Abstract
Cancer immune-therapy is an interesting avenue of studying the effects of deviating immune system responses to achieve the desired result. Lactobacilli are inhabitants of the GI tract which have shown beneficial health effects on various ailments including malignancies. Their mechanisms of action comprise a very intense area of research. In this study we evaluated the immunomodulatory effects of Lactobacillus acidophilus in in vivo model of breast cancer. Lactobacillus acidophilus (L.a) was isolated from traditional home-made yogurt and also from neonatal stool by aerobic overnight culture at 37°C in MRS broth. Delayed Type Hypersensitivity (DTH) assay was performed to find the best immunostimulant dose. 4T1 tumour bearing mice were treated with 2 × 108 cfu of isolated L. acidophilus and 20 mg/kg Cyclophosphamide for 15 consecutive days. Tumour volume was measured using a digital vernier calliper. Lymphocyte proliferation was done using MTT proliferation assay. Production of IFNγ, IL-4 and TGF-β from cultured Splenocytes was assessed in the presence of purified tumour antigen. According to results administration of L.a induced a significant decrease in tumour growth pattern (P value = 0.00). Significant alterations in splenocyte production of IFN-γ, IL-4 and TGf-β (P values < 0.05) and also lymphocyte proliferation in L.a treated animals was evident (P value < 0.05). This study indicated that oral administration of L.a is able to alter the cytokine production in tumour bearing mice into a Th1 protective pattern, favourable to anti tumour immunity. Reduced tumour growth rate and increased lymphocyte proliferation are also thus supportive. Further studies are required to elucidate the exact mechanism by which local actions of probiotics affect the systemic immune responses against transformed cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is one of the leading causes of death in women around the world with a very high degree of mortality and morbidity. Intensive researches in this area are being conducted. Still many aspects of this illness are obscure. Conventional treatments use cytotoxic drugs which have high levels of side effects, affecting the patient’s quality of life. Therefore today’s pharmacology is looking into treatments with low side effects and maximum efficiency. Immune-therapy is an interesting avenue of studying the effects of deviating immune system responses to achieve the desired result. Many immune modulations have been carried out by numerous studies to shift the responses from a Th2 pattern to a Th1, which is believed to be the principle causative of impaired response to malignancies. Many clinical and experimental studies have focused on DC based vaccine strategies [1–3], adoptive transfer of effector T cells [4–6], DNA vaccines containing designer cytokines and antigens [7, 8] and many more. All of these strategies are focusing on one thing that is built naturally through active immune responses. Many microorganisms initiate a strong Th1 response via various molecular patterns.
Probiotics are inhabitants of the GI tract possessing numerous health benefits. An appropriate definition to human nutrition has been outlined by Salminen et al. [9] describing a probiotic as, “a live microbial food ingredient that is beneficial to health”. Many probiotics are members of the genera Lactobacillus and Bifidobacteria. Many studies have outlined the beneficial effects of probiotics on various ailments such as food allergy [10, 11], inflammatory bowel disease (IBD) and Ulcerative Colitis (UC) [12, 13]. Probiotics exert their modulatory actions by different mechanisms. These include anti-genotoxicity and Inhibition of colonic enzyme activity which reduces the harmful effects of carcinogens which are activated via enzymatic actions in the colon [14], control of the growth of potentially harmful bacteria [9], interaction with colonocytes which results in enhanced homeostasis and the integrity of epithelial barrier through increasing the production of Zonula Occludens-2 (ZO-2)[15], immune system stimulation which is measured by increases in secretory and inflammatory responses in animals and man and production of physiologically active metabolites including short chain fatty acids which induce differentiation and apoptosis in vitro studies [16]. The immunomodulatory actions of probiotic are a very intense area of research. Many studies have utilised this effect for deviating the responses of immune system into the pattern of choice. It has been shown that probiotics are able to attach to entrocytes and be up taken by dendritic cells (DC) beneath the epithelial barrier and the M cells in Peyer Patches [17]. In vitro, T cell interactions with Lactobacillus rhamnosus primed DCs show less proliferation and production of cytokines [18]. This could be an indication for generation of regulatory DCs in the gut. How these mechanisms might affect systemic responses to cancer progression is to be investigated. With evidences at hand in their ability to shift the responses to Th1 [19], it is postulated that the probiotics may aid the immune system against cancer. Therefore in this study we evaluated the immunomodulatory effects of probiotics in in vivo model of breast cancer.
