Abstract
Introduction
Sarcoidosis is an inflammatory disease of unknown etiology. However, an infectious cause has been proposed suggesting a role for pattern-recognition receptors, such as Toll-like receptors (TLRs) and nucleotide-binding domain, leucin-rich repeat containing family proteins (NLRs), in the pathogenesis.
Objective
Our aim was to investigate whether differences in TLR2 and TLR4 expression, and the response to TLR2, TLR4, and NOD2 stimulation, are associated with sarcoidosis.
Materials and Methods
Blood mononuclear cells from sarcoidosis patients (n = 24) and healthy subjects (n = 19) were incubated with the TLR2 ligands PGN and Pam3CSK4, the TLR4 ligand LPS, the NOD2 ligand MDP, or medium alone. After 16 h, monocyte TLR2 and TLR4 expression and cytokine secretion, including TNFα, IL-1β, IL-6, IL-8, IL-10, and IL-12p70, were measured using flow cytometry and cytometric bead array.
Results
TLR2 and TLR4 expression at baseline was significantly higher in patients. Combined TLR2 and NOD2 stimulation induced a four-fold higher secretion of TNFα and a 13-fold higher secretion of IL-1β in patients. Additionally, there was a synergistic effect of TLR2 with NOD2 stimulation on induction of IL-1β in patients, whereas IL-10 was synergistically induced in healthy subjects.
Conclusion
Increased TLR expression and enhanced secretion of pro-inflammatory cytokines after combined TLR2 and NOD2 stimulation may be related to the pathogenesis of sarcoidosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcoidosis is a systemic inflammatory disease, primarily affecting the lungs. One subset of patients presents with Löfgren’s syndrome, i.e., erythema nodosum and/or ankle arthritis, fever, and bilateral hilar lymphadenopathy with or without lung parenchymal infiltration. These patients have an acute onset and a good prognosis and usually recover spontaneously within 2 years, whereas other patients with an insidious disease onset are at risk of developing pulmonary fibrosis. Bronchoalveolar lavage (BAL) fluid is characterized by increased numbers of lymphocytes, in particular activated CD4+ T cells, and macrophages. Biopsies from affected organs may reveal the hallmark of sarcoidosis, namely non-caseating epithelioid cell granulomas. The etiology is still unknown; nevertheless, epidemiological studies [1, 2] and findings of DNA from mycobacteria [3] and propionibacteria [4] in sarcoidosis tissue and lymph nodes indicate that sarcoidosis might be caused by an infection.
Pattern-recognition receptors (PRRs), such as Toll-like receptors (TLRs) and nucleotide-binding domain, leucin-rich repeat containing family proteins (NLRs), are key mediators of innate host defense. TLRs and NLRs are expressed by antigen presenting cells, e.g. dendritic cells (DC), macrophages, and monocytes, and upon ligation by conserved structures of microbes they induce innate immune responses and, via activation of DCs, influence adaptive immune responses. Most TLRs are expressed on cell surfaces, whereas NLRs are intracellular receptors [5]. TLR2 and TLR4 are involved in the recognition of bacterial cell wall components. TLR2 commonly recognizes molecules derived from gram-positive bacteria, e.g., peptidoglycan (PGN) and lipotechoic acid (LTA), whereas TLR4 binds molecules from gram-negative bacteria, e.g., lipopolysaccharide (LPS). If a bacterium is an agent initiating sarcoidosis, differences in the expression of, and function of these TLRs, might contribute to the pathogenesis.
Since sarcoidosis is a granulomatous disease, it is of particular interest that mutations in another pattern-recognition receptor, NOD2, has been associated with some granulomatous disorders, namely Crohn’s disease [6] and the rare diseases Blau syndrome (BS) [7] and early onset sarcoidosis (EOS) [8]. In Crohn’s disease, the inflammation is located in the gastrointestinal tract, whereas BS and EOS both share clinical features with sarcoidosis, including arthritis, skin rashes and uveitis, but without pulmonary involvement. The intracellular NOD2 receptor recognizes a breakdown product of peptidoglycan (PGN), namely muramyl dipeptide (MDP) [9]. Since MDP is conserved in PGN of almost all bacteria, NOD2 can be regarded as a general sensor of most bacteria.
