Abstract
Two new 3-D supramolecular compouds: [Cu2X2(Hpyba)2]n (X = Br (1), I (2), Hpyba = 4-pyridin-4-yl-benzoic acid) have been hydrothermally synthesized and structurally characterized. Although both compounds exhibit different space groups, they have a similar structure. Each {Cu2X2} cluster unit interconnects to form a 1D stair-step chain. The Saturated Hpyba ligands are regularly appended to both sides of main chain, linked via N atoms of ligands. These infinite chains are further linked by H-bonds and π–π interactions to form a 3-D supramolecular network structure. The thermal stability of 1 and 2 was investigated by thermogravimetric measurements.
Graphical Abstract
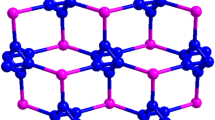
Two new 3-D supramolecular compouds [Cu2X2(Hpyba)2]n (X = Br(1), I(2), Hpyba = 4-pyridin-4-yl-benzoic acid) have been obtained under hydrothermal conditions. Both compounds exhibit a 1D stair-step chain structure based on the {Cu2X2} cluster units. These infinite chains are further linked by H-bonds and π–π interactions into forming a 3-D supramolecular network with two types of channels along different directions.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, the design and construction of larger supramolecular networks has attracted considerable attention, because of their intriguing architectures [1] and their potential applications in magnetism [2], luminescence [3], bimetallic catalysis [4], and molecular adsorption [5]. To construct these networks, the selection of the multifunctional organic ligands [6–8] is a key point to design and assemble the expected coordination polymers. As multifunctional bridging ligand, the isonicotinic acid (Hin) is a rigid linear ligand owing to the O and N donors on the opposite sides and can construct the extended frameworks with high structural stability and special topologies [9–14]. Based on these considerations, we chose Hin as the bridging ligand and successfully obtained a series of 3D Ln-TM wheel cluster polymers [Er7(μ3-O)(μ3-OH)6(bdc)3](in)9[Cu3X4] (X = Cl/Br, H2bdc = 1,2-benzenedicarboxylic acid) [10] and [Ln6(μ3-O)2](in)20[Cu8(μ4-I)2(μ2-I)3]·H3O (Ln = Y/Nd/Dy/Gd/Sm/Eu/Tb) [11], 3-D Ln-TM nanosized hydroxo lanthanide cluster polymers [Ln14(μ6-O)(μ3-OH)20(in)22Cu6Cl4(H2O)8]·6H2O (Ln = Y/Gd/Dy) [12], as well as 3-D Ln-TM pillared-layer polymers Ln2(bdc)2(in)2(H2O)2Cu·X (H2bdc = 1,2-benzenedicarboxylic acid; Ln = Eu/Sm/Nd, X = ClO4 −; Ln = Nd, X = Cl−) [13], Ln2Cu7I6(in)7(H2O)6·H2O (Ln = Ce/Sm) and Er4(OH)4Cu5I4(in)6(na)(2,5-pdc)·0.3H2O; na = nicotinic acid, 2,5-pdc = 2,5-pyridinedicarboxylic acid) [14]. The above-mentioned frameworks are all built from Ln and TM cluster linked by coordinate covalent bonds. Compared with the Hin ligand, 4-pyridin-4-yl-benzoic acid (Hpyba) has one more benzene ring and shows a longer linear length, which may make more open frameworks than the short Hin ligand. However, the investigations on the Hpyba ligand are rare, only three interpenetrating diamondoid lattices reported so far [15, 16]. Specially, there have no attempts to use the Hpyba ligand for making the supramolecular networks based on the combination of H-bonds and π–π interactions. As the continuing work in this system, we have extended our interesting from Hin to Hpyba ligand and successfully obtained two novel 1D stair-step chains built by the {Cu2X2} cluster units, [Cu2X2(Hpyba)2]n (X = Br (1), I (2)), by using Hpyba ligand. The chains in 1 and 2 are further linked each other by H-bonds and π–π interactions, forming 3-D supramolecular frameworks, respectively.
Experimental
Materials and Methods
All chemicals were commercially purchased and used without further purification. IR spectra (KBr pellets) were recorded on an ABB Bomen MB102 spectrometer over a range 400–4,000 cm−1. The thermogravimetric analysis was performed on a Mettler Toledo TGA/SDTA 851e analyzer in air atmosphere with a heating rate of 5 °C/min from 30 to 1,000 °C. The elemental analysis was carried out using the combustion method on an Elemental Vario EL III CHNOS elemental analyzer.
