Abstract
Caspase-8 deficiency (CED) was originally described in 2002 in two pediatric patients presenting with clinical manifestations resembling autoimmune lymphoproliferative syndrome (ALPS) accompanied by infections, and T, B and NK cell defects. Since then, no new CED patients were published. Here we report two adult siblings (Pt1 and Pt2) presenting in their late thirties with pulmonary hypertension leading to lung transplant (Pt1), and a complex neurological disease leading to multiple cranial nerves palsies (Pt2) as their main manifestations. A thorough clinical and immunological evaluation was performed at the Primary Immunodeficiency Clinic at NIH, followed by whole exome sequencing. The patients had multiorgan lymphocytic infiltration and granulomas, as well as clinical signs of immune deficiency/ immune dysregulation. Both siblings carried homozygous mutations in CASP8, c.1096C > T, p.248R > W. This was the same mutation described on the previously published CED patients, to whom these new patients were likely distantly related. We report two new CED patients presenting during adulthood with life-threatening end-organ lymphocyte infiltrates affecting the lungs, liver, spleen, bone marrow and central nervous system. This phenotype broadens the clinical spectrum of manifestations associated with this disease and warrants the search of CASP8 mutations in other cohorts of patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
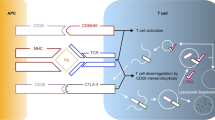
Apoptosis, a form of programmed cell death, helps to maintain a homeostatic balance in the immune system allowing potent responses to pathogens but avoiding autoimmunity. Apoptosis is controlled by caspases, a group of aspartate-specific cysteine proteases. In lymphocytes, the CD95 (FAS, APO-1) receptor triggers cell apoptosis by recruiting FAS-associated death domain (FADD), caspase-8 and caspase- 10 proteins into a death-inducing signaling complex (DISC). Recruitment into the DISC induces procaspase-8 oligomerization, followed by full processing into a highly active soluble tetramer that propagates the death signal, resulting in the demise of the cell [1].
In 2002, Chun et al. identified two patients with Caspase 8 deficiency (CED) [1]. The authors described two siblings, a 12-yr-old female and an 11-yr-old male born to a distantly consanguineous family, with some features of the autoimmune lymphoproliferative syndrome (ALPS), such as lymphadenopathy, splenomegaly slight elevation of the TCRαβ + CD4-CD8- T cells and defective lymphocyte apoptosis. In addition, and unlike other ALPS patients, these patients also presented with short stature, failure to thrive, asthma, recurrent sinopulmonary and cutaneous herpes simplex virus (HSV) infections, inverted CD4/CD8 ratios, slightly diminished IgG, IgA and IgM levels and poor responses to pneumococcal immunization, pointing to a mild combined immunodeficiency (Table 1). These patients were found to carry a homozygous mutation in the gene encoding caspase-8 (CASP8). In subsequent work, the same group demonstrated that caspase-8 was necessary for full activation of NF-kB downstream of the TCR, BCR, some NK and TLR receptors, explaining the underlying immunodeficiency state seen in the patients [2]. Since this original description, occurred over 12 years ago, no further CED patients were reported. Herein we report two new CED patients that presented during adulthood with manifestations mainly caused by end-organ lymphocytic infiltration, expanding the clinical findings of this disorder.
Patients, Materials and Methods
Patient Cohort
Patient 1 (Pt1, female, 42y.o.) and Patient 2 (Pt2, male, 41 y.o.) were two out of four children born to a reportedly non-consanguineous healthy couple. Pt1 and Pt2 were referred to the Primary Immunodeficiency Clinic (PID-C), National Institutes of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) for immune status evaluation. Pt1 could not travel to be physically evaluated at the PID-C because of her clinical condition, but her medical records, lymphocyte phenotype and genomic DNA were analyzed.