There are several reports that pathogens implicated in the pathogenesis of sarcoidosis contain ligands for the above-mentioned receptors. For example, Mycobacterium tuberculosis contains well-known ligands in particular for TLR2, but also for TLR4 and NOD2 [10, 11]. Propionibacterium acnes also expresses ligands for these three receptors [12, 13]. We hypothesized that the expression and function of TLRs and NLRs may be related to disease susceptibility, or to the clinical phenotype, e.g. whether a patient has Löfgren’s syndrome or not.
Monocytes are of importance in sarcoidosis since they mature into macrophages, making up a large part of the granulomas. Under the influence of cytokines and/or in the presence of pathogen products such as MDP they can also fuse to form the multinucleated giant cells (MGC) that are characteristic of granulomas, although the precise in vivo mechanism for this has not been defined. However, it has been suggested that newly arrived monocytes are important for MGC formation and that the in vitro fusion capacity of human monocytes is higher than after maturation to macrophages [14]. Interestingly, monocytes from sarcoidosis patients have been found to be more potent in their ability to induce MGC formation than monocytes from healthy controls or patients with other granulomatous disorders [15].
The aim of this study was to elucidate whether sarcoidosis patients and healthy subjects, or subgroups of sarcoidosis patients, differ with regard to their TLR expression on monocytes in peripheral blood, and whether there were any differences in the cytokine secretion when stimulating human mononuclear cells with TLR2, TLR4, and NOD2 ligands.
Materials and Methods
Subjects
A total of 24 sarcoidosis patients (median age 39 years, p25–p75 = 32–57 years), consisting of 14 men and ten women, participated in this study. They were all consecutive patients referred to the Respiratory Medicine Unit (Karolinska University Hospital, Stockholm, Sweden) for investigation. All patients were diagnosed with pulmonary sarcoidosis as determined by symptoms (such as fatigue, dyspnea on exertion, and dry coughing), chest radiography, and pulmonary function tests and the diagnosis was established using the criteria by the World Association of Sarcoidosis and other Granulomatous Disorders (WASOG) [16]. Thus, all patients had either a positive biopsy (showing non-caseating granuloma), a BAL CD4/CD8 ratio >3.5 or Löfgren’s syndrome (bilateral hilar lymphadenopathy with or without parenchymal infiltration, fever, erythema nodosum, and/or ankle arthritis) [17]. Only one patient was treated with oral steroids at the time of the study. Sixteen of the patients were never-smokers, four were ex-smokers and four were smokers. Clinical and BAL fluid characteristics are given in Table I. Written informed consent was obtained from all subjects, and the Regional Ethical Review Board approved the study. Nineteen healthy subjects (median age 27 years, p25–p75 = 27–43 years), consisting of five men and 14 women, were also included in the study; 12 in this group were never-smokers, seven were ex-smokers, and none were smokers. Due to limitations in numbers of blood cells, all patients and controls were not included in all experiments done. However, which individual was included in what experiment was randomly chosen. The exact numbers of patients and controls in each group are indicated in the figures.
Blood Samples
Whole blood from sarcoidosis patients and healthy subjects were collected in heparinized tubes. The blood was drawn in early morning in both groups. Peripheral blood mononuclear cells (PBMC) were isolated using Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) density gradient separation, followed by washing twice in phosphate buffered saline (PBS) solution. Isolated cells were diluted in PBS and counted in a Bürker chamber. Cell viability was assessed by counting in trypan blue. Cells to be used for antibody staining were kept in cold PBS, cells to be used for in vitro stimulations were immediately resuspended in complete medium (see below).
In Vitro Stimulation of Mononuclear Cells
Cells were plated at a concentration of 106 cells/ml in complete medium; RPMI-1640 medium (Sigma-Aldrich, Irvine, U.K.), supplemented with 1% penicillin streptomycin (Invitrogen Corporation, Paisley, Scotland), 1% l-glutamine (Sigma-Aldrich, Irvine, U.K.) and 2% heat-inactivated human AB serum (Sigma-Aldrich, Schnelldorf, Germany), in polypropylene tubes for 16 h at 37°C in humidified atmosphere of 5% CO2 in air. Pattern-recognition receptors were stimulated with their respective ligands according to Table II. Synergism between TLR2 and NOD2 pathways was assessed using a combination of Pam3CSK4 and MDP. Supernatants were thereafter collected, centrifuged for 10 min at room temperature followed by a second centrifugation at 4°C, and stored at −20°C until analysis of cytokine concentrations. Cells were washed twice in PBS before antibody staining. The optimal incubation time for detection of the selected cytokines was determined to be 16 h after testing cytokine concentrations at the following time points: 4, 14, 16, 18, 20, 22, and 24 h. Optimal ligand concentrations were also determined after testing a range of concentrations.
