Abstract
One of the applications of nanotechnology is use of carbon nanotubes for the targeted delivery of drug molecules. To demonstrate the physical and chemical properties of biomolecules and identify new material of drug properties, the interaction of carbon nanotubes (CNTs) with biomolecules is a subject of many investigations. CNTs is a synthetic compound with extraordinary mechanical, thermal, electrical, optical, and chemical properties widely applied for technological purposes. In this article we have tried to investigate thermodynamic parameters and dielectric effects in different solvents for one of the most famous anticancer drug “cisplatin” combined to SWCNT, by Monte Carlo and density functional theory (DFT) calculations. Cause of platinum element in cisplatin we have done calculations as Gibbs free energy, thermal enthalpy, thermal energy and entropy at 6-31G** basis set with SCRF model of solvent. In this work, the major point has been embedded that results of both two methods of Monte Carlo and DFT can overlap with each other and cisplatin- SWCNT is a suitable compound for drug delivery in different media.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, the majority of the investigation computational chemistry is focused on the examination of molecules submerged in various solvents and the interaction of anticancer drug with carbon nanotubes (CNTs) as well. The most tendency is toward the interaction between anticancer drug and single-walled carbon nanotubes (SWCNTs).Cis-diamminedichloroplatinum(II) (cisplatin) is a chemotherapy drug and it is used to treat some various types of cancers. These platinum complexes react in vivo, binding to and causing crosslinking of DNA, which ultimately triggers apoptosis.
The compound cis-PtCl2(NH3)2 was first described by M. Peyrone in 1844, and known for a long time as Peyrone’s salt [1]. The structure was deduced by Alfred Werner in 1893 [2]. In 1965, Rosenberg et al. discovered that electrolysis of platinum electrodes generated a soluble platinum complex which inhibited binary fission in Escherichia coli (E. coli) bacteria. Although bacterial cell growth was uninhibited, cell division was arrested, the bacteria growing as filaments up to 300 times their normal length [3]. The octahedral Pt(IV) complex cis PtCl4(NH3)2 was found to effective at forcing filamentous growth of E. coli cells. The square planar Pt(II) complex, cis PtCl2(NH3)2 turned out to be even more effective at forcing filamentous growth [4, 5].This finding led to the finding that cis PtCl2(NH3)2 was indeed highly effective at regressing the mass of sarcomas in rats [6].Confirmation of this finding, and extension of testing to other tumour cell lines launched the medicinal applications of cisplatin.
By trying cisplatin to contact DNA strand, its structure will be dechlorized simultaneously. Therefore, platinum of cisplatin add to guanine parts of DNA (Scheme 1) One of the chloride ligands is slowly displaced by water, in a process termed aquation. The aqua ligand in the resulting [PtCl(H2O)(NH3)2]+ is itself easily displaced, allowing the platinum atom to bind to bases. Of the bases on DNA, guanine is preferred. Subsequent to formation of [PtCl(guanine-DNA)(NH3)2]+, crosslinking can occur via displacement of the other chloride ligand, typically by another guanine [2]. Cisplatin crosslinks DNA in several different ways, interfering with cell division by mitosis. The damaged DNA elicits DNA repair mechanisms, which in turn activate apoptosis when repair proves impossible. Recently it was shown that the apoptosis induced by cisplatin on human colon cancer cells depends on the mitochondrial serine-protease Omi/Htra2 [7]. Since this was only demonstrated for colon carcinoma cells, it remains an open question if the Omi/Htra2 protein participates in the cisplatin-induced apoptosis in carcinomas from other tissues. Most notable among the changes in DNA are the 1,2-intrastrand cross-links with purine bases. These include 1,2-intrastrand d(GpG) adducts which form nearly 90% of the adducts and the less common 1,2-intrastrand d(ApG) adducts. 1,3-intrastrand d(GpXpG) adducts occur but are readily excised by the nucleotide excision repair (NER). Other adducts include inter-strand crosslinks and nonfunctional adducts that have been postulated to contribute to cisplatin’s activity. Interaction with cellular proteins, particularly HMG domain proteins, has also been advanced as a mechanism of interfering with mitosis, although this is probably not its primary method of action. Note that although cisplatin is frequently designated as an alkylating agent, it has no alkyl group and so cannot carry out alkylating reactions. It is correctly classified as alkylating-like.