Whole Exome Sequencing (WES)
The genomic DNA from Pt1, Pt2, unaffected sister 1, unaffected sister 2, father and mother were analyzed by whole exome sequencing (WES) in a Solid 4 platform (Life technologies, Carlsbad, CA). Paired end reads were aligned to the hg18 human reference genome using Bioscope software (Applied Biosystems); the non-coding regions of the reference genome were masked to avoid alignment with intergenic homologous sequences including pseudogene sequences, which would result in mapping qualities of zero. BAM files were soft-clipped to remove reads hanging off the end of the reference sequence, then sorted in karyotypic order and indexed using Picard tools (http://picard.sourceforge.net). We then applied GATK base quality score recalibration, indel realignment, and performed SNP and INDEL discovery and genotyping using standard hard filtering parameters or variant quality score recalibration [3, 4]. Multiple variant prioritization strategies were used to prioritize genetic variants, based on the phenotype of the patients and/or family members, the mode of inheritance, the severity of the disease, and variant conservation and/or population frequency. This was accomplished using a variety of variant annotation tools, including SeattleSeqAnnotation (http://snp.gs.washington.edu/SeattleSeqAnnotation/), ANNOVAR [5], SIFT [6], MutationTaster [7], and GeneDistiller 2 [8]. Sanger sequencing confirmed relevant results.
Functional Immunologic Evaluation
Immunoblotting: Cell lysates were prepared from EBV-transformed B cells from Pt2 and resolved on 4–12 % NuPAGE Bis–Tris gels (Invitrogen). Proteins were transferred on a nitrocellulose membrane and probed with the use of anti-Caspase 8 (Cell signaling) and anti-β-actin (Sigma-Aldrich) antibodies.
T cell proliferation: peripheral blood mononuclear cells (PBMCs) from Pt2 and a healthy control were loaded with 10 uM of CFSE and activated with soluble anti-CD3 and anti-CD28, with or without IL-2, for 3 days. CFSE dilution was then evaluated by flow cytometry. T cell activation and cytokine secretion: PBMCs from Pt2 and a healthy control were stimulated with anti-CD3 and anti-CD8 coated beads. CD25 expression was then evaluated by flow cytometry. IL-2 secretion in the supernatant was measured by ELISA. Toll-like receptor function: PBMCs from Pt2 and a healthy control were stimulated with a set of TLR ligands and different cytokines were measured in the supernatant by ELISA.
Results
Clinical and Laboratory Findings
Pt1: female, reported to be healthy until 2 m after delivering a normal baby girl at age 38. At that time she developed acute shortness of breath, diagnosed as pneumonia and treated with oral antibiotics. Dyspnea progressively worsened during the following months and she was eventually diagnosed with pulmonary hypertension and interstitial lung disease (no further studies were available for review). High dose steroids and sildenafil citrate failed to control her symptoms. At age 40 she was admitted with high-grade fevers and pancytopenia in the context of acute EBV infection and treated with one dose of intravenous immunoglobulin (IVIG) and filgrastim (pre-IVIG Ig levels: IgG 682 mg/dL, IgA 80 mg/dL, IgM 13 mg/dL) and eventually fully recovered from this episode. Two months later her Ig levels were normal (Table 1). During the following 2 years her lung condition continued to deteriorate, and at age 42 she successfully received a lung transplant. Reports from the explanted lung biopsies highlighted the presence of multiple necrotizing and non-necrotizing granulomas, and interstitial lung disease with lymphoid clusters, while no microorganisms were seen or grown from the tissues (slides were not available for review). Eight months after her lung transplant, and while on steroids, mofetil mycophenolate and tacrolimus as anti-rejection medication, she developed a Nocardia asteroides meningoencephalitis that was her cause of death.
Pt2: male, healthy until age 37 when he presented with a complex neurologic syndrome characterized by anisocoria, solid food dysphagia, and right side throbbing migraines, followed by recurrent lung infections, liver and spleen nodular organomegaly. Neurologic exam showed left trigeminal neuralgia, left IX, X and XII palsy/paresis, right mydriasis, and right III, IV and VI palsy/paresis. Neurologic images showed a 1 cm3 mass at the Meckel’s cave that was surgically resected (Fig. 1), and abdomen CT scans showed multinodular liver and spleen lesions (Fig. 2). No inflammation, infection or malignancy was detected from CSF lumbar puncture analysis, but the mass biopsy showed a necrotizing granuloma (Fig. 1). A thin needle liver biopsy was not informative at that time (only fibrous tissue was visualized; microbiology cultures were also negative). Both his neurologic manifestations and his liver-spleen nodular disease responded impressively to oral methyl prednisone (60 mg/day) (Fig. 2). During follow up, flares of his neurologic manifestations responded to increased immunosupression over his baseline daily corticosteroids (20 mg). The patient presented multiple aspiration pneumonias and eventually developed bronchiectasis (Fig. 2). Staphylococcus aureus and Pseudomonas aeruginosa were repeatedly isolated from sputum samples and he was placed on IVIG therapy. Immunoglubulin levels pre-IVIG replacement were IgG 992 mg/dL, IgA 369 mg/dL, IgM 25 mg/dL, IgE 87 IU/mL; no antibody functional tests were determined. During follow up, IgG trough levels where found to be as low as 580 mg/dL while the patient was maintained on daily methyl prednisone (60 mg) and mofetil mycophenolate (1,500 mg). CFTR genetic analysis showed no mutations, and neutrophil respiratory burst testing was also normal. During his evaluation at the NIH (Table 1), and while on a daily corticosteroids maintaining dose (20 mg), a bone marrow aspirate and biopsy showed clusters of small CD3+ CD8+ Perforin + T cells infiltrates, scattered CD20+ B cells and increased CD138+ plasma cells. T-cell and B-cell clonal diseases were rule out by DNA-based TCR and BCR rearrangement studies; karyotype was also normal on his bone marrow; plasma protein immunofixation studies did not show clonal bands. A liver biopsy showed mild portal and lobular inflammation with CD3+ CD8+ Perforin + GranzymeB + TIAl + Ki-67+ lymphocyte infiltration of sinuses, and sinusoidal dilatation (Fig. 1). One year after his NIH evaluation, the patient died due to progressive neurological and pulmonary complications. An autopsy was not performed.