Immunofluorescent Staining of Monocytes and Flow Cytometry
Approximately 5 × 105 cells diluted in cold PBS were double-stained with either anti-CD14 and anti-TLR2 (or isotype control) or with anti-CD14 and anti-TLR4 (or isotype control), day zero and day one, respectively; see Table III. After a 20-min incubation, cells were washed twice in PBS solution followed by re-suspension in cell-fixation solution (5 ml of 10× buffered fixative containing less than 10% formaldehyde and 1% sodium azide (BD Bioscience, Erembodegem, Belgium) diluted in 45 ml of ultra clean water). Typical forward scatter (FSC) and side scatter (SSC) in combination with CD14 positive cells was used in order to gate the monocytes. TLR2 and TLR4 expression was analyzed within the next 24 h using CellQuest software on a FACSCalibur (BD, Mountain View, CA, USA). TLR expression was quantified as the mean fluorescence intensity (MFI) values, after deduction of the isotype control MFI values.
Quantification of Secreted Cytokines
The Cytometric Bead Array kit (BD Bioscience, Pharmingen, San Diego, CA, USA) was used to quantify levels of secreted cytokines in mononuclear cell supernatants. Briefly, these kits contain micro-particles with six various fluorescence intensities, each coated with antibodies to a particular cytokine, allowing simultaneous measurement of several soluble proteins after labeling with a second, fluorescently labeled anti-cytokine antibody. The Human Inflammation kit enabled detection of TNFα, IL-1β, IL-6, IL-8, IL-10, and IL-12p70 protein levels. Assays were performed according to manufacturer’s instructions and cytokine secretion was analyzed using BD Cytometric Bead Array software on a FACS Calibur (BD, Mountain View, CA, USA).
Statistical Analysis
The Mann–Whitney U-test was used for comparison of receptor expression and protein levels between sarcoidosis patients and healthy subjects. Wilcoxon’s matched pairs test was used for within-group comparisons. Investigation of synergistic induction of cytokines was made by comparing the sum of the cytokine concentrations after separate TLR2 and NOD2 stimulation, respectively, with the cytokine concentration after stimulation with a combination of TLR2 and NOD2 ligands. A p value of <0.05 was considered as significant. Statistical analyses were performed with GraphPad PRISM 4.03 (GraphPad Software Inc., San Diego, CA, USA).
Results
TLR2 and TLR4 Expression on Peripheral Blood Monocytes
Mononuclear cells from sarcoidosis patients and healthy subjects were stained for TLR2, TLR4, and the monocyte marker CD14 (Fig. 1). Monocyte TLR2 and TLR4 expression was determined at baseline and after a culture period of 16 h in medium alone or with the TLR2 ligands PGN or Pam3CSK4, the TLR4 ligand LPS, the NOD2 ligand MDP, or Pam3CSK4 and MDP in combination.
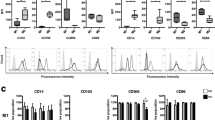
Representative FACS plots of monocytes and TLR staining on day zero. Gating of monocytes in a FSC–SSC dot plot, showing mononuclear cells from peripheral blood (in freshly obtained samples) (a). Dot plot showing the gate for CD14+ monocytes, used for the analysis of TLR expression (b). Histogram of TLR2 expression on CD14+ monocytes. Dotted line is the isotype control; black line is anti-TLR2 (c). Histogram of TLR4 expression on CD14+ monocytes. Dotted line is the isotype control; black line is anti-TLR4 (d)
TLR2 Expression
Sarcoidosis patients had a significantly higher baseline TLR2 expression compared to healthy subjects (p = 0.0062) as shown in Fig. 2a. After a culture period of 16 h in medium, the TLR2 expression was increased approximately two-fold in both sarcoidosis patients and healthy subjects, as shown in Fig. 2b. The significant difference (p = 0.015) was sustained between groups.