Synthesis of [Cu2Br2(Hpyba)2]n (1)
A mixture of La(C2O4)3·9H2O (0.2 mmol, 0.1417 g), CuBr2(0.2 mmol, 0.0445 g), Hpyba(0.5 mmol, 0.0997 g), H2O (10 mL, 0.22 mmol) and three drops of HClO4 with the pH value of about 2.0 was sealed in a 30 mL Teflon-lined bomb at 170 °C for 5 days then cooled to room temperature, orange parallelepiped-shape crystals of 1 were recovered by filtration, washed with distilled water and dried at ambient temperature (Yield, 80%). Elemental Analysis: Calcd. for C24H18Br2Cu2N2O4: C 42.03, H 2.63, N 4.09. Found: C 42.05, H 2.61, N 4.08.
Synthesis of [Cu2I2(Hpyba)2]n (2)
Compound 2 was prepared by a similar method as the synthesis of 1 except that CuBr2 was replaced by CuI. The resulting orange crystals of 2 suitable for X-ray structural analysis were filtered off, and dried (Yield, 75%). Elemental Analysis: Calcd. for C24H18Cu2I2N2O4: C 36.95, H 2.31, N 3.59. Found: C 36.97, H 2.31, N, 3.57.
X-ray Crystallography
Intensity data was collected on a Scxmini CCD diffractometer with a graphite- monochromatized Mo Kα radiation (λ = 0.71073 Å) at 293(2) K by using the ω scan mode. Orange crystals for 1 and 2 were used for data collection. An absorption correction was applied for both compounds using a multi-scan correction method. The structures were solved by Direct Methods using the SHELXS-97 program [17]. The refinement was performed against F 2 using the SHELXL-97 program [18]. All non-H atoms were refined anisotropically. The H atoms residing on the ligands were placed geometrically and refined isotropically. Experimental details for the structural determinations for 1 and 2 are presented in Table 1. Selected bond distance and angle data for 1 and 2 are listed in Table 2.
Result and Discussion
Structure Description
1 crystallizes in the monoclinic with space group C2/c, while 2 belongs to the triclinic with space group P ī. As shown in Fig. 1, they exhibit different asymmetric units. The asymmetric unit of 1 consists of one Cu+, one Br− and one Hpyba ligand, while 2 has two Cu+, two Br− and two Hpyba ligands in the asymmetric unit. In 1, each Cu+ ion has a tetrahedral geometry comprised of three Br− ions and one N from one Hpyba bridging ligand. The Cu–N distance is 2.023(4) Å, and Cu–Br bond lengths vary from 2.472(1) to 2.540(6) Å. These values are consistent with the already reproted literatures [19–21]. Each Br− ion displays μ 3-Br coordinate mode, two Cu+ ions are bridged by two Br− to form dimeric {Cu2Br2} cluster, which is further orderly linked by double Cu–Br bonds to obtain a 1-D stair-step chain along the b-axis (Fig. 2a). The separations between adjacent Cu ions are ranging from 2.808(4) to 2.891(7) Å, which shows a weaker Cu–Cu interaction. The Hpyba ligands are parallelly appended to both sides of the chain, connected via the N atoms.
There are the obvious H-bonded interactions in 1. Adjacent Hpyba molecules are head-to-head linked together via two self-complementary O–H···O H-bonds (3.113(8) Å) to generate a 2-D layer, where the [CuBr]n chains adopt −ABA– linking mode (Fig. 2a). These layers are further linked by the combination of C–H···Br (3.718(5) Å) and C–H···O (3.085(4) Å) H-bonds to form a 3-D supramolecular network, in which the layers display –AAA– mode stacking along b-axis (Fig. 3a). More interestingly, there exist two kinds of channels: rectangular (R) channels and square (S) channels. Rectangular channels are constructed from both O–H···O and C–H···Br H-bonds, while square channels are only built of C–H···Br H-bonds. In addition, the π–π stacking interactions (3.835(6) Å) between the aromatic rings also play an important role in stabling the whole crystal structure.