Histopathological findings in late-onset CED. a) The Meckel’s cave mass biopsy resected from Pt2 showed a necrotizing granuloma (black arrow); b) Bone marrow biopsy from Pt2 showed CD3+/CD8+/Perforin + T cell infiltrates, CD20+ B cells (despite their absence on peripheral blood), and CD138+ plasma cells; c) Liver histopathology slides from Pt2 demonstrated sinusoidal lymphocytosis (white arrow) and sinusoidal dilatation (black arrow). Liver lymphocytic infiltrates consisted mainly on CD3+/ CD8+ T cells expressing cytotoxic markers Perforin, Granzyme-B, and TIA-1, as well as proliferation marker Ki-67
Genetic Analysis
The number of variants detected by WES for the two patients, one healthy sibling and both parents ranged from 33913 to 35146. Twenty-nine single nucleotide variants and no insertions or deletion were inherited in an autosomal recessive manner, including CASP8 NM_033355 c.742C > T, p.R248W (alternate notation based on other transcripts: NM_033356 c.697C > T, p.R233W; NM_001080124 c.697C > T, p.R233W; NM_001080125 c.919C > T, p.R307W; NM_033358 c.677C > T, p.T226M; NM_001228 c.793C > T, p.R265W). The CASP8 variant was homozygous in both patients and heterozygous in both parents, while the unaffected sibling was homozygous wild type at this position. Sanger sequencing confirmed the CASP8 variant and familial segregation. The CASP8 NM_033355 c.742C > T, p.R248W variant was previously reported by Chun et al. for the two originally described caspase-8 deficient patients [1]. Although the family denied consanguinity, extensive pedigree analysis revealed a paternal distant relationship to the original CED patients, Single nucleotide polymorphism analysis of the maternally and the paternally inherited mutated CASP8 alleles showed no differences between them, suggesting identity by descent (not shown). The remaining 29 SNVs that were inherited in an autosomal recessive manner were common SNPs and/or predicted to be benign, except for a novel variant detected in ZEB2 (ZEB2 NM_001171653 c.2048C > T p.S683F; NM_014795 c.2120C > T, p.S707F). As ZEB2 mutations are associated with Mowat-Wilson syndrome (OMIM #235730), an autosomal dominant complex developmental disorder that has no phenotypic overlap with the phenotypes of the family members in this study, we concluded that this variant was likely a non-functional, incidental finding.
Immunologic Defects
Similar to the previous patients carrying the same mutation on CASP8, immunoblot analysis on protein lysates from Pt2 showed markedly diminished Caspase 8 expression (Fig. 3a). Given the previously reported important role of caspase-8 in T cell activation, we evaluated lymphocyte activation and proliferation in Pt2 and a healthy control individual. Upon activation with anti-CD3 and anti-CD28 beads, the patient cell’s showed diminished expression of the activation marker CD25 (Fig. 3b, left panels). Addition of IL-2 partially corrected the defect. Likewise, lymphocyte proliferation was almost abolished in the patient, as evaluated by CFSE dilution (Fig. 3a, right panels). Supplementary IL-2 also partially restored the proliferative capacity.