TLR2 expression on peripheral blood monocytes of healthy subjects (white boxes) and sarcoidosis patients (grey boxes). Graphs depict TLR2 expression at baseline (a), after a 16 h incubation in medium (b), and after a 16 h stimulation with MDP (c), PGN (d), Pam3CSK4 (e), LPS (f), MDP and Pam3CSK4 in combination (g). Boxes show median and interquartile range, whiskers show range. Numbers within parentheses state the number of healthy subjects and sarcoidosis patients in each analysis. Mann–Whitney U-test, *p < 0.05, **p < 0.01
Figure 2c shows the TLR2 expression after cells had been stimulated with MDP. Also in this case, sarcoidosis patients had higher receptor expression compared to healthy subjects (p = 0.0048). No differences in TLR2 expression were seen between sarcoidosis patients and healthy subjects after stimulation with PGN (Fig. 2d), Pam3CSK4 (Fig. 2e), LPS (Fig. 2f), or Pam3CSK4 and MDP in combination (Fig. 2g).
Compared to receptor expression after culture in medium alone, stimulation of mononuclear cells for 16 h with MDP, Pam3CSK4, or MDP and Pam3CSK4 in combination increased the TLR2 expression in sarcoidosis patients as well as in healthy subjects (Fig. 3). We did not observe any differences with regard to smoking history.
TLR2 expression on peripheral blood monocytes of healthy subjects (a) and sarcoidosis patients (b) after in vitro stimulation with the ligands indicated on the x-axis. Boxes show median and interquartile range, whiskers show range. Numbers within parentheses state the number of healthy subjects and sarcoidosis patients in each analysis. Wilcoxon’s matched pairs test, *p < 0.05, **p < 0.01, ***p < 0.001
TLR4 Expression
As shown in Fig. 4a, baseline TLR4 expression was significantly higher in sarcoidosis patients than in healthy subjects (p = 0.047). After a culture period of 16 h in medium, the receptor expression was increased in both groups, as shown in Fig. 4b. The significant difference between the groups was sustained, (p = 0.0094). With MDP and with PGN stimulation, the difference in TLR4 expression between patients and healthy subjects was also sustained (p = 0.013) and (p = 0.043), respectively, see Fig. 4c and d.
TLR4 expression on peripheral blood monocytes of healthy subjects (white boxes) and sarcoidosis patients (grey boxes). Graphs depict TLR4 expression at baseline (a), after a 16 h incubation in medium (b), and after a 16 h stimulation with MDP (c), PGN (d), Pam3CSK4 (e), LPS (f), MDP and Pam3CSK4 in combination (g). Boxes show median and interquartile range, whiskers show range. Numbers within parentheses state the number of healthy subjects and sarcoidosis patients in each analysis. Mann–Whitney U-test, *p < 0.05, **p < 0.01
However, similar to TLR2 expression, no differences in TLR4 expression were seen between sarcoidosis patients and healthy subjects after stimulation with Pam3CSK4 (Fig. 4e), LPS (Fig. 4f), or Pam3CSK4 and MDP in combination (Fig. 4g).
Compared to receptor expression after culture in medium alone, stimulation with MDP significantly decreased the TLR4 expression in both groups, and it also decreased after combined stimulation with MDP and Pam3CSK4 (although significantly so only in healthy controls). TLR4 also decreased in both groups after LPS stimulation (however, not statistically significant; Fig. 5). Similar to TLR2, we did not observe any differences with regard to smoking history.
TLR4 expression on peripheral blood monocytes of healthy subjects (a) and sarcoidosis patients (b) after in vitro stimulation with the ligands indicated on the x-axis. Boxes show median and interquartile range, whiskers show range. Numbers within parentheses state the number of healthy subjects and sarcoidosis patients in each analysis. Wilcoxon’s matched pairs test, *p < 0.05, **p < 0.01
Cytokine Profile in Patients and Healthy Subjects
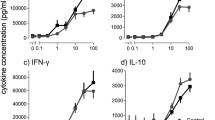
The levels of secreted cytokines are depicted in Fig. 6. There were no significant differences after stimulation with individual ligands for TLR2 (PGN and Pam3CSK4), TLR4 (LPS) or NOD2 (MDP) alone. In contrast, when using a combination of Pam3CSK4 and MDP, i.e., ligands for TLR2 and NOD2, respectively, several differences between patients and controls were observed. Sarcoidosis patients had a four-fold higher (p = 0.0093) secretion of TNFα (median 529 pg/ml vs. 132 pg/ml) and a 13-fold higher (p = 0.035) secretion of IL-1β (1219 pg/ml vs. 92 pg/ml) than healthy subjects after combined Pam3CSK4 and MDP stimulation, as shown in Fig. 6a–b. There were tendencies to a higher secretion of IL-8 and IL-10 in sarcoidosis patients after Pam3CSK4 stimulation alone (Fig. 6d–e). The secretion of IL-12p70 was below the detection limit of the assay (data not shown). Cytokine secretion after Pam3CSK4 stimulation did not correlate with the expression of TLR2, either at base line or after Pam3CSK4 stimulation (data not shown).