Carbon nanotubes (SWNTs) are very prevalent in today’s world of medical research and are being highly researched in the fields of efficient drug delivery and biosensing methods for disease treatment and health monitoring. The use of SWNTs in drug delivery and biosensing technology has the potential to revolutionalize medicine. Functionalization of SWNTs has proven to enhance solubility and allow for efficient tumor targeting/drug delivery.
SWNTs have several unique chemical, size, optical, electrical and structural properties that make them attractive as drug delivery and biosensing platforms for the treatment of various diseases [8, 9] and the noninvasive monitoring of blood levels and other chemical properties of the human body, respectively [10]. SWNTs are considered to be ideal candidates due to their remarkable structure-dependent properties, including high tensile strength and surface area, together with their high electric and thermal conductivity, and their high stability in suspension when they are properly functionalized [11, 12].
SWNTs can be bind to the polymers and biological system such as DNA, carbohydrates and drugs [13]. Recently literatures have shown that anticancer drugs binds to SWNTs with covalent and non-covalent conjugations [14–16], but the details of these interactions have yet many questions.
In this article, the Cisplatin interaction with open-end of SWCNT and water effects on this intersection have been investigated in drug delivery.
This study has been carried out using quantum mechanics (QM) method to increase the practical application of cisplatin-SWCNT system. We believe that this investigation could be used in nanotechnology as well as other drug delivery.
Theoretical Background and Computational Methods
Monte Carlo Simulation
The Monte Carlo (MC) method samples phase space by generating random configurations from a Boltzmann distribution at a given temperature. Averages computed from a properly equilibrated MC simulation corresponding to thermodynamic ensemble averages. Thus, the MC method can be used to find average energies and equilibrium structural properties of complex interacting systems [17].
For some systems, this method provides a more direct route to equilibrium structural and thermodynamic properties and an alternate approach to the generation of stable conformations [18, 19].
In principle, one could define the time scale of MC dynamics based on the frequency of local conformational jumps.
In this study the accuracy of MC algorithm is determined by random displacement on cisplatin-SWNT model in mixed solvents (dielectric constant = 1, 32.63, 36.42808, 40.27192, 44.07, 47.86808, 51.71192, 59.30808, 63.15192, 66.95, 70.74808, 74.59192, 78.39) using by Hyperchem-7 program package [20].
The effect of temperature in MC simulations is primarily to modulate the strength of intermolecular interactions, since temperature enters the simulation only through the Boltzmann factor (exp(-∆E/κT)). Therefore, in MC method, computation of energy was sufficient to simulate the structure of cisplatin- SWCNT at 298, 300, 305, 310 and 315 K temperatures.
DFT Method
In this investigation density functional theory (DFT) method on cisplatin-SWCNT implemented in Gaussian 03 [21] for the vacuum and different solvents (water, dimethylsulfoxide(DMSO), nitro methane, acetonitrile, dichloroethane, dichloromethane, tetrahydrofuran(THF), aniline, chlorobenzene, chloroform, diethyl ether, toluene, benzene, carbon tetrachloride, cyclohexane and heptane) which provided logical accuracy and are particularly suitable for the study of defects in a wide range of materials [22]. DFT is based on a theorem due to Hohenberg and Kohn, which states that all ground state properties are functions of the total electronic charge density ρ(r) [23, 24].
There are several different DFT functional, available differing primarily in the choice of the basis functions, in which, the electronic wave functions are expanded and the scheme of integration [23].In this work DFT with B3LYP keyword have been employed for calculations using 6-31G** basis sets. B3LYP corresponds to the approximation method that makes use of Beck-Style parameters density functional theory [25] with the Lee–Yang–Parr correlation functional [26].
To account for the solvent effects, the self-consistent reaction field (SCRF) method is most commonly used for different systems [27].Hence, SCRF based on Onsager model used to include the effects of the solvents on DNA-cisplatin interaction with open-end of SWCNT in drug delivery.