Caspase-8 expression, lymphocyte proliferation and cytokine production in late-onset CED WT, wild type healthy control; Pam, Pam3CSK4, a TLR1 /TLR2 agonist; HKLM, heat-killed Listeria monocytogenes, a TLR2 agonist; LPS, lipopolysaccharide, a TLR4 agonist; Flagellin, a TLR5 agonist; FSL, Pam2CGDPKHPKSF, a TLR6/ TLR2 agonist; ODN, ODN2006 (type B), a TLR9 agonist
Evaluation of the patient’s T cells cytokine secretion capacity revealed low production of IL-2, IL-6 and IL-10 upon T cell activation but normal secretion of TNF-α A similar pattern could also be demonstrated upon TLR stimulation (Fig. 3c), showing a markedly diminished IL-10 secretion after activation of several TLRs, while production of IL-6 and TNF-α was comparable to the normal control.
Because of the almost complete lack of B and NK cells in Pt2, functional evaluation of these cells could not be performed. Apoptosis studies were attempted twice, but failed due to technical difficulties.
Conclusions
Since its original description in 2002, no new cases of CED have been reported [1]. Pediatric onset, recurrent herpes infections, mild hypogammaglobulinemia with poor antibody responses, inverted CD4/CD8 ratios, and apoptosis defects were some of the hallmarks associated to CASP8 mutations in that original report. The two new CED patients described in this manuscript share some of the clinical features (e.g., viral infection susceptibility), and the same mutation with the original patients (to whom they are distantly related), but certainly broaden the spectrum of clinical manifestations associated with CED: adult onset, life-threatening end organ lymphocytic infiltration (as shown on the CNS, liver, bone marrow, and lungs tissues available and analyzed) and (severely) decreased CD4, B and NK cells in peripheral blood, as detected in both of our patients.
It is well known that clinical phenotypes on primary immunodeficiency diseases could vary widely even for patients carrying genetic defects on the same gene. Patients with mutations in WAS gene could present with different clinical phenotypes depending on the mutation type or the level of residual protein expression and function [9]. Moreover, siblings with Cartilage-Hair Hypoplasia syndrome carrying identical mutations in RMRP could also present with strikingly diverse clinical manifestations, some with dwarfism an others with normal height [10]. Interestingly, and although the clinical manifestations displayed by these two new CED patients did not match those reported on the original set of CED patients, or even (young) targeted caspase-8 deficient mice, they do strongly resemble the phenotype described on adult mice with targeted caspase-8 deficiency [11–13].
Mice deficient in caspase-8 show embryonic lethality; however, mice with a targeted caspase-8 mutation restricted to the T cell or B cell lineages, are able to survive [11–13]. Interestingly, both the previously published as well as our CED patients had severely diminished but not absent Caspase-8 expression. Although speculative, this might suggest that at least a minimal Caspase-8 protein expression and /or function is required for surviving the embryonic stage in humans. Mice with targeted T cell and B cell caspase-8 deficiency present normal thymocyte development but a marked decrease in peripheral blood T-cells. Besides, when challenged with the lymphocytic choriomeningitis virus (LCMV), these animals showed a significantly impaired immune response to the infection that included impaired CD8 cell expansion and an abrogated ability to generate virus-specific CD8+ cytotoxic T-cells. While these features are described in young mice, older mice develop a surprisingly different phenotype characterized by a lethal lymphoproliferative and lymphoinfiltrative immune disorder presenting with lymphoadenopathy, splenomegaly, and end-organ infiltration by T cells (lung, liver and kidneys) showing up-regulated activation markers and increased proliferation rates even in the absence of any infection or stimulation [2, 11]. Moreover, failure to express caspase-8 on dendritic cells leads to an enhanced intrinsic activation and, subsequently, more mature and auto-reactive lymphocytes [14]. On the other hand, mice lacking expression of caspase-8 on B cells also have an age-dependent phenotype. Although B cells in these animals showed no developmental defects early on life, they presented with attenuated antibody production upon in vivo and ex vivo stimulation, and diminished B cell survival due to increased apoptosis in response to Toll-like receptor 3 and 4 stimulation [12, 13]. During long term follow up these mice also developed massive splenomegaly and lymphadenopathy, as well as infiltrating tumors resembling human multiple myeloma. Moreover, caspase-8 expression in B cells was essential to maintain B cell cytokinesis and prevent B cell lymphomagenesis [15].