Secretion of TNFα (a), IL-1β (b), IL-6 (c), IL-8 (d) and IL-10 (e) from human mononuclear cells of healthy subjects (white boxes) and sarcoidosis patients (grey boxes) after stimulation with ligands indicated on the x-axis. Boxes show median and interquartile range, whiskers show range. Numbers within parentheses state the number of healthy subjects and sarcoidosis patients, respectively, in each analysis. Mann–Whitney U-test, *p < 0.05, **p < 0.01
Synergistic Induction of Cytokine Secretion
Figure 7 shows the synergistic induction of cytokine secretion after combined TLR2 and NOD2 stimulation. Only patients and controls where data were obtained for all three stimuli (Pam3CSK4, MDP, or combined Pam3CSK4 and MDP) are included in the comparisons. A synergy was present if the cytokine level after stimulation with the combination of Pam3CSK4 and MDP was significantly higher than the sum of cytokine levels after separate Pam3CSK4 and MDP stimulations. There was a synergistic induction of IL-1β (Fig. 7b) in sarcoidosis patients, while in contrast healthy subjects had a synergistic induction of IL-10 (Fig. 7e). In healthy subjects there was also a synergistic induction of IL-6 (Fig. 7c), and a similar tendency was seen in patients. The secretion of IL-12p70 was below the detection limit of the assay (data not shown). Cytokine secretion after combined TLR2 and NOD2 stimulation did not correlate with the expression of TLR2, either at base line or after combined TLR2 and NOD2 stimulation (data not shown).
Synergistic effect of MDP with Pam3CSK4 on induction of TNFα (a), IL-1β (b), IL-6 (c), IL-8 (d) and IL-10 (e) in human mononuclear cells of healthy subjects (white boxes) and sarcoidosis patients (grey boxes). Comparisons within each subject group (healthy subjects and sarcoidosis patients) refer to the sum of the cytokine concentrations after separate MDP and Pam3CSK4 stimulation, respectively, versus the cytokine concentration after stimulation with a combination of MDP and Pam3CSK4. Only patients and controls were data were obtained for all three stimuli (Pam3CSK4, MDP, or Pam3CSK4 and MDP in combination) are included in the comparison. Boxes show median and interquartile range, whiskers show range. Numbers within parentheses state the number of healthy subjects and sarcoidosis patients in each analysis. Wilcoxon’s matched pairs test, *p < 0.05, **p < 0.01
TLR and Cytokine Profile in Patient Subgroups
Clinical and BAL fluid characteristics for patients with (n = 8) and without (n = 16) Löfgren’s syndrome are given in Table E1 in the online data supplement. Comparing TLR expression and cytokine secretion as described above, there were no significant differences between these patient subgroups (see Figures E1 and E2 in the online data supplement). However, for some ligands the number of Löfgren’s syndrome patients was too low to draw any firm conclusions. There were also no apparent differences in these aspects between patients who did or did not express the HLA-DRB1*03 allele. Only one patient was under steroid treatment at the time of sampling. However, this individual did not stand out in any particular way regarding TLR expression or cytokine profile.
Discussion
In the present study, we demonstrated a significantly higher TLR2 and TLR4 expression on blood monocytes of sarcoidosis patients compared to healthy subjects. Furthermore, an increased secretion of the pro-inflammatory cytokines TNFα and IL-1β was seen in sarcoidosis patients after combined TLR2 and NOD2 stimulation, as well as different patterns of synergistic cytokine secretion after simultaneous stimulation of TLR2 and NOD2. These differences may be of relevance for how a putative sarcoidosis pathogen interacts with the immune system, and thus for disease susceptibility.