Material and Methods
Mice
8–10 week old Balb/C inbred female mice (Pasteur Institute, Iran) were used, housed five to a cage with access to autoclaved standard mouse chow at libitum. . All animals received humane care. The animal protocol was reviewed and approved by the Animal Care and Research Committee of the Tarbiat Modares University.
Isolation of Lactobacillus
Lactobacillus acidophilus strain was used in this study as a probiotic that was isolated from traditional home-made yogurt as standard bacteria (Standard Strain) and also from neonatal stool (Stool Strain) as a normal flora of GI. Before administration, L. acidophilus strain was grown aerobically overnight at 37°C in DeMan, Rogosa, Sharpe, Oxoid (MRS) broth. The next day, bacteria were harvested with centrifugation, washed twice in sterile phosphate buffered saline, and adjusted to a cell density of 2 × 108 colony-forming units (cfu) per millilitre.
Delayed Type Hypersensitivity (DTH)
Twenty eight to ten week old mice were sensitised subcutaneously (SC) with109 sheep red blood cells (SRBC) and divided randomly into 4 groups of five. 3 groups were treated daily with 0.1 mL of the L.a solution containing 108, 2 × 108 and 3 × 108 cfu via the orogastric route by a feeding canula for a 7 day period. The remaining group (as control) were treated with phosphate buffer saline in the same route, volume, and time. On day 8 of sensitization, the sensitized animals were challenged with 108 sRBCs injected subcutaneously on the left hind foot pad. The increase in the foot pad thickness was measured 24, 48, and 72 h after the antigen challenged by vernier calliper (Mitutoyo, Japan). Each measurement was repeated twice. The results were expressed as the mean percentage increase in the foot pad thickness. The results were calculated according to the following formula.
Breast Cancer Model
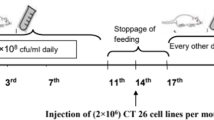
4–6 week old Balb/c mice were transplanted in the left flank with 7 × 105–106 cells of 4T1 breast cancer cell line (Pasteur Institute, Tehran, Iran). The tumors developed on the 8th day, after which we started therapy at the 11th day. The animals were sacrificed at the 30th day. 4T1 breast cancer cell line develops in Balb/c mice (syngenic). 4T1 mammary carcinoma derived from Balb/c mice shares many characteristics with naturally occurring human breast cancer. By the time the primary tumor is palpable, 4T1 tumor cells have spontaneously spread to various distant organs. Surgical removal of palpable primary tumors from the mammary glands does not affect the growth of distal metastases and pulmonary metastasis is the major cause of death in 4T1-bearing mice. For these reasons, 4T1 in the immune-competent and syngeneic Balb/c mouse is recognized as a most challenging breast tumor model in which to evaluate the efficacy of novel immunotherapeutics.
Treatments with L. acidophilus and Cyclophosphamide
The tumours grew for approximately 8 days, after which animals were divided into groups of 5 mice. Experimental groups were treated with 1) 2 × 108 cfu L. acidophilus isolated from yogurt and 2) neonatal stool and 3) injected intraperitoneally with 20 mg/kg Cyclophosphamide (CYC) (Endoxan®). PBS was used as control in a total volume of 0.2 ml. Treatment was carried out for 15 consecutive days.
Tumour Volume Measurement
Tumour volume was measured using a digital vernier calliper (Mitutoyo, Japan) and calculated using the following formula:
where L = length and W = width
Mononuclear Cell (MNC) Separation
According to the results from DTH, tumour-bearing mice were treated with 2 × 108 cfu L. acidophilus isolated from yogurt, stool and 20 mg/kg CYC and PBS as control for 15 consecutive days. On day 16 blood samples were drawn and mice were sacrificed by cervical dislocation and spleen was resected. Spleen was removed under sterile conditions and suspended in PBS containing 2 % FBS. RBCs were lysed with lysis buffer. Single-cell suspension was prepared and adjusted to 3 × 106 cells/ml in RPMI 1640 (Gibco) supplemented with 5 % FCS, 4 mM L-glutamine, 25 mM HEPES, 0.1 mM non-essential amino acid, 1 mM sodium pyrovate, 50 μm 2ME. The number of isolated cells from the spleen was in a similar range among different samples (3–5 × 106 cell/ml), and the viability of cells was an average of 95 % according to trypan blue staining.