Interestingly, our immunologic results suggest that CASP8 mutations not only impair adequate TCR and TLR signaling as previously described, but also skew the immune responses towards a pro-inflammatory pattern, which may explain the abundant formation of T cell infiltrates seen in these patients. However, these data (and also some of the clinical manifestations in our patients) should be cautiously interpreted: when evaluated at NIH, Pt2 was under strong immunosuppressive therapy that likely affected his quantitative and functional immunologic studies. The fact that despite this immunosuppressive therapy, i) some of the tests performed were normal while others were abnormal and correlated with previously reported defects in CED (e.g., poor T cell activation and IL-2 secretion, as well as poor NFkB signaling [1, 2]), ii) another restricted set of tests demonstrated an abnormal balance between inflammatory vs. anti-inflammatory cytokines, and iii) these results correlated with and partially explained his clinical phenotype, suggest (although do not confirm) they are likely related to his underlying condition (CED) and not simply based on the immunosuppressive therapy.
The description of two new CED patients with new clinical manifestations diagnosed through a WES approach, warrants the search for CASP8 mutations in patients with similar phenotypes (e.g., adult onset CVID with granulomatous disease, sarcoidosis, ALPS-like lymphoproliferative disorders, or even the recently described CTLA4 haploinsufficient patients [16]), and also highlights the utility of WES on detecting new clinical phenotypes associated to known genetic disorders.
References
Chun HJ, Zheng L, Ahmad M, Wang J, Speirs CK, Siegel RM, et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature. 2002;419(6905):395–9.
Su H, Bidere N, Zheng L, Cubre A, Sakai K, Dale J, et al. Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science. 2005;307(5714):1465–8.
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–303.
DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43(5):491–8.
Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38(16):e164.
Ng PC, Henikoff S. Predicting the effects of amino acid substitutions on protein function. Annu Rev Genomics Hum Genet. 2006;7:61–80.
Schwarz JM, Rodelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods. 2010;7(8):575–6.
Seelow D, Schwarz JM, Schuelke M. GeneDistiller–distilling candidate genes from linkage intervals. PLoS One. 2008;3(12):e3874.
Ochs HD, Filipovich AH, Veys P, Cowan MJ, Kapoor N. Wiskott-Aldrich syndrome: diagnosis, clinical and laboratory manifestations, and treatment. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2009;15(1 Suppl):84–90.
Makitie O, Kaitila I. Cartilage-hair hypoplasia–clinical manifestations in 108 Finnish patients. Eur J Pediatr. 1993;152(3):211–7.
Salmena L, Lemmers B, Hakem A, Matysiak-Zablocki E, Murakami K, Au PY, et al. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 2003;17(7):883–95.
Lemmers B, Salmena L, Bidere N, Su H, Matysiak-Zablocki E, Murakami K, et al. Essential role for caspase-8 in Toll-like receptors and NFkappaB signaling. J Biol Chem. 2007;282(10):7416–23.
Beisner DR, Ch'en IL, Kolla RV, Hoffmann A, Hedrick SM. Cutting edge: innate immunity conferred by B cells is regulated by caspase-8. J Immunol. 2005;175(6):3469–73.
Cuda CM, Misharin AV, Gierut AK, Saber R, Haines 3rd GK, Hutcheson J, et al. Caspase-8 acts as a molecular rheostat to limit RIPK1- and MyD88-mediated dendritic cell activation. J Immunol. 2014;192(12):5548–60.
Hakem A, El Ghamrasni S, Maire G, Lemmers B, Karaskova J, Jurisicova A, et al. Caspase-8 is essential for maintaining chromosomal stability and suppressing B-cell lymphomagenesis. Blood. 2012;119(15):3495–502.
Kuehn HS, Ouyang W, Lo B, Deenick EK, Niemela JE, Avery DT, et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 2014.
Acknowledgements
The content of this article does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. Supported by the Intramural Research Program of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Julie Niemela, Hye Sun Kuehn, João B. Oliveira and Sergio D. Rosenzweig contributed equally to this work.
Rights and permissions
About this article
Cite this article
Niemela, J., Kuehn, H.S., Kelly, C. et al. Caspase-8 Deficiency Presenting as Late-Onset Multi-Organ Lymphocytic Infiltration with Granulomas in two Adult Siblings. J Clin Immunol 35, 348–355 (2015). https://doi.org/10.1007/s10875-015-0150-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-015-0150-8