So far, the etiology of sarcoidosis is unknown. However, previous studies indicating an infectious cause may provide a role for PRRs in the pathogenic process. Mycobacterium tuberculosis has been proposed being an agent causing sarcoidosis, and support for this comes from findings of mycobacterial DNA in sarcoidosis tissue and lymph nodes [3]. The case for a mycobacterial etiology was strengthened by recent finding by Song et al. of protease-resistant antigens in sarcoidosis tissue, among them M. tuberculosis catalase–peroxidase, and that these antigens are targets of IgG antibodies in patients [18]. Several TLR ligands derived from mycobacteria are known, including the cell wall component lipoarabinomannan (LAM) and mannosylated phosphatidylinositol (PIM), both recognized by TLR2 [19]. In addition, ligands for TLR4 and NOD2 are also present in M. tuberculosis [10, 11], and ligands for all three receptors are likewise present in another candidate pathogen, Propionibacterium acnes [12, 13]. TLRs have a central role in the induction of immune responses to invading microbial pathogens, as supported by numerous studies in both humans and animals [20].
One explanation why sarcoidosis patients express higher baseline TLR2 and TLR4 than healthy subjects could be that sarcoidosis patients constitutively express higher levels of the receptors, although studies performed to date seem to suggest that polymorphisms in TLR2 and TLR4 only play a minor role in sarcoidosis [21, 22]. However, the results of linkage analysis indicate that an unidentified polymorphism of TLR4, or in the vicinity of that gene, is associated with sarcoidosis [23]. Environmental exposure may also alter TLR expression, as exemplified by the higher expression of TLR2 mRNA in blood cells of children growing up on farms [24]. TLR levels can also be affected by smoking. Droemann et al. reported that alveolar macrophages of smokers express lower levels of TLR2 than cells from healthy non-smokers [25]. However, we did not observe any differences related to smoking status, which is in agreement with the results regarding smoking and blood monocytes in Droemann’s study. Since smoking is associated with a reduced risk of developing sarcoidosis [26], Droemann’s findings may support our hypothesis that a higher TLR2 expression could have a role in the pathogenesis of sarcoidosis. Chronic infection may result in modified TLR expression. TLR expression can also be altered by inflammation per se, e.g. via release of endogenous TLR ligands or by elevated TNFα levels [27].
The increased TLR2 and TLR4 expression in sarcoidosis patients may have consequences for host–pathogen interaction. It may be speculated that one consequence could be a more intense immune response to an otherwise harmless microbe. We attempted to determine TLR expression on alveolar macrophages, obtained by BAL of sarcoidosis patients. However, the low level of expression above background isotype control staining prevented us from obtaining reliable data. That alveolar macrophages have a lower expression of TLR2 and TLR4 compared to monocytes was previously shown [25].
The finding that stimulation of one PRR may alter the expression of other such receptors is intriguing. It has previously been observed that TLR4 stimulation can lead to increased TLR2 and decreased TLR4 expression [25, 28]. This was the case also in our experiments, although not statistically significant. However, to our knowledge, results of TLR2 and TLR4 expression after stimulation with Pam3CSK4, MDP, or Pam3CSK4 in combination with MDP have not previously been reported. We found that all of these TLR2 and NOD2 stimuli up-regulated the TLR2 expression in sarcoidosis patients as well as in healthy subjects. Regarding TLR4, MDP down-regulated the expression in both groups. However, combined Pam3CSK4 and MDP stimulation significantly down-regulated TLR4 only in healthy subjects. Such a regulation of receptor expression may be a way to fine-tune the specificity and sensitivity of PRRs, although this needs to be investigated. Furthermore, it was intriguing to see that incubation in medium alone could up-regulate the monocytic TLR2 and TLR4 expression. However, our data in this respect agree very well with a previous study of TLR2 expression [29]. This alteration in receptor expression may be a result of plastic adherence, since previous studies have shown that plastic adherence can up-regulate cell surface marker expression and cytokine gene expression in monocytes and macrophages [30, 31].
In contrast to the granulomatous diseases mentioned in the introduction [6–8], Martin et al. sequenced NOD2 gene exons from DNA obtained from sarcoidosis patients, but did not find any mutations associated with sarcoidosis [32]. Most other studies also did not report any NOD2 mutations to be associated with sarcoidosis [33, 34]. Nevertheless, an alteration in the NOD2 signaling pathway may contribute to the pathogenesis also in sarcoidosis. Our data suggest that such an altered NOD2 function may exist in sarcoidosis patients, at least regarding pathways that involve simultaneous recognition of TLR2 and NOD2 ligands.