In Onsager model, solute whose charge distribution is represented by a simple dipole, that is embedded in a typically spherical cavity and interacts with the solvent. The energy of these interactions E int that is equal to the difference between its solvated and isolated energies [28] is given by:
Therefore, for electroneutral dipolar solute molecules the electronic Schrodinger equation could be solved using the self-consistent field method as follows:
where H 0 is the total electronic Hamiltonian for the isolated molecule, Ψ denotes the electronic wave function [28], and V is the reaction field potential [28] that given by:
where \( \hat{\mu } \) denotes the dipole moment operator and \( \varphi |\hat{\mu } |\varphi \) indicates the expectation value of total dipole moment of the solute molecule [29], ε is the solvent static dielectric constant and a 0 is cavity radius [30].
In Onsager calculation additional specific parameters including: cavity radius and number of points per sphere are necessary. In the Onsager calculations the cavity radius derived from molecular volume calculations [28].
In this article, the interaction energy, total dipole moment and thermodynamic properties of DNA-cisplatin interaction binded to open-end of SWCNT in water, dimethylsulfoxide(DMSO), nitromethane, acetonitrile, dichloroethane, dichloromethane, tetrahydrofuran(THF), aniline, chlorobenzene, chloroform, diethylether, toluene, benzene, carbon tetrachloride, cyclohexane, heptane, vacuum have been explored with B3LYP method using 6-31G** basis set.
Results and Discussion
MC Study
The combination of CNTs with biologically important structures, such as DNA or polypeptides, is particularly intriguing since it opens the door to novel biotechnology and nanotechnology applications [31]. SWNTs have been considered as the leading candidate for nanodevice applications because of their one-dimensional electronic band structure, molecular size, biocompatibility and controllable property of electrical conductivity and reversible response to biochemical reagents [32]. Therefore, the interaction of biomolecules with SWNTs has generated a great deal of interest in the past few years [33]. SWNTs can be bind to the polymers and biological system such as DNA and carbohydrates [13].
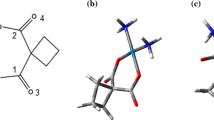
Understanding of different molecular processes in chemistry and biochemistry is possible by the interaction between the solute and the solvent molecules. The structure of CNT is in the form of a hexagonal mesh which is similar to a graphite sheet and it bears a carbon atom accommodated in the vertex of each mesh. The sheet is rolled and its two edges are joined continuously. Combination of cisplatin drug and single-walled carbon nanotube has been modeled for drug delivery (Fig. 1). Therefore, in this article, thermodynamic values on interaction of cisplatin with SWCNT have been investigated in different dielectric constants. First, we exercised different force fields to determine energy and other kinds of thermodynamic parameters on interaction of cisplatin with SWCNT.
In this study MC calculations on cisplatin added to guanine parts of DNA combined to SWNT (cisplatin-SWNT) for investigation of dielectric effects at different media such as water, dimethylsulfoxide(DMSO), nitro methane, acetonitrile, dichloroethane, dichloromethane, tetrahydrofuran(THF), aniline, chlorobenzene, chloroform, diethyl ether, toluene, benzene, carbon tetrachloride, cyclohexane, heptane, vacuum have carried out at 298 K up to 315 K span (Fig. 1).
MC method is one of the most broadly and commonly used numerical technique, with application in statistical physics, quantum mechanics, field theory, and others (17).
When we changed media namely pure methanol (dielectric constant = 32.63) to different proportional mixture of “methanol + water” and also pure water solvent (dielectric constant = 78.39), energy predictable decreasing of cisplatin-SWCNT has been appeared because by increasing dielectric constant, energy will decrease too and therefore we had the most stable situation for (cisplatin-SWCNT) at pure water medium (as shown in Fig. 2). At vacuum phase calculations (dielectric constant = 1) we had a jump at energy parameter. It means that our structure experienced the most unstable status. Also we have specified potential energy and correlation coefficient for all 14 dielectric constants in which cisplatin-SWCNT was solved at those media that listed in Tables 1 and 2 at 298, 300, 302, 305 and 310 K and it has been plotted the potential energy in MC method at 300 K.
By values of potential energy (kcal/mol) and time (ps) for superlative correlation coefficient at all media mentioned above, we realize that potential energies have a considerable and the same changes between 0 and 15 ps and also after 25 ps these changes have smooth gradient approximately.
