phase BiOCl. The less intensive diffraction peaks appeared at 2θ = 30.5° and 2θ = 35.8° in the XRD patterns in Fig. 1c, d can be indexed to the cubic phase Fe3O4 (JCPDS card no. 74-0748). However, the traces of Fe3O4 phase in the XRD pattern of the 10 wt% Fe3O4/BiOCl nanocomposite photocatalyst could not be detected due to less amount of Fe3O4 incorporated.
The morphology and particle size of pure BiOCl and Fe3O4/BiOCl nanocomposite photocatalyst prepared with different amounts of Fe3O4 at room temperature were observed by TEM and the results are represented in Fig. 2. In Fig. 2a, the TEM image of pure BiOCl powders is shown. As can be seen, each flower-like BiOCl nanostructure is composed of several nanoplates with the widths of 100–300 nm and the thicknesses of 10–20 nm. Figure 2b shows the HRTEM image of a single BiOCl plate-like nanostructure, which indicates that the BiOCl plate-like nanostructure was formed as a single-crystal structure. The lattice fringes of 0.275 nm in the observed crystallite agrees well with the (110) lattice plane. The TEM images of Fe3O4/BiOCl nanocomposite photocatalysts with different amounts of Fe3O4 (5 wt% (c), 10 wt% (d), and 20 wt% (e)) are shown in Fig. 2c–e. The TEM images evidence that the overall morphology of pure BiOCl was not changed with the incorporation of Fe3O4. That is, flower-like BiOCl nanostructures are still composed of several nanoplates with the thicknesses of 10–20 nm.
Nevertheless, the Fe3O4 nanoparticles with the size of 5–10 nm were homogenously dispersed on the surface of BiOCl nanoplates. The number of Fe3O4 nanoparticles increases with increasing the amount of Fe3O4 up to 20 wt%, as shown in Fig. 2c–e. Figure 2f shows a magnified TEM image of 10 wt% Fe3O4/BiOCl nanocomposite photocatalyst, which clearly indicates the presence of Fe3O4 nanoparticles on the surface of BiOCl nanoplates.
Energy-dispersive X-ray spectroscopy was used to elucidate the elemental composition of the Fe3O4/BiOCl nanocomposite photocatalyst. Figure 3 shows the EDS spectra of pure BiOCl (a) and Fe3O4/BiOCl nanocomposite photocatalysts with different amounts of Fe3O4: 5 wt% (b), 10 wt% (c) and 20 wt% (d). According to the EDS spectra in Fig. 3, pure BiOCl only contains Bi, Cl and O, whereas the Fe3O4/BiOCl nanocomposite photocatalysts mainly contain Bi, Fe, Cl and O. The elemental ratio of Bi:Fe in the Fe3O4/BiOCl nanocomposite photocatalyst prepared with 5, 10 and 20 wt% Fe3O4, is 7.8:1, 5.5:1, and 2.76:1.
The UV–Vis diffuse reflectance spectra of pure BiOCl and Fe3O4/BiOCl nanocomposite photocatalysts with different amounts of Fe3O4 (5, 10 and 20 wt%) are shown in Fig. 4.
As expected, pure BiOCl powders clearly show an absorption edge at ca. 360 nm, implying that pure BiOCl can only be excited under UV light irradiation. In contrast, the Fe3O4/BiOCl nanocomposite photocatalysts show a strong light absorbance in the range of 250–800 nm, evidencing that the Fe3O4/BiOCl nanocomposite photocatalysts can respond to the UV and visible light. This was achieved by the incorporation of Fe3O4 into BiOCl. Moreover, the visible light absorbance increases with increasing the amount of Fe3O4 incorporated into BiOCl.