Tumor Antigen Preparation
Tumours cell line suspension was washed with saline and prepared. Tumour suspension was then subjected to three rounds of freezing (−170°C) and thawing (37°C). It was then sonicated with a power of 4 W for 30 s followed by a 30-s incubation period for three consecutive times. In order to inhibit serine proteases, 1 mMPhenylmethylsulfonyl fluoride (PMSF) was added to the cell lysates. Finally, the extract was dialysed and filtered. Protein concentration of the extract was determined using micro Bradford method and then stored in −20° until use.
Lymphocyte Proliferation Assay
Splenic MNCs were cultured in RPMI 1640 (as described above). Proliferation assay was done using MTT proliferation assay protocol. Briefly, 100 μl of cell suspensions was dispensed in96 well flat bottom micro plates (Nunc, Denmark). 6 wells were considered for each sample. 20 μl of tumour antigen was added to three wells of each sample. Plates were incubated in 37°C (separate wells were cultured with untreated normal splenocytes and incubated with PHA as positive control). After 48 h at 37 ° C and 90 % humidity and 5 % CO2 cells were labeled with 5 μl/ml MTT. Cells were reincubated for additional 4 h at 37°C. Labeling medium was removed using 10 min centrifuge (Eppendorf) at 300 g. Cells were fixed using 100 μl DMSO solution and stored at room temperature for 5 min. Absorbance of the samples was measured using ELISA reader at 540 nm. Results are presented as stimulation index (SI), using the following formula:
Splenocyte Cytokine Production
Isolated spleen MNCs were cultured in 24 well plates (Nunc,Denmark) as mentioned above. 20 μl of purified tumour antigen was added to each and 72 h after plates were centrifuged (Eppendorf 300 g, 10 min) and supernatants were collected and were kept at −70°C until use. IFNγ, IL-4 and TGF-β concentrations were measured using R&D DuoSet ELISA Development kit according to the manufacturers’ protocols. Each sample was analyzed in duplicates.
Statistical Analyses
Each experiment was performed in duplicate or triplicate. The results are expressed as mean ± SE. The P-value of 0.05 was considered the statistical significance of the results using SPSS software version 19.
Results
Delayed-Type Hypersensitivity (DTH) Assay
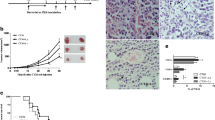
Treatment with L.a has shown that it has the ability to augment the cellular immune responses. Thus in this matter, to assess the overall effect of L.a on the cellular immune system, three doses were administered and compared. As seen in Fig. 1, it is evident that the three different doses of L.a showed distinct patterns of response. In 24 h intervals after left foot pad challenge, swelling was measured as in difference between left and right foot pad expressed in percentage. After 72 h, mice treated with 2 × 108 cfu of L. Acidophilus responded significantly to antigen challenge as compared to doses of 108 and 3 × 108 (P-value = .394, .029 and .353 compared to control, in the ascending order of dose).
Delayed type responses in mice (n = 5 for each group) pre-sensitized with sheep RBC is shown as the percentage of increased swelling in left foot pad compared to right food pad as control. Data is presented as mean ± SEM. Results clearly showed that 2 × 108 cfu of L.a has a greater influence on immune response (P-value = 0.029) con = control
Tumour Volume
The volume of tumours was measured throughout the treatment using a digital vernier calliper. The pattern of tumour growth is shown in Fig. 2. CYC was used as an approved treatment and as positive control in this study. As expected, it showed significant control of tumour growth compared to control mice treated with PBS and other treatment groups. However efficient control of tumour growth was observed in administration of L.a (P-value = 0.06 on day 10 and 0.000 on day 15 of treatment.)
Tumour growth was measured every five days by digital calliper. Tumour volume was calculated using the formula described. Data represent the means ± SEM for five animals per group. SD = standard strain CYC = cyclophosphmide, ST = stool, CON = control (P value < .000 as indicated with ***). The comparison indicated is between treatment groups and the negative control group. No significant difference was observed between groups treated with L. a and CYC
Lymphocyte Proliferation Assay
The results of the proliferation assays are shown in Fig. 3. The data reflect the mean values of triplicates after stimulation with specific tumour antigen. Comparing the results of both groups, the data clearly demonstrated significant difference between the treatment groups and with the control.