Consequently the best correlation coefficient for most of solvents were carried out at 302 K up to 310 K namely the best correlation coefficient will appear at near natural human temperature. Complete elaboration is reported in Tables 1, 2 and Fig. 2.
Also, we have seen that with increasing of dielectric constant in different media, the complex system (cisplatin-SWCNT) has become more stable (Fig. 2).
As a result, the most stable situation was relating to cisplatin-SWNT interaction with pure water solvent. According to last part, we expect that pure water is a best solvent for this model in drug delivery.
Ab Initio Study
The Gibbs free energies, thermal enthalpies, thermal energies and entropies calculated in different temperatures (298, 300, 305 and 310 K) at B3LYP/6-31G** level of method in vacuum and also some solvents that are listed in Table 3. According to values carried out, entropy has a low quantity and against the enthalpy cannot have a significant role on free energy Gibbs. Therefore, enthalpy terms have the significant role in (cisplatin-SWCNT) reactions.
By increasing temperature from 298 to 310 K, Gibbs free energy at all solvents has been decreased. Indeed interaction between cisplatin-SWCNT and all solvents at high temperature are higher (as shown in Table 3a; Fig. 3). As we expected by increasing temperature entropy changes is increasing too (as shown in Table 2d). Besides them, by arranging solvents from vacuum (ε = 1) toward water (ε = 78.39) listed in Table 3 for Gibbs free energy in different temperatures (T = 298, 300, 305 and 310 K) and optimized energy in Table 4, we have notified that the interaction of cisplatin-SWCNT complex with water solvent is the most stable compound (shown in Fig. 4). The same explanation could be taken for enthalpy and energy too (Table 4b, c).
The lack of experimental demonstration and its importance in theoretical simulations was a motivation for us to investigate dipole moments from a theoretical point of view. Dipole moment is a parameter that demonstrated charge distribution in all atoms of a system so we attended that by increasing dielectric constant of the solvent (from dielectric constant 1.92 by Heptane toward water with dielectric constant 78.39) and increasing polarizability, dipole moment has follow ascendant approach (Table 4) and caused of high interaction between polarized solvent and anticancer drug, the most permanent situation will appear at high dielectric constant and the dipole moment converged under different conditions (Fig. 4).
In addition, the orientation of molecules of water on the surfaces of cisplatin-SWCNT can influence the orientation of dipole moment. The reason is that a dipole in the molecule will induce a dipole in the medium, and the electric field applied by the solvent dipole will in turn interact with the molecular dipole leading to net stabilization.
Thus, the results achieved within the Onsager self—consistent reaction field (SCRF) model seemed quite sensitive to the polarity of the encircling solvent. Also, we can say that, by increase of molecule dipole moment, the interaction between molecules of solvent and solute will be increased.
Therefore, it could be realized that our observed dipole moment has been directed linearly with increasing of dielectric constant and this observation supported the stability of (cisplatin-SWCNT) at various media.
Conclusion
In the treatment of cancer patients, a perception of the pathophysiology of cancer and contact of anticancer drugs has the main role.
In this research, we have studied the effects of various solvents and temperatures on interaction of anticancer drug, cisplatin, with SWCNT as model for drug delivery.
Monte Carlo Calculation
By increasing dielectric, system (cisplatin and mediums) will be more stable.
We have superlative correlation coefficient in water 302 K up to 310 K that is near human body temperature. The lowest potential energy is related to pure water.
Ab Initio Calculation
By decreasing Gibbs free energy we will see increasing of entropy and we have exothermic reaction between cisplatin-SWCNT and media. More stable situation of cisplatin-SWCNT happened at high temperature (310 K) and water solvent (dielectric constant = 78.39).By increasing dielectric constant of solvents, system has been polarized more and we had more stable situation.