Magnetic separation provides an effective route for removing and recycling magnetic nanoparticles. The magnetic properties of Fe3O4/BiOCl composite photocatalysts were analyzed using a vibrating sample magnetometer at room temperature. Figure 5 shows the magnetization M versus applied field H curves of Fe3O4/BiOCl nanocomposite photocatalysts prepared with 5, 10 and 20 wt% Fe3O4. From the M-H curves, a ferromagnetic behavior is observed with narrow hysteresis loops as well as small values of coercivity and remanence owing to the presence of ultrafine magnetite nanocrystals. The saturation magnetization values of 5, 10, and 20 wt% Fe3O4/BiOCl nanocomposite photocatalysts are found to be 5.1, 10.9, and 19.8 emu/g, respectively. The strong magnetization of the Fe3O4/BiVO4 nanocomposite photocatalysts indicates that the nanocomposite photocatalysts can be easily separated from solution by applying an external magnetic field.
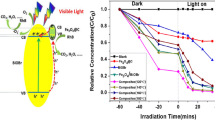
The photocatalytic activities of pure BiOCl and Fe3O4/BiOCl nanocomposite photocatalysts were evaluated by the degradation of RhB over the samples under visible light irradiation (λ > 420 nm) at room temperature. The temporal changes in the concentration of RhB were monitored by examining the variation in maximal absorption in UV–Vis spectra at 554 nm. The results from the photodegradation experiments are plotted in Fig. 6a. For comparison, we also conducted the experiments for the direct photolysis of RhB (blank experiment) under identical experimental conditions. Apparently, a blank experiment in the absence of photocatalyst shows no considerable change in the RhB concentration within 30 min of photocatalytic reaction. In contrast, 91.0 % RhB dye was degraded in the presence of pure BiOCl nanostructures under visible light irradiation within 30 min. It implies that the incorporation of 5−20 wt% Fe3O4 nanoparticles into BiOCl obviously enhances the photocatalytic activity of the Fe3O4/BiOCl nanocomposite photocatalyst. Interestingly, the 10 wt% Fe3O4/BiOCl nanocomposite photocatalyst shows the highest photocatalytic efficiency, 99.7 % RhB dye degraded under visible light irradiation within 30 min. According to the Fe3O4 amount, the degradation of RhB dye over the as-prepared nanocomposite photocatalyst follows the order: 99.7 % (10 wt% Fe3O4) > 99.5 % (5 wt% Fe3O4) > 99.2 % (20 wt% Fe3O4). The UV–Vis spectra of RhB aqueous solution after photodegradation experiment over 10 wt% Fe3O4/BiOCl nanocomposite photocatalyst are plotted in Fig. 6b as a function of irradiation time under visible light. As is shown, the absorption peak at 554 nm decreases dramatically as the irradiation time increases, and nearly disappears within 30 min.
a Degradation profiles of RhB over pure BiOCl Fe3O4/BiOCl nanocomposite photocatalysts with different amounts of Fe3O4: 5 wt%, 10 wt%, 20 wt% (C 0 = 20 mg/L, catalyst 1.0 g), b UV–Vis spectra of RhB aqueous solution after photodegradation experiment over 10 wt% Fe3O4/BiOCl, c reaction kinetics of degradation of the RhB over the as-prepared samples under visible light irradiation and d five successive RhB photodegradation runs using the 10 wt% Fe3O4/BiOCl
In order to quantitatively investigate the reaction kinetics of degradation of the RhB over the as-prepared samples under visible light irradiation, the following pseudo-first-order kinetic model is used [23, 24]:
where C t and C 0 are the concentrations of RhB at irradiation time t and 0 (the time to obtain absorption–desorption equilibrium) in the aqueous solution, respectively. K is the pseudo-first-order rate constant, which can be obtained from the decrease of peak intensity at 554 nm with irradiation time. As shown in Fig. 6c, a liner relationship of −ln(Ct/C0) with irradiation time demonstrates that the pseudo-first-order rate constants K calculated from the data shown in Fig. 6c are 0.086, 0.211, 0.215, 0.165 min−1 for pure BiOCl and Fe3O4/BiOCl nanocomposite photocatalysts with 5, 10 and 20 wt% Fe3O4, respectively. According to the pseudo-first-order rate constants, the degradation rate of RhB over the as-prepared samples can be placed in the following order: 10 wt% Fe3O4/BiOCl > 5 wt% Fe3O4/BiOCl > 20 wt% Fe3O4/BiOCl > pure BiOCl.