Comparing the proliferation of splenocyts among treated and control group showed a significant increase in proliferation in L. acidophilus treated mice (P-value = 0.05 and .02 respectively for standard bacteria (SD) and stool derived bacteria (ST) as indicated with *). Positive control was treated with PHA and showed significant deference with other groups both controls and treatment groups (p-value < 0.000 indicated with ***). Error bars represent ±2 SEM. SD = standard strain, CYC = cyclophosphmide, ST = stool, CON = control. C + represents the SI for cells treated with Phytohemagglutinin (PHA) as positive control. The comparison indicated is between treatment groups and the negative control group (n = 5 per group)
Splenocyte Cytokine Production
The cytokine pattern of splenocytes in tumour-bearing animals treated with L. acidophilus was evaluated and compared to control (as described above). Results showed a reduced production of IL-4 and increased production of IFN-γ. Increased production of IFN-γ in groups treated with L.a was statistically significant and additionally no difference was observed between treatment groups (P-value = .04, .08 and .7 for mice treated with standard (SD), stool (ST) and cyclophosphamide (CYC) respectively). Lower production of IL-4 in L.a treated groups however not statistically significant (P-value > 0.05). Decrease in IL-4 production in mice treated with CYC was significant with a p-value of 0.008 and also had a significant difference with mice treated with L.a. Significant lower production of TGF-β in probiotic treated groups was observed with no significant differences between groups of treatment (Fig. 4).
Mice (n = 5) were treated with L. acidophilus extracted from standard yogurt (SD), neonatal stool (ST) and cyclophosphamide (CYC) for 15 days which after spleen cells were isolated and the levels of IFN-γ, IL-4 and TGF-β produced from splenocytes stimulated by tumour antigen extract was evaluated. Data represents mean ± SEM. The comparison indicated is between treatment groups and the negative control group
Discussion
In the gut lactobacilli and other probiotics are responsible for homeostasis of gut epithelium via various mechanisms one of which includes their anti-inflammatory actions which prevents excessive inflammatory damage to epithelial tissues [20]. Cell wall components of lactobacilli and other probiotics, such as peptidoglycans and lipoteichoic acid, induce maturation and activation of DCs via toll-like receptor (TLR) 2, as well as producing various proinflammatory cytokines and chemokines [20–22]. Specific probiotic strains, in particular Lactobacillus strains, have been shown to interact with DCs and to induce strain-specific effects [23]. Their beneficial health effects have been studied in various disease and natural response settings. For instance they are capable of inducing production of IFN-γ in vivo and in vitro models [24, 25].
Proliferation of lymphocytes is the first indication of immune system activation by antigen. In this study we have shown that mice treated with L.a had a greater increase in splenocyte proliferation in response to tumour antigen. This increased proliferation has been reported previously [26].
Natural killer cells are major source of IFN-γ which studies have shown that L.a is able to activate NK cells. They are also active participants in anti-tumour immunity. Therefore one possible mechanism by which L.a could have impacts on tumour growth control is by activating these innate anti-cancer cells. Takagi et al. have demonstrated that administration of Lactobacillus casei Shirota (LcS) in mice is able to delay the onset of carcinogen induced tumor and reduced tumor incidence [27]. They showed that these effects were attributed to enhanced NK activity. Other studies have shown similar results by using heat killed strains [28]. Yasdi et al. in similar experimental setting have shown that L.a is able to increase the levels of IL-12 in cultured splenocytes [29]. Murosaki et al. have also shown the increase in IL-12 production via daily consumption of lactobacilli in tumour-bearing mice is required to exert an anti-tumour effect at the late stage of tumour development when the IL-12 production is considerably impaired [30, 31]. This cytokine is responsible for production of IFN-γ from stimulated T cells and enhancing the shift to a protective Th1 pattern which favours anti tumour immunity. The balance between Th1 and Th2 cytokine production can determine the direction and outcome of an immune response. As expected, this study showed that administration of lactobacilli was able to augment the production of IFN-γ from splenocyte. Increased production of IFN-γ in turn has other anti-cancer effects including anti-angiogenesis and increased NK activity. Thereby a continuous cascade of cytokine production and cellular activation, by definition, are responsible for L.a anti cancer mechanisms. These speculations surely are subject to further investigations. Our results showed that lactobacilli did not affect the production of IL-4 in treated tumour bearing animals. This finding is confirmed in a previous study by Gill et al. in which implied that regular ingestion of Lactobacillus strains is able to enhance the capacity of murine splenic leukocytes to produce IFN-γ following mitogenic stimulation, while IL-4 or IL-5 production is unaffected [32].
