Abstract
Natural killer (NK) cells are important effectors of the innate immune system that help control viral infections and tumorigenesis. However, the relationship between NK cell function and HIV disease progression remains poorly defined. In this study, we examined the function of NK cells in Chinese patients who were HIV-infected but treatment-naïve. These individuals include primary HIV-infected patients (PHIs), typical progressors (TPs), and long-term nonprogressors (LTNPs). We observed an increase of CD56dim NK cells in PHIs, but the production of interferon-gamma (IFN-γ) and CD107a expression in PHIs were not altered compared with normal control subjects (NCs). However, the NK cells from LTNPs exhibited increased activities in IFN-γ production, CD107a expression and granzyme B change after K562 stimulation compared with NCs. Furthermore, the percentage of IFN-γ+CD107a− NK cells in LTNPs was higher than that in TPs, PHIs and NCs; levels of IFN-γ production in LTNP NK cells exhibited an inverse correlation with viral loads. Similar correlations, however, were not observed in the PHI and TP groups. Taken together, these data demonstrate that enhanced NK cell function may contribute to the control of HIV infection, and increased IFN-γ secretion may be associated with delayed disease progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural killer (NK) cells are large granular lymphocytes that play important roles in the innate immune response. There is an emerging consensus that, in addition to the adaptive B- and T-cell responses, innate immune responses are critical to the initial containment of viral replication and play a pivotal role in shaping subsequent adaptive immune responses [1].
The human NK cell surface phenotype defines them as a group of lymphocytes lacking the CD3 antigen but expressing the CD56 antigen [2]. The surface densities of CD56 can further distinguish NK cells into CD56dim (expressing lower levels of CD56) and CD56bright (expressing higher levels of CD56) subsets. These two NK cell subsets are functionally distinct, but can secrete cytokines such as IFN-γ and generate strong cytotoxicity [3, 4].
Interferon gamma (IFN-γ) is considered the prototypic NK cell cytokine, and its production by NK cells is known to induce antiviral responses and shape the Th1 immune response [5]. NK cells also contain high concentrations of cytolytic granules in their cytoplasm, such as granzyme B, that promote apoptosis of target cells and suppression of viral replication [6]. As degranulation occurs, secretory lysosomes are released, and lysosome-associated membrane protein-1 (LAMP-1, CD107a) is transported to the surface of NK cells, acting as a functional marker for NK cell activity [7].
Though NK cells are known to be essential to the control of certain tumors and viral infections, the precise role that NK cells play in HIV infection has not been fully elucidated [8]. Several lines of evidence have suggested that early virological and immunological events may influence the determination of a viral setpoint and the rate of disease progression [9], and NK cells have been shown to be active and proliferative during the early stages of HIV infection [10]. Several studies reported an increase of cytolytic CD56dim NK cells in acute HIV infections, however, chronic viremic HIV infection is associated with reduced percentage of CD3−CD56+ NK cells [11, 12]. Viremic HIV-1 infection is also associated with dramatic changes in the surface distribution of NK cell receptors [13]. In addition, NK cell function is also altered dramatically during HIV infection. However, data related to NK cell function during HIV infection seem contradictory. For example, several studies have reported a reduction in NK cell activity during HIV infection [14–16], but other studies reported elevated NK cell activity in HIV infected subjects [11, 17–19].
A small proportion of antiretroviral therapy (ART)-naïve HIV patients have been found capable of maintaining low viral loads, stable CD4+ T-cell counts (greater than 500 cells/μl) and disease-free conditions for 10 years or more. These individuals are known as the long-term nonprogressors (LTNPs) and represent 1 % to 5 % of all HIV-infected subjects [20, 21]. The reason for this prolonged disease progression is unclear. Some studies have suggested that some viral factors may contribute to the inhibition of infection but host factors also play a role. In particular, HLA-B57+ LTNP patients exhibit stronger CD8+CTL function than do typical progressors (TPs) [22, 23]. LTNPs also generate a strong CD4+ T cell response specific to HIV [24]. Recent evidence has convincingly linked the expression of KIR3DS1 and its ligand –HLA-Bw4*80I – to slower HIV-1 disease progression [25]. Still, information regarding the NK cell function of LTNPs has rarely been reported. Although studies investigating NK cells have found some evidence of their involvement in anti-HIV activity, changes in NK cell function during HIV infection among LTNPs are poorly defined. In this study, we described, for the first time, NK cell function in ART-naïve, primary HIV-infected patients (PHIs), LTNPs and TPs. We systematically used an array of techniques to quantify intracellular cytokine levels, CD107a expression, and the change of granzyme B for the assessment of NK cell function. Our specific aims were to identify NK cell factors associated with the control of HIV infection.