We also aimed to investigate the occurrence of any functional differences between sarcoidosis patients and healthy subjects with regard to the effects of TLR and NOD2 stimulation. By using the synthetic TLR2 ligand Pam3CSK4, we avoided any risk of endotoxin contamination, which has complicated the interpretation of previous studies using purified TLR2 ligands. Importantly, it also prevented indirect stimulation of NOD2 with MDP, the degradation product of PGN. There were no significant differences in cytokine secretion between patients and controls after stimulation of mononuclear cells with individual ligands for TLR2, TLR4, or NOD2 using our protocol for stimulation. In contrast, our results demonstrate a stronger secretion of the pro-inflammatory cytokines TNFα and IL-1β in sarcoidosis patients after combined TLR2 and NOD2 stimulation. This is very likely of relevance for the in vivo situation during an infection, since most microbes possess ligands for both TLRs and NLRs. With TLRs as sensors of bacteria on the cell surface and NLRs as sensors of their presence inside the cytosol, these PRRs complement each other for the detection of bacterial infection.
TNFα and IL-1β are both important in the initiation of the inflammatory response as well as for the formation of granuloma in patients with active sarcoidosis. The main producers of TNFα are alveolar macrophages, monocytes, and T cells, and increased levels have been reported to correlate with prolonged course of disease [35]. The primary sources of IL-1β are macrophages, monocytes, and dendritic cells, all present inside the granuloma. Protein levels of TNFα and IL-1β in BAL fluid has been shown to be significantly higher in sarcoidosis patients compared to healthy subjects [36].
The lack of detectable significant differences with regard to TLR expression and cytokine secretion when comparing subgroups of patients (Löfgren’s syndrome versus non-Löfgren’s syndrome) may suggest that alterations in these respects are associated with a general susceptibility to, or consequences of, sarcoidosis, but not to different clinical phenotypes of this disease. This is also supported by the lack of differences between patients who did or did not express the HLA-DRB1*03 allele, which was previously shown to be associated with a good prognosis in Scandinavian sarcoidosis patients [37].
We found a synergistic induction of cytokine secretion after combined TLR2 and NOD2 stimulation. Notably, the pattern differed between patients and controls, with sarcoidosis patients having a synergistic induction of the pro-inflammatory cytokine IL-1β, in contrast to healthy subjects where the immunoregulatory cytokine IL-10 was induced in that manner. IL-6 was synergistically induced in both groups, yet statistically significant only in healthy subjects.
Previously, it has been reported that TLR2 and NOD2 ligation can induce these cytokines, as well as TNFα, in a synergistic way [11, 38, 39], and TLR2 may also, under certain circumstances, act as a negative regulator of NOD2 [40]; but to our knowledge, such phenomena have not been studied with regard to any human disease with the exception of Crohn’s disease [39, 41]. Interestingly, in that disorder, the synergy was not observed in patients with the NOD2 3020insC mutation, and it was suggested that the relative lack of IL-10 induction in patients could explain the development of chronic intestinal inflammation, since IL-10 is a powerful anti-inflammatory cytokine. In that context, it is noteworthy that in the present study, a synergistic induction of IL-10 was observed in healthy subjects, but not in sarcoidosis patients. It is possible that this is of relevance for the development of pulmonary inflammation in sarcoidosis. One might hypothesize that the increased TLR2 expression in sarcoidosis may be of importance for the altered patterns of cytokine induction. Alternatively, other factors may be essential since we did not observe any correlation between TLR expression and cytokine secretion. Mechanisms previously implicated in TLR–NLR synergy include shared signaling pathways and NOD2-induced up-regulation of the adaptor molecule MyD88, involved in TLR signaling [42]. With respect to mycobacteria, it is of interest that TLR2 and NOD2 were found to be non-redundant for the recognition of M. tuberculosis, and that there were synergistic interactions between these two receptors with regard to cytokine induction [11]. TLR2 and NOD2 interactions merit further investigations in sarcoidosis.
To summarize, we show that sarcoidosis patients are characterized by higher expression of TLR2 and TLR4 on blood monocytes and a more pronounced pro-inflammatory cytokine profile after combined TLR2 and NOD2 stimulation than healthy subjects. Our results suggest that an altered profile of TLRs and NLRs might play a role in the pathogenesis of sarcoidosis.
References
Parkes SA, Baker SB, Bourdillon RE, Murray CR, Rakshit M. Epidemiology of sarcoidosis in the Isle of Man—1: a case controlled study. Thorax 1987;42(6):420–6.