To study the photostability of Fe3O4/BiOCl nanocomposite photocatalyst after photodegradation experiments under visible light irradiation, the samples were collected, dried, and reused in five successive photodegradation experiments. Figure 6d shows the results of five successive RhB photodegradation runs using the 10 wt% Fe3O4/BiOCl nanocomposite photocatalyst under the identical experimental conditions. It can be seen from Fig. 6d that the 10 wt% Fe3O4/BiOCl nanocomposite photocatalyst retains its high photocatalytic ability in all five successive runs. Each run maintaining > 97 % of RhB photodegradation lasted for 30 min. As an example shown in the inset of Fig. 6b, when the Fe3O4/BiOCl composite photocatalyst was dispersed in water, the suspension turns into a black color, and upon applying an external magnetic field, the tiny black powders were readily harvested within 30 s and the solution became transparent.
Based on the results shown above, pure BiOCl and Fe3O4/BiOCl nanocomposite photocatalysts have good photocatalytic activity under visible light irradiation. By considering its absorption edge, pure BiOCl can only absorb UV light (λ < 360 nm). Hence, visible light (λ > 420 nm) could not excite BiOCl to produce reactive radicals because of its wide band gap, and the degradation of RhB over pure BiOCl through a photocatalytic pathway was negligible. Nevertheless, 91 % RhB was degraded by pure BiOCl within 30 min under visible light, while 99.7 % RhB was degraded by 10 wt% Fe3O4/BiOCl nanocomposite photocatalyst within 30 min under visible light. The RhB degradation mechanism of photosensitization pathway over BiOCl under visible light irradiation has been reported previously. In the photosensitization pathway, RhB dye is considered as a photosensitizer, whereas the BiOCl plays the key roles of electron carriers and electron acceptors. For the photocatalysis of pure BiOCl, the RhB first absorbs the light energy to produce singlet and triplet states (denoted as RhB*) [25], and then the electrons can transfer to the CB of BiOCl and react with O2 to generate the O2 − and ·OH. The RhB* is converted to the radical cation ·RhB+ that ultimately reacts with reactive oxygen radicals to yield the degraded products. Therefore, the RhB dye can be degraded by BiOCl with a wide gap of about 3.4 eV under visible-light irradiation. The photocatalytic activity of Fe3O4/BiOCl nanocomposite photocatalyst is higher than that of pure BiOCl due to the synergetic effect of Fe3O4 and BiOCl. After the incorporation of Fe3O4 nanoparticles, the Fe3O4/BiOCl nanocomposite photocatalyst becomes more effective in absorbing visible light than pure BiOCl that will enhance the photocatalytic activity for the degradation of organic dyes under visible light irradiation. Furthermore, the coupling of a narrow-band-gap semiconductor with a wide-band-gap semiconductor not only enhances the optical absorption ability of a photocatalyst by extending the absorption range of solar spectrum but also facilitates the separation of the photogenerated carriers under internal field induced by different electronic band structures [24]. Therefore, the Fe3O4/BiOCl nanocomposite photocatalyst shows higher photocatalytic activity than single ones under visible light irradiation. The photocatalytic mechanism can be described as follows:
Conclusions
In summary, magnetically recoverable Fe3O4/BiOCl nanocomposite photocatalysts were fabricated by a simple chemical coprecipitation method at room temperature. The amount of Fe3O4 incorporated into BiOCl was varied from 0 to 20 wt%. The photocatalytic activity of Fe3O4/BiOCl nanocomposite photocatalysts was evaluated by the photodegradation of RhB over the samples under visible light irradiation. The 10 wt% Fe3O4/BiOCl nanocomposite photocatalyst shows the highest photocatalytic efficiency among the samples. The Fe3O4/BiOCl nanocomposite photocatalyst was stable under visible light irradiation to efficiently degrade RhB molecules after five cycles and could be easily recovered with a magnet after each cycle.
References
M. S. Bazarjani, M. Hojamberdiev, K. Morita, G. Zhu, G. Cherkashinin, C. Fasel, T. Herrmann, H. Breitzke, A. Gurlo, and R. Riedel (2013). J. Am. Chem. Soc. 135, 4467.