Materials and Methods
Subjects
Fifty-three subjects were asked to participate in this study, including 35 HIV-infected individuals and 18 HIV-antibody-negative normal controls. The 35 HIV-infected individuals, ages 18 to 55, who were studied included PHIs and chronic HIV-infected subjects (both LTNPs and TPs). Primary HIV infection was defined as an initial HIV infection within 3 months. An HIV LTNP was defined as an HIV-infected individual who had remained asymptomatic for ten or more years with CD4+ T cell counts greater than 500 cells/μl in the absence of antiretroviral therapy. HIV TPs were defined as HIV-infected individuals who had CD4+ T cell counts less than 500 cells/μl. According to these criteria, 10 PHIs were enrolled, and the group had a median CD4+ T cell count of 484 cells/μl (range from 166 cells/μl to 774 cells/μl) and a median viral load of 52,000 copies/ml (range from 728 copies/ml to 10,000,000 copies/ml). The group of 12 TPs who were enrolled had a median CD4+ T cell count of 279 cells/μl (range from 115 cells/μl to 422 cells/μl) and a median viral load of 72,050 copies/ml (range from 1770 copies/ml to 355,000 copies/ml). The 13 LTNPs who were recruited for the study had a median CD4+ T cell count of 647 cells/μl (range from 481 cells/μl to 1389 cells/μl) and a median viral load of 3940 copies/ml (range from 40 copies/ml to 449,000 copies/ml) (Table I). The 18 HIV-antibody-negative normal controls (NCs) were enrolled according to the following criteria: negative for HIV antibodies, normal blood cell count, normal hemoglobin levels, normal liver function, and no history of immunologic diseases.
The Research and Ethics Committee of The First Affiliated Hospital of China Medical University approved the study, which was conducted according to Declaration of Helsinki principles. Each study participant gave written informed consent for his participation in the study.
Assessment of NK Cell Function
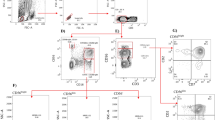
Cryopreserved peripheral blood mononuclear cells (PBMCs) that were isolated from anticoagulated whole blood were incubated for 6 h with the MHCnull K562 cell line (ATCC) at an E:T ratio of 5:1 in the presence of PE-conjugated anti-CD107a (BD Biosciences) and monensin (Becton Dickinson). After incubation, cells were stained to assess expression of both Percp-conjugated anti-CD3 and PE-cy7-conjugated anti-CD56 antibodies (BD Biosciences). The cells were made permeable by applying Perm/Wash (Becton Dickinson) for 10 min, stained with FITC-conjugated anti-IFN-γ or FITC-conjugated anti-granzyme B (BD Biosciences) for 30 min at 4 °C, washed, and then fixed in 2 % formaldehyde. Expressions of CD107a, IFN-γ production and granzyme B content were determined by using flow cytometry (Fig. 1). The content change of granzyme B was calculated by the percentage of granzyme B without stimulation minus the percentage of granzyme B with K562 stimulation.
The gating strategy for identification of NK cells subsets and functional analysis. Total NK cells and its subsets were identified according to the surface expression of CD3−CD56+ (CD56dim + CD56bright), CD3−CD56dim and CD3−CD56bright, and total NK cell or its subsets function were determined by the surface expression of CD107a, or the intracellular expression of IFN-γ or change of granzyme B content after K562 stimulation (unstimulated content of granzyme B minus the content of granzyme B after K562 stimulation)
Determination of CD4+ T Cell Counts
CD4+ T cell counts were measured by using a FACSCalibur flow cytometer (Becton Dickinson). A single-platform lyse-no-wash procedure was performed using Trucount tubes and TriTEST anti-CD4-FITC/CD8-PE/CD3-PerCP reagents (Becton Dickinson). Trucount Control Beads (low, medium and high beads; Becton Dickinson) were used to control the quality and accuracy of the CD4+ T cell counts.