References
M. Peyrone (1844). Ann. Chemie. Pharm. 51(1), 1.
T. Stephen (2005). C&EN News 83, 25.
B. Rosenberg, L. Van Camp, and T. Krigas (1965). Nature 205(4972), 698.
B. Rosenberg, L. Van Camp, E. B. Grimley, and A. J. Thomson (1967). J. Biol. Chem. 242(6), 1347.
A. J. Thomson, D. A. Christie, and E. M. Tansey (2007). Wellcome Trust Witnesses to Twentieth Century Medicine 30, 6.
B. Rosenberg, L. Vancamp, J. E. Trosko, and V. H. Mansour (1969). Nature 222(5191), 385.
F. G. Pruefer, F. Lizarraga, V. Maldonado, and J. Melendez-Zajgla (2008). J. Chemother. 20, 348.
A. Bianco, K. Kostarelos, and M. Prato (2005). Curr. Opin. Biotechnol. 9, 674.
M. Khalehian, M. Zahmatkesh, F. Mollaamin, and M. Monajjemi (2011). Fullerenes, Nanotubes, and Carbon Nanostructures 19(4), 251.
B. Ghalandari, M. Monajjemi, and F. Mollaamin (2011). J. Comput. Theor. Nanosci. 8(7), 1212.
M. Monajjemi, L. Mahdavian, and F. Mollaamin (2008). Bull. Chem. Soc. Ethiop. 22(2), 1.
M. Monajjemi, L. Mahdavian, F. Mollaamin, and M. Khaleghian (2009). Russ. J. Inorg. Chem. 54(9), 14651473.
T. Ramanathan, F. T. Fisher, R. S. Ruoff, and L. C. Brinson (2005). Chem. Mater. 17(6), 1290.
A. Star, E. Tu, J. Niemann, J. Christophe, P. Gabriel, C. S. Joiner, and C. Valcke (2006). Proc. Nat. Acad. Sci. 103(4), 921.
C. Hu, Y. Zhang, G. Bao, Y. Zhang, M. Liu, and Z. L. Wang (2005). J. Phys. Chem. B 109(43), 20072.
M. E. Hughes, E. Brandin, and J. A. Golovchenko (2007). Nano. Lett. 7(5), 1191.
M. Kastner (2010). Commun. Nonlinear Sci. Numer. Simul. 15, 1589.
M. Monajjemi, S. Ketabi, H. Zadeh, and A. Amiri (2006). Biochemistry (Mosc) 71(S1), S1.
M. Monajjemi, S. Ketabi, and A. Amiri (2006). Russ. J. Phys. Chem. A 80(S1), S55.
Hypercube, Inc., Gainesville, FL, USA.
M. J. Frisch et al, in GAUSSIAN 03, Revision C.02 (Gaussian Inc., Wallingford, CT, 2004).
M. Monajjemi, M. H. Razavian, F. Mollaamin, F. Naderi, and B. Honarparvar (2008). Russ. J. Phys. Chem. A 82(13), 113.
F. Mollaamin, M. T. Baei, M. Monajjemi, R. Zhiani, and B. Honarparvar (2008). Russ. J. Phys. Chem. A 82(13), 2354.
A. Maiti (2008). Microelectronics 39(20), 208.
D. Srivastava and S. N. Atluri (2002). Comput. Model. Eng. Sci. 3(5), 531.
A. D. Beck (1993). J. Chem. Phys. 98(7), 5648.
M. Monajjemi, B. H. Honarparvar, H. Haeri, and M. Heshmat (2006). Russ. J. Phys. Chem. C 80(1), S40.
A. Tsolakidis and E. Kaxiras (2005). J. Phys. Chem. A 109(10), 2373.
A. Szarecka, J. Rychlewski, and U. Rychlewska (1998). Comput. Method Sci. Technol. 4, 25.
M. Karelson and A. Lomaka (2001). Arkivoc. III, 51.
G. Lu, P. Maragakis, and E. Kaxiras (2005). Nano. Lett. 5(5), 897.
X. Li, Y. Peng, and X. Qu (2006). Nucl. Acids Res. 34(13), 3670.
X. Zhao and J. K. Johnson (2007). J. Am. Chem Soc. 129(34), 10438.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Monajjemi, M., Mollaamin, F. Molecular Modeling Study of Drug-DNA Combined to Single Walled Carbon Nanotube. J Clust Sci 23, 259–272 (2012). https://doi.org/10.1007/s10876-011-0426-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-011-0426-y