M. Hojamberdiev, G. Zhu, A. Eminov, and K. Okada (2013). J. Clust. Sci. 24, 97.
G. Zhu and W. Que (2013). J. Clust. Sci. 24, 531.
M. Shokouhimehr, Y. Piao, J. Kim, Y. Jang, and T. Hyeon (2007). Angew. Chem. 119, 7169.
W. L. Huang (2012). Comp. Mater. Sci. 55, 166.
Y. Zhiyong, D. Bahnemann, R. Dillert, S. Lin, and L. Liqin (2012). J. Mol. Catal. A 365, 1.
S. Wu, C. Wang, Y. Cui, W. Hao, T. Wang, and P. Brault (2011). Mater. Lett. 65, 1344.
X. Zhang, Z. Ai, F. Jia, and L. Zhang (2008). J. Phys. Chem. C 112, 747.
J. Ma, X. Liu, J. Lian, X. Duan, and W. Zheng (2010). Cryst. Growth Des. 10, 2522.
K. Zhang, J. Liang, S. Wang, J. Liu, K. Ren, X. Zheng, H. Luo, Y. Peng, X. Zou, X. Bo, J. Li, and X. Yu (2012). Cryst. Growth Des. 12, 793.
X. F. Chang, M. A. Gondal, A. A. Al-Saadi, M. A. Ali, H. F. Shen, Q. Zhou, J. Zhang, M. P. Du, Y. S. Liu, and G. B. Ji (2012). J. Colloid. Interface Sci. 377, 291.
Z. Q. Shi, Y. Wang, C. M. Fan, Y. F. Wang, and G. Y. Ding (2011). Trans. Nonferrous Met. Soc. China. 21, 2254.
S. Xuan, W. Jiang, X. Gong, Y. Hu, and Z. Chen (2009). J. Phys. Chem. C 113, 553.
Q. Dai, D. Berman, K. Virwani, J. Frommer, P.-O. Jubert, M. Lam, T. Topuria, W. Imaino, and A. Nelson (2010). Nano Lett. 10, 3216.
G. Maris, O. Shklyarevskii, L. Jdira, J. G. H. Hermsen, and S. Speller (2006). Surf. Sci. 600, 5084.
H. Gu, K. Xu, C. Xu, and B. Xu (2006). Chem. Commun. 9, 941.
M. Babic, D. Horák, M. Trchová, P. Jendelová, K. Glogarová, P. Lesný, V. Herynek, M. Hájek, and E. Syková (2008). Bioconjug. Chem. 19, 740.
X. Xu, X. Shen, G. Zhu, L. Jing, X. Liu, and K. Chen (1012). Chem. Eng. J. 200–202, 521.
Q. Yu, C. Zhou, and X. Wang (2008). J. Mol. Catal. A 283, 23.
X. Huang, G. Wang, M. Yang, W. Guo, and H. Gao (2011). Mater. Lett. 65, 2887.
G. Shi, B. Sun, Z. Jin, J. Liu, and M. Li (2012). Sensors Actuators B 171–172, 699.
D. Chen, Y. Li, J. Zhang, J-z Zhou, Y. Guo, and H. Liu (2012). Chem. Eng. J. 185–186, 120.
M. Hojamberdiev, G. Zhu, P. Sujaridworakun, S. Jinawath, P. Liu, and J.-P. Zhou (2012). Powder Technol. 218, 140.
B. Cao, J. Peng, and Y. Xu (2013). J. Clust. Sci. (in press).
J. Xiong, G. Cheng, G. Li, F. Qin, and R. Chen (2011). RSC Adv. 1, 1542.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Program Nos. 51102160 and 51272148), the Fundamental Research Funds for the Central Universities (Program No. GK201102027) and the State Ministry of-Education College Students’Innovative Projects (Program No. 201210781002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tan, C., Zhu, G., Hojamberdiev, M. et al. Room Temperature Synthesis and Photocatalytic Activity of Magnetically Recoverable Fe3O4/BiOCl Nanocomposite Photocatalysts. J Clust Sci 24, 1115–1126 (2013). https://doi.org/10.1007/s10876-013-0602-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-013-0602-3