Though 1 and 2 exhibit similar 3-D supramolecular network, there still exist some differences between them. The first is the different [CuX]n chain arrangement modes and H-bond directions (Fig. 2), the second is their channels extending along the b-/a-axis, and different stacking modes with –AAA–/–ABA– for 1 and 2, respectively (Fig. 3). Such differences are mainly due to their different space groups and the different dihedral angles between adjacent aromatic rings of the ligand (38.7(1)° for 1 and 18.7(2)-44.0(2)° for 2) [15].
Although numerous 1-D [CuX]n stair-step chains have been reported, their extended structures mainly focus on 1-D chains and 2-D lamellar. For instance, the auxiliary ligands without function groups are monodentate, they directly bond to 1-D CuX skeletal core as decorating groups, which only display similar 1-D chain, such as [CuI(2-Me-Py)]n and [CuI(2,4-Me2-Py)]n [22]. If the auxiliary ligands contain more than two N-donors, the 1-D stair-step chains converted into 2-D lamellars through the bridging ligands via Cu–N bonds, as exemplified by [(CuCl)2Pyz]n (Pyz = pyrazine), [23] [(CuX)2Phz]n (X = Cl, Br) [24] and [(CuX)2Bpy]n (X = Cl, Br; Bpy = 2,2′-Bipyridine) [25]. The auxiliary ligands possess functional groups (such as O–H, –NH2 and –COOH), the 1-D stair-step chains can be further assembled into 2-D lamellars, such as [CuI(3-pyridinealdoxime)]n, [CuI(isonicotinamide)]n [26], [CuX(6-Me-3-HO2CPy)] (X = Cl, Br) [27], and [CuI(4-acetylpyridine)]n [21]. While the 1-D stair-step chains in both 1 and 2 are extended to 3-D architectures via H-bonds and π–π stacking interactions, mainly because two aromatic rings occur twist, which is manifested by the dihedral angles between two aromatic rings (38.7(1)° for 1 and 18.7(2)-44.0(2)° for 2), the X and O atoms of 1-D [CuX(Hpyba)]n are easy involved in intermolecular C–H···Br and C–H···O H-bonds with adjacent chains. More, there are the obvious π–π stacking interactions between the parallel aromatic rings. Therefore, both 1 and 2 represent a new structural motif.
IR Spectroscopy
In 1 and 2, the characteristic features of Hpyba ligand dominate the IR spectrum. The strong vibrations at 1,573–1,595 cm−1 and 1,404–1,420 cm−1 are corresponding to the asymmetric and symmetric stretching vibrations of the carboxylate group, respectively. Bands in the 1,000–1,420 cm−1 range are attributed to v(C–N) and v(C–C) vibrations. The δO–C–O vibration in plane occurs in middle intensity peaks from 697 to 858.65 cm−1.
Thermal Properties
The TG analysis was performed in air atmosphere with a heating rate of 10 °C/min in the temperature range 30–1,000 °C for 1 and 2 (Fig. 4). The TG curve of 1 shows that it is thermally stable up to 200 °C. Above this temperature, three steps of weight loss were observed until 595 °C. The first two steps in the temperature range from 200 to 330 °C correspond to the partial combustion of Hpyba per-formula unit. The third step in the range of 330–595 °C is ascribed to the release of bridging Br− ions and the combustion of the remaining ligands. Therefore, the total weight loss of 76.9% (calcd: 76.7%) is attributable to the loss of Br− ions and the combustion of ligands. The remaining weight (23.1%) is close to the percentage (23.3%) of Cu and O components, indicating that the final product is possibly CuO, which is formed by the oxidation of the Cu+ ion under the air atmosphere. The TG curve of 2 shows that the first weight loss in the range of 260–355 °C corresponds to the partial combustion of Hpyba ligand. The second weight loss between 350 and 490 °C is ascribed to the loss of bridging I− ions and the combustion of the remaining ligands. The remaining weight (21.4%) is close to the percentage (21.1%) of Cu and O components, indicating that the final product is possibly CuO formed by the oxidation of the Cu+ ion under the air atmosphere.
Conclusions
Two novel copper(I) halide coordination polymers: [Cu2X2(Hpyba)2]n (X = Br(1), I(2), Hpyba = 4-pyridin-4-yl-benzoic acid) have been prepared by hydrothermal methods. They exhibit a 1D stair-step chain structure based on the {Cu2X2} cluster units. These infinite chains are extended to a 3-D supramolecular network via H-bonds and π–π interactions, which displays a new structural motif. Further work will be focused on the construction of multi-dimensional frameworks based on the combination of Ln clusters and TM clusters.