HIV Viral Load Measurement
Plasma HIV RNA was measured via RT-PCR using the COBAS®AmpliPrep®/COBAS Taqman(Roche Diagnostic Systems). The detection range of the assay was between 40 copies/ml and10,000,000 copies/ml. HIV RNA copy numbers were calculated according to the manufacturer’s reference standards.
Statistical Analysis
The nonparametric Mann–Whitney U test was used between two-group comparisons. All analyses were carried out using SPSS 11.5 software and P values less than 0.05 were considered significant. Associations between NK cell function and viral loads or CD4+ T cell counts were determined by the Spearman rank test.
Results
Characteristics of NK Cell Subsets in HIV Infection
Subsets of NK cells were detected with flow cytometry in our study population, which included PHIs, LTNPs, TPs and NCs. We observed a dramatic increase of CD56dim subset in the PHI group compared with normal controls (P = 0.014) and TPs (P = 0.005). The proportion of CD56dim NK cells in TPs was lower than that in NCs (P = 0.047) (Fig. 2a). However, there was no difference in the proportions of CD56dim NK cells and CD56bright NK cells between PHIs and LTNPs (P = 0.077, P = 0.291) (Fig. 2a, b).
Changes of the distribution in the NK cell subsets during HIV infection. The percentage of CD56dim NK cell subset (a) and CD56bright NK cell subset (b) in CD3− lymphocytes were compared between each two groups among NCs, PHIs, TPs and LTNPs. On CD56dim NK cell subset, the difference has been shown with p value. There was no difference on the CD56 bright NK cell subset between two-group comparisons
Functional Activities of NK Cells During HIV Infection
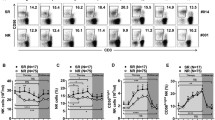
We compared the functional activities of total NK cells and two NK cell subsets in normal controls and HIV-infected groups. PBMC samples were cultured either with the K562 cell line or without K562. CD107a expression, IFN-γ production and changes in the granzyme B content were measured to reflect functional alteration of total NK cells and the two NK cell subsets. Representative flow cytometry figures are shown in Fig. 3.
In PHI group, total NK cell activity related to IFN-γ production was similar to that of NCs and TPs but lower than that of LTNPs (P = 0.034) (Fig. 4a). However, IFN-γ production of the CD56dim NK cells in PHIs was also lower than that of TPs and LTNPs (P = 0.034, P = 0.001) (Fig. 4b). CD107a expression in total NK cells or CD56dim NK cells showed no difference compared to NCs and TPs, however, for the CD56bright NK subset, PHIs presented with higher levels of CD107a than did NCs, TPs and LTNPs (P < 0.001, P = 0.008, P < 0.001) (Fig. 4c). After K562 stimulation, a marked decrease in the staining of granzyme B, in comparison with cells without K562 stimulation, was observed, likely resulting from stimulation-triggered degranuation of granzyme B. However, It is also possible, although unlikely, that the stimulation caused a decrease in granzyme B production. The granzyme B content change after K562 stimulation for total NK cells in PHIs was similar to that in NCs but lower than that in LTNPs (P = 0.03) (Fig. 4a). A similar comparison could be found for CD56dim NK subset (Fig. 4b), but there was no difference between the groups for CD56bright NK subset (Fig. 4c).
Changes in NK cell function during HIV-1 infection. a Single function of NK cells which was detected by IFN-γ production, CD107a expression and content change of granzyme B respectively. b Single function of CD56dim NK cells which was detected by IFN-γ production, CD107a expression and content change of granzyme B respectively. c Single function of CD56bright NK cells which was detected by IFN-γ production, CD107a expression and content change of granzyme B respectively. d Combinating function of NK cells which was detected by IFN-γ production and CD107a expression
In LTNPs, IFN-γ production of total NK cells was higher than that of NCs (P = 0.034). Furthermore, IFN-γ production was higher among both CD56dim NK and CD56bright NK subsets compared to that of NCs (P = 0.028, P = 0.032) (Fig. 4a, b, c). The expression of CD107a of total NK cells in LTNPs was greater than that of NCs (P = 0.045). Similarly, CD107a expression by the CD56dim NK subset was higher than that in NCs (P = 0.037) (Fig. 4a, b). LNTPs also exhibited an increased content change in granzyme B for total NK cells compared with that of TPs and NCs (P = 0.034, P = 0.003), and this change in granzyme B was also true for the CD56dim NK subset (P = 0.044, P = 0.002) (Fig. 4a, b). There was no difference between the groups regarding granzyme B for CD56bright NK subset (Fig. 4c).
