Abstract
Protein kinase C delta (PRKCD) has essential functions in controlling B-cell proliferation and apoptosis, development of B-cell tolerance and NK-cell cytolitic activity. Human PRKCD deficiency was recently identified to be causative for an autoimmune lymphoproliferative syndrome like disorder with significant B-cell proliferation particularly of immature B cells. Here we report a child with a novel mutation in PRKCD gene who presented with CMV infection and an early onset SLE-like disorder which was successfully treated with hydroxychloroquine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protein kinase C delta deficiency is a newly described immune disregulative syndrome with lupus-like features and increased autoimmunity. PRKCD is essential in B cell homeostasis in humans as shown in PKCδ knockout mice with increased autoimmunity, lymphoproliferation and lupus-like skin rash [1–3]. Clinical manifestations of the so far reported 5 cases include lymphoproliferation and autoimmunity like juvenile systemic lupus erythematosus (SLE) [4–6]. Within this context, we report a child with a novel mutation in PRKCD gene with early onset SLE-like skin disease who was successfully controlled by hydroxychloroquine.
Case Report
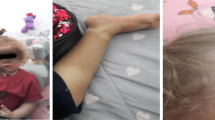
A 3.5 year old male was born to a first degree cousin union of healthy parents who was admitted to the neonatal intensive care unit due to respiratory distress for 2 weeks. Past medical history revealed multiple hospital admissions due to recurrent fever at infancy. Only two out of those admissions concluded with myositis and gastroenteritis. In addition, intermittent diarrhea was remarkable since 6 months of age which spontaneously resolved in 2 months. However, the etiology remained unknown. At 8 months, he presented with erythematosus skin rash accompanied by fever and thrombocytopenia. Physical examination at admission revealed partial alopecia, a hyperpigmented skin rash predominantly in sun-exposed areas, cervical lymphadenomegaly, hepatosplenomegaly and mild hypotonia (Fig. 1a). PCR-based molecular analysis revealed a CMV infection which had been successfully treated with gancyclovir and intravenous gammaglobulins (IVIG), relapsed a year later.
At 18 months, serum immunoglobulin levels were normal except for markedly elevated IgM levels. Detailed immunophenotyping revealed normal T and NK and increased B cells predominantly high naive and CD21low, yet diminished switched memory subset (Fig. 1c and d, Table 1). T cell proliferation and NK cell cytolytic activity was assessed demonstrating a slight decrease of T cell proliferation and a moderate decrease in NK-cytolytic activity compared to healthy control (Table 1). EBV PCR was found to be negative. Specific antibody responses were only evaluated for hepatitis B antibody which was negative prior to IVIG treatment. Autoantibodies including ANA, anti-dsDNA, anti-thyroglobulin and anti-thyroid peroxidase were negative, whereas serum C3 and C4 were found to be low. Urinary analysis revealed no hematuria or proteinuria. Skin biopsy revealed basal membrane degeneration and apoptosis compatible with vasculitis. Intravenous immunoglobulin substitution, antibacterial prophylaxis and topical steroids were started at 2.5 years of age and associated with a decreased frequency of infections, while the skin lesions remained unchanged. During follow-up, the patient developed ANA positivity and the diagnosis of cutaneous lupus erythematosus was confirmed by skin biopsy. Accordingly, oral hydroxychloroquine therapy was initiated. After 2 months under this therapy, skin lesions resolved almost completely together with a significant reduction in lymph node, spleen and liver size (Fig. 1b). Additionally, repeated flow cytometric analysis, during hydroxychloroquine treatment demonstrated a remarkable decrease in CD21lowCD38low and increase in switched memory B cells, after 5 and 7 months of therapy, respectively (Fig. 1e and f, Table 1).
To identify the underlying genetic defect whole-exome sequencing was performed and a missense mutation in PRKCD gene exon 9 (c.742G > A, p.Gly248Ser) was detected (Fig. 2a). To assess functional consequences of PRKCD deficiency, expression of myristoylated alanine-rich C kinase substrate (MARCKS), a major PKC target was evaluated [6]. Our patient demonstrated a slight decrease in PRKCD levels with no change in total MARCKS expression; however, the levels of phosphorylated MARCKS (p-MARCKS) seem to be substantially decreased, when compared to normal donor controls (Fig. 2b). The detail of the methods was given in the Supplementary file.
Perfect segregation of the single base substitution (c.742G > A; p.G248S) is shown in the patient, the parents and two nonaffected siblings (a). The solid symbol indicates the homozygous affected subject, half-filled symbols refer to heterozygous carrier and empty symbols represent a wildtype status. Male and female subjects are distinguished by squares and circles, respectively. Western blot analysis (b) was performed on unstimulated (−) and PMA stimulated (+) immortalized B-cell lines, from two normal donor controls (lane 1–4), a previously published patient with PRKCD deficiency (lane 5 and 6) and the index patient (lane 7 and 8). The index patient shows a slight decrease in PRKCD levels, no change in total myristoylated alanine-rich C kinase substrate (MARCKS) expression, however, the levels of phosphorylated MARCKS (p-MARCKS) seem to be substantially decreased, when compared to normal donor controls
Currently the patient is under hydroxychloroquin treatment, TMP-SMX and IVIG prophylaxis and free of any infections including CMV.