TGF-β is Th2 cytokine with an anti-inflammatory property which induces the production of IL-10 from tumour associated macrophages (TAM) and aid the shift to Th2 pattern in tumour microenvironment [33]. TGF-β produced from tumour cells acts on the surrounding stromal cells, immune cells, endothelial cells and smooth muscle cells, causing immunosuppression and angiogenesis in the microenvironment [34]. TGFβ is a key enforcer of immune tolerance, and tumors that produce high levels of this cytokine may be shielded from immune surveillance and is involved inhibition of proliferation of T cells [35]. This study showed that L.a is able to decrease the production of TGF-β from splenocytes in culture. Taken together, our findings suggest that probiotics La may play a role in attenuating tumour growth by altering the cytokine milieu and anti-tumoural cell activation during 4T1 cell carcinogenesis. It is possible to speculate that lactobacilli or other genera of probiotics could be used as an adjuvant treatment during anticancer chemotherapy.
References
Gilboa E. DC-based cancer vaccines. J Clin Investig. 2007;117(5):1195.
Smits ELJM, Anguille S, Cools N, Berneman ZN, Van Tendeloo VFI. Dendritic cell-based cancer gene therapy. Hum Gene Ther. 2009;20(10):1106–18.
Huang FP, Chen YX, To CKW. Guiding the “misguided”–functional conditioning of dendritic cells for the DC–based immunotherapy against tumours. Eur J Immunol. 2011.
Smith FO, Klapper JA, Wunderlich JR, Rosenberg SA, Dudley ME. Impact of a recombinant fowlpox vaccine on the efficacy of adoptive cell therapy with tumor infiltrating lymphocytes in a patient with metastatic melanoma. J Immunother. 2009;32(8):870.
Mondino A, Dardalhon V, Michelini RH, Loisel-Meyer S, Taylor N. Redirecting the immune response: role of adoptive T cell therapy. Hum Gene Ther. 2010;21(5):533–41.
Klebanoff CA, Gattinoni L, Palmer DC, Muranski P, Ji Y, Hinrichs CS, et al. Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice. Clin Cancer Res. 2011;17(16):5343.
Kirkwood JM, Lee S, Moschos SJ, Albertini MR, Michalak JC, Sander C, et al. Immunogenicity and antitumor effects of vaccination with peptide vaccine+/− granulocyte-monocyte colony-stimulating factor and/or IFN-α2b in advanced metastatic melanoma: eastern cooperative oncology group phase II trial E1696. Clin Cancer Res. 2009;15(4):1443–51.
Zarei S, Schwenter F, Luy P, Aurrand-Lions M, Morel P, Kopf M, et al. Role of GM-CSF signaling in cell-based tumor immunization. Blood. 2009;113(26):6658–68.
Collado MC, Isolauri E, Salminen S, Sanz Y. The impact of probiotic on gut health. Curr Drug Metabol. 2009;10(1):68–78.
Schiavi E, Barletta B, Barone A, Butteroni C, Corinti S, Di Felice G. Probiotic treatment induces intestinal regulatory dendritic and T cells, and counter-regulates Th2 responses and anaphylaxis in a mouse model of food allergy. Clin Transl Allergy. 2011;1:1.
Kalliomäki M, Isolauri E. Role of intestinal flora in the development of allergy. Curr Opin Allergy Clin Immunol. 2003;3(1):15.
Fedorak RN, Dieleman LA, Madsen KL. Prebiotics, probiotics, antibiotics, and nutritional therapies in IBD. Inflamm Bowel Dis. 2011:123–50.
Stephani J, Radulovic K, Niess JH. Gut Microbiota, Probiotics and Inflammatory Bowel Disease. Archivum immunologiae et therapiae experimentalis. 2011:1–17.
Liong MT. Roles of probiotics and prebiotics in colon cancer prevention: postulated mechanisms and in-vivo evidence. Int J Mol Sci. 2008;9(5):854–63.
Oelschlaeger TA. Mechanisms of probiotic actions-a review. Int J Med Microbiol. 2010;300(1):57–62.
Gill H, Prasad J. Probiotics, immunomodulation, and health benefits. Bioact Compon Milk. 2008:423–54.
Chiba Y, Shida K, Nagata S, Wada M, Bian L, Wang C, et al. Well–controlled proinflammatory cytokine responses of Peyer’s patch cells to probiotic lactobacillus casei. Immunology. 2010;130(3):352–62.
Braat H, van den Brande J, van Tol E, Hommes D, Peppelenbosch M, van Deventer S. Lactobacillus rhamnosus induces peripheral hyporesponsiveness in stimulated CD4+ T cells via modulation of dendritic cell function. Am J Clin Nutr. 2004;80(6):1618.