Supporting Information
CCDC-723838 and 723839 contains the supplementary crystallographic data for 1 and 2. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/ retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk).
References
J. Xia, B. Zhao, and H.-S. Wang (2007). Inorg. Chem. 46, 3450.
J.-W. Cheng, S.-T. Zheng, and G.-Y. Yang (2007). Dalton. Trans. 36, 4059.
W.-H. Zhu, Z.-M. Wang, and S. Gao (2007). Inorg. Chem. 46, 1337.
S. Kitagawa, R. Kitaura, and S. Noro (2004). Angew. Chem. Int. Ed. 43, 2334.
G.-X. Liu, K. Zhu, and H. Chen (2008). Cryst. Eng. Commun. 10, 1527.
H.-L. Gao, L. Yi, and B. Ding (2006). Inorg. Chem. 45, 481.
X. Gu and D. Xue (2007). Inorg. Chem. 46, 5349.
N. Zheng, X. Bu, and H. Lu (2005). J. Am. Chem. Soc. 127, 11963.
J.-W. Cheng, J. Zhang, and G.-Y. Yang (2007). Inorg. Chem. 46, 10261.
J.-W. Cheng, J. Zhang, and G.-Y. Yang (2006). Angew. Chem. Int. Ed. 45, 73.
J.-W. Cheng, S.-T. Zheng, and G.-Y. Yang (2008). Chem. Eur. J 14, 88.
M.-B. Zhang, J. Zhang, and G.-Y. Yang (2005). Angew. Chem. Int. Ed. 44, 1385.
J.-W. Cheng, S.-T. Zheng, and G.-Y. Yang (2007). Inorg. Chem. 46, 10534.
J.-W. Cheng, S.-T. Zheng, and G.-Y. Yang (2008). Inorg. Chem. 47, 4930.
T.-B. Lu and R.-L. Luck (2003). Inorg. Chim. Acta. 351, 345.
O. R. Evans and W.-B. Lin (2001). Chem. Mater. 13, 2705.
G. M. Sheldrick, SHELXS-97, Program for Crystal Structure Solution (University of Göttingen, Gottingen, 1997).
G. M. Sheldrick, SHELXL-97, Program for Crystal Structure Refinement (University of Göttingen, Göttingen, 1997).
J.-H. Yu, J.-Q. Xu, L. Ye, and H. Ding (2002). Inorg. Chem. Commun. 5, 572.
J. Xiang, Y. Yin, and P. Mei (2007). Inorg. Chem. Commun. 10, 1168.
E. Cariati, D. Roberto, R. Ugo, P. C. Ford, S. Galli, and A. Sironi (2002). Chem. Mater. 14, 5116.
N. P. Rath, J. L. Maxwell, and E. M. Holt (1986). J. Chem. Soc. Dalton. Trans. 2449.
S. Kawata, S. Kitagawa, H. Kumagai, S. Iwabuchi, and M. Katada (1998). Inorg. Chim. Acta. 267, 143.
M. Munakata, T. K. Sowa, M. Maekawa, A. Honda, and S. Kitagawa (1994). J. Chem. Soc. Dalton. Trans. 2771.
J.-Y. Lu, B.-R. Cabrera, R.-J. Wang, and J. Li (1999). Inorg. Chem. 38, 4608.
C. B. Aakeröy, A. M. Beatty, D. S. Leinen, and K. R. Lorimer (2000). Chem. Commun. 935.
C. B. Aakeröy, A. M. Beatty, and K. R. Lorimer (2000). J. Chem. Soc. Dalton. Trans. 3869.
Acknowledgements
The authors are thankful for the financial supports from the National Natural Science Fund for Distinguished Young Scholars of China (no. 20725101), the NNSF of China (nos. 50872133 and 20821061), the 973 Program (no. 2006CB932904), the NSF of Fujian Province (nos. E0510030 and 2008F3120) and the Knowledge Innovation Program from CAS (no. KJCX2.YW.H01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jia, XL., Zhou, J., Zheng, ST. et al. Two 3-D Supramolecular Networks Containing Dimeric {Cu2X2} Cluster Units. J Clust Sci 20, 555–563 (2009). https://doi.org/10.1007/s10876-009-0260-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-009-0260-7