Discussion
Although only few patients were identified so far with PRKCD deficiency, they show a significant heterogeneity with regard to clinical manifestations [4–6]. The early clinical onset of the disease and prominent autoimmunity in PRKCD deficiency was demonstrated to be associated with an abnormal B cell compartment, characterized by increased naive and diminished memory cells as well as high number of CD21lowCD38low cells [4–6]. Although, T cell functions were reported to be preserved in previous patients, we detected subtle defect in the proliferative capacity to mitogens in our case [6]. Persistent CMV infection may be the result of defective function and/or low numbers of NK cells as mentioned in some previous reports [4–6]. The underlying mechanism of NK cell deficiency needs to be elucidated.
Of note, the application of hydroxychloroquine in this patient established a favorable improvement in clinical symptoms. This improvement was associated with a reduction of CD21lowCD38lowB cells. This population of cells is reported to be increased in common variable immune deficient patients with autoimmunity and splenomegaly [7] and has also been observed previously for PRKCD deficiency [6]. Although, the mechanistic action of hydroxychloroquine has not yet been fully elucidated, it is known to have immunomodulatory, anti-inflammatory, anti-proliferative and photoprotective effects [8]. Recent data on the mechanism of hydroxychloroquine revealed an immunomodulatory effect through inhibition of autophagy in effector memory T cells thereby inducing apoptosis of these cells [9]. Various reports showed that hydroxychloroquine inhibits proliferative responses to T-cell mitogens and alloantigens and reduce some proinflammatory cytokines such as interleukin (IL)-6 and tumor necrosis factor alpha [10, 11]. Goldman et al. showed that hydroxychloroquine treatment reduced B cell receptor induced calcium signaling and completely inhibited intracellular response in higher doses [8]. As PRKCD modulates the activation of caspase-3 and downstream of the caspase activation, it has a regulatory role in cell apoptosis [12]. In the case of PRKCD deficiency altered apoptosis may cause uncontrolled B cell proliferation and autoinflammatory response [5]. Hydroxychloroquine use in PRKCD deficient patient presented here modulated the number of autoreactive B cells probably leading to improvement in skin finding and lymphoproliferation.
In essence, the trio of early onset autoimmunity, lymphoproliferation and lupus like features accompanied with B cell abnormalities should raise suspicion for PRKCD deficiency in children. Use of hydroxychloroquine in such patients may help to achieve good clinical control.
References
Miyamoto A, Nakayama K, Imaki H, Hirose S, Jiang Y, Abe M, et al. Increased proliferation of B cells and auto-immunity in mice lacking protein kinase C delta. Nature. 2002;416:865–9.
Fujii T, Garcia-Bermejo ML, Bernabo JL, Caamano J, Ohba M, Kuroki T, et al. Involvement of protein kinase C delta (PKC delta) in phorbol ester-induced apoptosis in LNCaP prostate cancer cells. Lack of proteolytic cleavage of PKCdelta. J Biol Chem. 2000;275:7574–82.
Guo B, Su TT, Rawlings DJ. Protein kinase C family functions in B-cell activation. Curr Opin Immunol. 2004;16:367–73.
Belot A, Kasher PR, Trotter EW, Foray AP, Debaud AL, Rice GI, et al. Protein kinase cδ deficiency causes mendelian systemic lupus erythematosus with B cell-defective apoptosis and hyperproliferation. Arthritis Rheum. 2013;65:2161–71.
Kuehn HS, Niemela JE, Rangel-Santos A, Zhang M, Pittaluga S, Stoddard JL, et al. Loss-of-function of the protein kinase C δ (PKCδ) causes a B-cell lymphoproliferative syndrome in humans. Blood. 2013;121:3117–25.
Salzer E, Santos-Valente E, Klaver S, Ban SA, Emminger W, Prengemann NK, et al. B-cell deficiency and severe autoimmunity caused by deficiency of protein kinase C δ. Blood. 2013;121:3112–6.
Wehr C, Kivioja T, Schmitt C, Ferry B, Witte T, Eren E, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111:77–85.
Goldman FD, Gilman AL, Hollenback C, Kato RM, Premack BA, Rawlings DJ. Hydroxychloroquine inhibits calcium signals in T cells: a new mechanism to explain its immunomodulatory properties. Blood. 2000;95:3460–6.
Van Loosdregt J, Spreafico R, Rossetti M, Prakken BJ, Lotz M, Albani S. Hydroxychloroquine preferentially induces apoptosis of CD45RO+ effector T cells by inhibiting autophagy: a possible mechanism for therapeutic modulation of T cells. J Allergy Clin Immunol. 2013;131:1443–6.
Gilman AL, Beams F, Tefft M, Mazumder A. The effect of hydroxychloroquine on alloreactivity and its potential use for graft-versus-host disease. Bone Marrow Transplant. 1996;17:1069–75.
Sperber K, Quraishi H, Kalb TH, Panja A, Stecher V, Mayer L. Selective regulation of cytokine secretion by hydroxychloroquine: inhibition of interleukin-1-alpha (IL-1-a) and IL-6 in human monocytes and T cells. J Rheumatol. 1993;20:803–8.
Basu A. Involvement of protein kinase C-delta in DNA damage-induced apoptosis. J Cell Mol Med. 2003;7:341–50.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Kiykim, A., Ogulur, I., Baris, S. et al. Potentially Beneficial Effect of Hydroxychloroquine in a Patient with a Novel Mutation in Protein Kinase Cδ Deficiency. J Clin Immunol 35, 523–526 (2015). https://doi.org/10.1007/s10875-015-0178-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-015-0178-9