Torii A, Torii S, Fujiwara S, Tanaka H, Inagaki N, Nagai H. Lactobacillus acidophilus strain L-92 regulates the production of Th1 cytokine as well as Th2 cytokines. Allergol Int. 2007;56(3):293–301.
Wallace TD, Bradley S, Buckley ND, Green-Johnson JM. Interactions of lactic acid bacteria with human intestinal epithelial cells: effects on cytokine production. J Food Protect. 2003;66(3):466–72.
Haller D, Bode C, Hammes W, Pfeifer A, Schiffrin E, Blum S. Non-pathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. Gut. 2000;47(1):79.
Galdeano CM, Perdigon G. The probiotic bacterium lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clin Vaccine Immunol. 2006;13(2):219–26.
Maassen C, van Holten-Neelen C, Balk F, Heijne den Bak-Glashouwer MJ, Leer RJ, Laman JD, et al. Strain-dependent induction of cytokine profiles in the gut by orally administered lactobacillus strains. Vaccine. 2000;18(23):2613–23.
Prescott S, Wickens K, Westcott L, Jung W, Currie H, Black P, et al. Supplementation with lactobacillus rhamnosus or bifidobacterium lactis probiotics in pregnancy increases cord blood interferon–γ and breast milk transforming growth factor–β and immunoglobin A detection. Clin Exp Allergy. 2008;38(10):1606–14.
Chuang L, Wu KG, Pai C, Hsieh PS, Tsai JJ, Yen JH, et al. Heat-killed cells of lactobacilli skew the immune response toward T helper 1 polarization in mouse splenocytes and dendritic cell-treated T cells. J Agric Food Chem. 2007;55(26):11080–6.
Easo J, Measham J, Munroe J, Green-Johnson J. Immunostimulatory actions of lactobacilli: mitogenic induction of antibody production and spleen cell proliferation by lactobacillus delbrueckii subsp. Bulgaricus and lactobacillus acidophilus. Food Agric Immunol. 2002;14(1):73–83.
Takagi A, Matsuzaki T, Sato M, Nomoto K, Morotomi M, Yokokura T. Enhancement of natural killer cytotoxicity delayed murine carcinogenesis by a probiotic microorganism. Carcinogenesis. 2001;22(4):599.
Cheon S, Lee KW, Kim KE, Park JK, Park S, Kim C, et al. Heat-killed Lactobacillus acidophilus La205 enhances NK cell cytotoxicity through increased granule exocytosis. Immunology Letters. 2011.
Yazdi MH, Dallal MMS, Hassan ZM, Holakuyee M, Amiri SA, Abolhassani M, et al. Oral administration of lactobacillus acidophilus induces IL-12 production in spleen cell culture of BALB/c mice bearing transplanted breast tumour. Br J Nutr. 2010;104(2):227–32.
Murosaki S, Muroyama K, Yamamoto Y, Yoshikai Y. Antitumor effect of heat-killed lactobacillus plantarum L-137 through restoration of impaired interleukin-12 production in tumor-bearing mice. Canc Immunol Immunother. 2000;49(3):157–64.
O’Hara RJ, Greenman J, MacDonald AW, Gaskell KM, Topping KP, Duthie GS, et al. Advanced colorectal cancer is associated with impaired interleukin 12 and enhanced interleukin 10 production. Clin Cancer Res. 1998;4(8):1943–8.
Gill HS, Rutherfurd KJ, Prasad J, Gopal PK. Enhancement of natural and acquired immunity by lactobacillus rhamnosus (HN001), lactobacillus acidophilus (HN017) and bifidobacterium lactis (HN019). Br J Nutr. 2000;83(02):167–76.
Maeda H, Kuwahara H, Ichimura Y, Ohtsuki M, Kurakata S, Shiraishi A. TGF-beta enhances macrophage ability to produce IL-10 in normal and tumor-bearing mice. J Immunol. 1995;155(10):4926.
Massagué J. TGF [beta] in cancer. Cell. 2008;134(2):215–30.
Maeda H, Shiraishi A. TGF-beta contributes to the shift toward Th2-type responses through direct and IL-10-mediated pathways in tumor-bearing mice. J Immunol. 1996;156(1):73.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maroof, H., Hassan, Z.M., Mobarez, A.M. et al. Lactobacillus acidophilus Could Modulate the Immune Response Against Breast Cancer in Murine Model. J Clin Immunol 32, 1353–1359 (2012). https://doi.org/10.1007/s10875-012-9708-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-012-9708-x