We also observed the combinational expression of IFN-γ and CD107a. The results showed that the percentage of IFN-γ+CD107a+ NK cells in PHIs was lower than that in NCs, TPs and LTNPs (P = 0.02, P = 0.008, P = 0.041). The proportion of IFN-γ−CD107a+ NK cells in the three HIV-infected groups (PHIs, TPs and LTNPs) was higher than that in normal controls (P = 0.033, P = 0.016, P = 0.024, respectively). While the level of IFN-γ+CD107a− NK cells in LTNPs experienced a greater increase compared to that in TPs, PHIs and NCs (P = 0.005, P = 0.001, P < 0.001, respectively) (Fig. 4d).
Correlation of NK Cell Function in HIV-Infected Individuals with CD4+ T Cell Counts and Viral Loads
HIV Viral loads and CD4+T cell counts are generally used as markers of HIV disease progression. The correlation of these markers with NK cell functions, as determined by IFN-γ production, CD107a expression, and the content change in granzyme B, were investigated in HIV LTNPs, PHIs, and TPs. No correlations were found between any of the NK cell functional activities and CD4+ T counts in LTNPs. However, IFN-γ production for total NK cells in LTNP exhibited a significant inverse correlation with viral loads (r = −0.762, P = 0.002) (data no shown), especially for the CD56dim subset (r = −0.802, P = 0.001) (Fig. 5a). There was no observable correlation between total NK cell IFN-γ production and viral loads in PHIs and TPs. In addition, IFN-γ production of the CD56dim subset in TPs showed a positive correlation with viral loads (r = 0.614, P = 0.034), but there was no correlation in PHIs (r = −0.195, P = 0.589) (Fig. 5b, c). It indicated that the correlation between IFN-γ production of NK cells and markers of HIV-1 disease progression in PHIs and TPs was quite different from that in LTNPs. There was no significant correlation between CD107a expression or granzyme B content change with viral loads in TPs and LTNPs (data not shown). Furthermore, we found that the IFN-γ production of total NK cells and CD56dim subset is lower in high viral load group (lgvl > 3.5) compared with low viral load group (lgvl < 3.5) (P = 0.011, P = 0.03) (Fig. 6).
Correlation between the level of IFN-γ expression on CD56dim subset with viral loads. a The correlation between IFN-γ production and viral loads on CD56dim subset of LTNPs. b The correlation between IFN-γ production and viral loads on CD56dim subset of TPs. c The correlation between IFN-γ production and viral loads on CD56dim subset of PHIs
Discussion
Although some studies have described associations between NK cell dysfunction and HIV infection, specific characteristics of NK cells in PHIs and LTNPs require further clarification. In this study, we investigated the functions and subsets of NK cells in primary HIV infection and in chronic infection, specifically investigating NK cell function in TPs and LTNPs.
Our results indicated an increase in CD56dim NK cells in PHI group, which is consistent with other studies that observed increased percentage of CD56dim NK cells in the early stages of HIV infection [11, 26]. One possible explanation for this increase is that these additional CD56dim NK cells may be derived from differentiating CD56bright NK cells in PHIs; we have found that CD56bright levels were lowers in PHIs. Another possible explanation may be that there is an increased replication of CD56dim NK cells after the acute infection. Several studies have indicated a dramatic reduction in the proportion of CD56dim NK cells in chronic HIV infection while other studies reported no difference between the CD56dim NK cell levels of chronically infected HIV patients and NCs [1]. Our data showed that the CD56dim NK subset in chronically infected TPs was lower than that of the NCs. This difference is likely due to the patients being studied during different stages of their infection or during different stages of disease progression. Although significant changes in the distribution of NK cell subsets has been observed in TPs, there was no difference between the distributions of NK cell subsets in LTNPs and NCs. The mechanism by which NK cell subset alteration occurs remains unclear. Several reports have demonstrated that the reduction of CD56dim NK cells appears to be partially attributable to the emergence of a novel subset of NK cells that is not easily detectable in HIV-negative normal individuals, known as CD56−CD16+ NK cells [11, 27].
To elucidate the functional properties of NK cells, we evaluated the functions of total NK cells and NK cell subsets in primary infection and chronic infection (LTNPs and TPs) by measuring intracellular IFN-γ production, CD107a expression and content change of granzyme B. We observed that the cytokine production or degranulation or granzyme B content change for total NK cells in the PHI group were similar to that in the NCs and TPs but was lower than that in LTNPs. However, Galit Alter et al. reported elevated IFN-γ production of NK cells in acute HIV infection [28]. The disagreement between these data and our findings may be due to the different infection times of the subjects being studied. Their data was observed during the seronegative acute infection phase, while our study was in the seropositive primary infection phase. In addition to total NK cell function, the function of NK cell subsets in PHIs was also observed. The CD56bright NK cells, generally known to be less mature and perform immunoregulatory functions, have the ability to degranulate and produce cytokines and chemokines [29]. We observed that the CD56bright population of NK cells demonstrated significantly increased expression of CD107a in the PHI group compared with NCs, TP and LTNP groups. This increase may be a compensatory mechanism for the dysfunction of the CD56dim NK cells in these subjects. Another study found that the CD56bright cells in fact had the ability to degranulate at rates comparable to the CD56dim cells [29], which agreed with our findings. Obviously, it would be ideal to perform a longitudinal analysis of NK cell function if the PBMC samples available from multiple time points for each patient. Although the polyfunction of NK cells has been reported [30], it has rarely been studied in LTNPs, NCs and TPs. We analyzed NK cell function by examining IFN-γ production and CD107a degranulation together. We found that the percentage of IFN-γ+CD107a+ NK cell in PHIs were lower than that in NCs, TPs and LTNPs. The NK cells at primary infection time may have been exhausted from controling the virus, leading to a lowered or absent response when stimulated with K562.
LTNPs are a special group of HIV-infected individuals who exhibit delayed disease progression. Studying LTNPs can provide unique insight into successful immunological control of HIV infection. Early work demonstrated that both host and viral factors appear to be involved in determining rates of HIV disease progression. However, there is increasing evidence that the presence of a vigorous immune response against HIV may play a pivotal role in prolonging the asymptomatic phase of the infection [31]. It is found that LTNPs exhibit peculiar phenotypic features that are associated with high levels of IFN-γ and activation markers [8, 32]. In addition to our findings of higher levels of IFN-γ production by the NK cells of LTNPs – which is in accordance with the findings of Vieillard et al. [32] – we also discovered that NK cells from LTNPs exhibit higher expressions of CD107a than did NCs and much more content change of granzyme B after stimulation than did TPs and NCs. This finding suggests that the functional activity of NK cells may be one of the factors facilitating viral control by LTNPs.
Previous studies focused on a single functional characteristic of NK cells during HIV infection. It is essential to perform combinational analyses of NK cell function so that we can offer a more integrated description of their activity and immune contributions. Interestingly, we observed an increase in the proportion of IFN-γ+CD107a− NK cells in LTNPs, which was much higher than in TPs, PHIs and NCs. On the other hand, the proportions of IFN-γ−CD107a+ NK cells in TPs and PHIs were higher than that in NCs. This indicates that higher rates of IFN-γ+CD107a− and IFN-γ+CD107a+ NK cells are unique to LTNPs, while higher rates of IFN-γ−CD107a+ NK cells and lower rates of IFN-γ production are more common in TPs and PHIs.
We analyzed the correlation of the functional activity of the CD56dim NK subset with viral loads and CD4+ T cell counts in the HIV-infected subjects. In the PHI group, we observed no correlation between the IFN-γ production of the CD56dim NK cells and viral load, but the IFN-γ production of the CD56dim NK cells in LTNPs exhibited a significant inverse correlation with viral loads, which suggests that NK cells may perform a direct antiviral function in LTNPs. The IFN-γ production in TPs positively correlates with viral loads. However, the mechanism of this relationship is unknown, though we speculate that it may be similar to that of cytotoxic T lymphocytes(CTL) function among TPs, which is higher in viremic patients and lower in aviremic patients [33]. Nevertheless, because of the cross-sectional data we use, there is a limitation of our analysis.
The mechanism behind the elevation of the functional activity of NK cells in LTNPs is unclear. Some researchers investigated the relationship between NK cell surface phenotypes and functional outcomes, and observed that NKG2C, KIR2DL1/S1and KIR2DL2, L3/S2 were expressed on NK cells of LTNPs [8, 32]. However, these receptors were also present in viremic HIV patients and, therefore, cannot explain the increased activity of NK cells in LTNPs [8, 32]. KIR3DL1 – one of the inhibitory KIR alleles expressed by NK cells, in combination with HLA-B alleles that encode molecules with isoleucine at position 80 (HLA-B Bw4-80Ile) – has been associated with delayed disease progression in individuals infected with HIV-1 [25]. However, carrier frequencies of KIR3DL1 are high among Chinese populations [34], so KIR3DL1 probably does not contribute to the delay of disease progression, at least among HIV-infected Chinese. In contrast, KIR3DS1, an active receptor of NK cells, is much higher in LTNPs than in TPs in another independent study we performed (unpublished), which offers a possible explanation for the elevation of NK cell function in LTNPs.
Some studies have noted that the cytotoxicity of NK cells in TPs was impaired [8, 32]. However, other studies and our data did not observe this impairment. This discrepancy is likely due to the study subjects at different periods of infection as well as differences in the methods used to stimulate NK cells. In our research, cryopreserved PBMCs and K562 cells were used to measure the functionality of NK cells. It was very likely to have dead cells and nonspecific staining in flow cytometry analysis. Although we examined the survival rates of the cells (more than 90 %), it would be more rigorous if we removed the interference from dead cells by viability dye.
In conclusion, we have demonstrated that the levels of the CD56dim NK subset increased in PHIs. Additionally, there were no differences between the IFN-γ production and CD107a expressions of PHIs compared with that of NCs. NK cells from LTNPs exhibited increased activities in terms of IFN-γ production, CD107a expression, and granzyme B content change compared with that of the NCs or TPs. In addition, IFN-γ+CD107a− NK cell levels in LTNPs were higher than that in TPs, PHIs and NCs. High IFN-γ secretion is likely associated with delayed disease progression. Levels of IFN-γ production by the NK cells of LTNPs exhibited a significant inverse correlation with viral load. Taken together, increased NK cell function likely contributes to the control of HIV infection.
References
Alter G, Altfeld M. NK cell function in HIV-1 infection. Curr Mol Med. 2006;6:621–9.
Lanier LL, Testi R, Bindl J, Phillips JH. Identity of Leu-19 (CD56) leukocyte differentiation antigen and neural cell adhesion molecule. J Exp Med. 1989;169:2233–8.
Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97:3146–51.
Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40.
Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–9.
Afonina IS, Cullen SP, Martin SJ. Cytotoxic and non-cytotoxic roles of the CTL/NK protease granzyme B. Immunol Rev. 2010;235:105–16.
Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22.
O’Connor GM, Holmes A, Mulcahy F, Gardiner CM. Natural Killer cells from long-term non-progressor HIV patients are characterized by altered phenotype and function. Clin Immunol. 2007;124:277–83.
Alter G, Altfeld M. NK cells in HIV-1 infection: evidence for their role in the control of HIV-1 infection. J Intern Med. 2009;265:29–42.
Biron CA, Turgiss LR, Welsh RM. Increase in NK cell number and turnover rate during acute viral infection. J Immunol. 1983;131:1539–45.
Alter G, Teigen N, Davis BT, Addo MM, Suscovich TJ, et al. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood. 2005;106:3366–9.
Titanji K, Sammicheli S, De Milito A, Mantegani P, Fortis C, et al. Altered distribution of natural killer cell subsets identified by CD56, CD27 and CD70 in primary and chronic human immunodeficiency virus-1 infection. Immunology. 2008;123:164–70.
De Maria A, Moretta L. NK cell function in HIV-1 infection. Curr HIV Res. 2008;6:433–40.
Ahmad R, Sindhu ST, Tran P, Toma E, Morisset R, et al. Modulation of expression of the MHC class I-binding natural killer cell receptors, and NK activity in relation to viral load in HIV-infected/AIDS patients. J Med Virol. 2001;65:431–40.
Mavilio D, Benjamin J, Daucher M, Lombardo G, Kottilil S, et al. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc Natl Acad Sci U S A. 2003;100:15011–6.
Azzoni L, Papasavvas E, Chehimi J, Kostman JR, Mounzer K, et al. Sustained impairment of IFN-gamma secretion in suppressed HIV-infected patients despite mature NK cell recovery: evidence for a defective reconstitution of innate immunity. J Immunol. 2002;168:5764–70.
Ironson G, Balbin E, Solomon G, Fahey J, Klimas N, et al. Relative preservation of natural killer cell cytotoxicity and number in healthy AIDS patients with low CD4 cell counts. AIDS. 2001;15:2065–73.
Parato KG, Kumar A, Badley AD, Sanchez-Dardon JL, Chambers KA, et al. Normalization of natural killer cell function and phenotype with effective anti-HIV therapy and the role of IL-10. AIDS. 2002;16:1251–6.
Alter G, Malenfant JM, Delabre RM, Burgett NC, Yu XG, et al. Increased natural killer cell activity in viremic HIV-1 infection. J Immunol. 2004;173:5305–11.
Salgado M, Rallon NI, Rodes B, Lopez M, Soriano V, et al. Long-term non-progressors display a greater number of Th17 cells than HIV-infected typical progressors. Clin Immunol. 2011;139:110–4.
Van Duyne R, Guendel I, Kehn-Hall K, Easley R, Klase Z, et al. The identification of unique serum proteins of HIV-1 latently infected long-term non-progressor patients. AIDS Res Ther. 2010;7:21.
Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–9.
Harrer T, Harrer E, Kalams SA, Elbeik T, Staprans SI, et al. Strong cytotoxic T cell and weak neutralizing antibody responses in a subset of persons with stable nonprogressing HIV type 1 infection. AIDS Res Hum Retroviruses. 1996;12:585–92.
Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, et al. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–50.
Martin MP, Gao X, Lee JH, Nelson GW, Detels R, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31:429–34.
Alter G, Teigen N, Ahern R, Streeck H, Meier A, et al. Evolution of innate and adaptive effector cell functions during acute HIV-1 infection. J Infect Dis. 2007;195:1452–60.
Mavilio D, Lombardo G, Benjamin J, Kim D, Follman D, et al. Characterization of CD56-/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci U S A. 2005;102:2886–91.
Chang JJ, Altfeld M. Innate immune activation in primary HIV-1 infection. J Infect Dis. 2010;202 Suppl 2:S297–301.
Eller MA, Eller LA, Ouma BJ, Thelian D, Gonzalez VD, et al. Elevated natural killer cell activity despite altered functional and phenotypic profile in Ugandans with HIV-1 clade A or clade D infection. J Acquir Immune Defic Syndr. 2009;51:380–9.
Kamya P, Tallon B, Melendez-Pena C, Parsons MS, Migueles SA, et al. Inhibitory killer immunoglobulin-like receptors to self HLA-B and HLA-C ligands contribute differentially to natural killer cell functional potential in HIV infected slow progressors. Clin Immunol. 2012;143:246–55.
Paroli M, Propato A, Accapezzato D, Francavilla V, Schiaffella E, et al. The immunology of HIV-infected long-term non-progressors–a current view. Immunol Lett. 2001;79:127–9.
Vieillard V, Fausther-Bovendo H, Samri A, Debre P. Specific phenotypic and functional features of natural killer cells from HIV-infected long-term nonprogressors and HIV controllers. J Acquir Immune Defic Syndr. 2010;53:564–73.
Zhang Z, Zhao QX, Fu JL, Yao JX, He Y, et al. Characteristics of HIV-1-specific CD8 T-cell responses and their role in loss of viremia in children chronically infected with HIV-1 undergoing highly active antiretroviral therapy. Chin Med J (Engl). 2006;119:1949–57.
Wu GQ, Zhao YM, Lai XY, Yang KL, Zhu FM, et al. Distribution of killer-cell immunoglobulin-like receptor genes in Eastern mainland Chinese Han and Taiwanese Han populations. Tissue Antigens. 2009;74:499–507.
Acknowledgments
The authors wish to express their gratitude for the generosity of the patients who participated in this study. This work was supported by a research grant, the Mega Projects of National Science Research for the 12th Five-Year Plan (2012ZX10001-006); Liaoning Province Department of Higher Education Program for Excellence in Research (LR2012027). The authors appreciated the help from Christina Liao for editing the English words and grammers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yongjun Jiang and Hong Shang contributed equally to this article.
Rights and permissions
About this article
Cite this article
Jiang, Y., Zhou, F., Tian, Y. et al. Higher NK Cell IFN-γ Production is Associated with Delayed HIV Disease Progression in LTNPs. J Clin Immunol 33, 1376–1385 (2013). https://doi.org/10.1007/s10875-013-9930-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-013-9930-1