Hosoda Y, Yamaguchi M, Hiraga Y. Global epidemiology of sarcoidosis. What story do prevalence and incidence tell us? Clin Chest Med 1997;18(4):681–94. doi:10.1016/S0272-5231(05)70412-3.
Saboor SA, Johnson NM, McFadden J. Detection of mycobacterial DNA in sarcoidosis and tuberculosis with polymerase chain reaction. Lancet 1992;339(8800):1012–5. doi:10.1016/0140-6736(92)90535-B.
Abe C, Iwai K, Mikami R, Hosoda Y. Frequent isolation of Propionibacterium acnes from sarcoidosis lymph nodes. Zentralbl Bakteriol Mikrobiol Hyg [A] 1984;256(4):541–7.
Mitchell JA, Paul-Clark MJ, Clarke GW, McMaster SK, Cartwright N. Critical role of toll-like receptors and nucleotide oligomerisation domain in the regulation of health and disease. J Endocrinol 2007;193(3):323–30. doi:10.1677/JOE-07-0067.
Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 2001;411(6837):603–6. doi:10.1038/35079114.
Miceli-Richard C, Lesage S, Rybojad M, Prieur AM, Manouvrier-Hanu S, Hafner R, et al. CARD15 mutations in Blau syndrome. Nat Genet 2001;29(1):19–20. doi:10.1038/ng720.
Kanazawa N, Okafuji I, Kambe N, Nishikomori R, Nakata-Hizume M, Nagai S, et al. Early-onset sarcoidosis and CARD15 mutations with constitutive nuclear factor-kappaB activation: common genetic etiology with Blau syndrome. Blood 2005;105(3):1195–7. doi:10.1182/blood-2004-07-2972.
Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J Biol Chem 2003;278(8):5509–12. doi:10.1074/jbc.C200673200.
Krutzik SR, Modlin RL. The role of Toll-like receptors in combating mycobacteria. Semin Immunol 2004;16(1):35–41. doi:10.1016/j.smim.2003.10.005.
Ferwerda G, Girardin SE, Kullberg BJ, Le Bourhis L, de Jong DJ, Langenberg DM, et al. NOD2 and toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog 2005;1(3):279–85. doi:10.1371/journal.ppat.0010034.
Nagy I, Pivarcsi A, Koreck A, Szell M, Urban E, Kemeny L. Distinct strains of Propionibacterium acnes induce selective human beta-defensin-2 and interleukin-8 expression in human keratinocytes through toll-like receptors. J Invest Dermatol 2005;124(5):931–8. doi:10.1111/j.0022-202X.2005.23705.x.
Tanabe T, Ishige I, Suzuki Y, Aita Y, Furukawa A, Ishige Y, et al. Sarcoidosis and NOD1 variation with impaired recognition of intracellular Propionibacterium acnes. Biochim Biophys Acta 2006;1762(9):794–801.
Most J, Neumayer HP, Dierich MP. Cytokine-induced generation of multinucleated giant cells in vitro requires interferon-gamma and expression of LFA-1. Eur J Immunol 1990;20(8):1661–7. doi:10.1002/eji.1830200807.
Mizuno K, Okamoto H, Horio T. Heightened ability of monocytes from sarcoidosis patients to form multi-nucleated giant cells in vitro by supernatants of concanavalin A-stimulated mononuclear cells. Clin Exp Immunol 2001;126(1):151–6. doi:10.1046/j.1365-2249.2001.01655.x.
Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999;160(2):736–55.
Lofgren S, Lundback H. The bilateral hilar lymphoma syndrome; a study of the relation to tuberculosis and sarcoidosis in 212 cases. Acta Med Scand 1952;142(4):265–73.
Song Z, Marzilli L, Greenlee BM, Chen ES, Silver RF, Askin FB, et al. Mycobacterial catalase–peroxidase is a tissue antigen and target of the adaptive immune response in systemic sarcoidosis. J Exp Med 2005;201(5):755–67. doi:10.1084/jem.20040429.
Jones BW, Means TK, Heldwein KA, Keen MA, Hill PJ, Belisle JT, et al. Different Toll-like receptor agonists induce distinct macrophage responses. J Leukoc Biol 2001;69(6):1036–44.
Philpott DJ, Girardin SE. The role of Toll-like receptors and Nod proteins in bacterial infection. Mol Immunol 2004;41(11):1099–108. doi:10.1016/j.molimm.2004.06.012.
Veltkamp M, Grutters JC, van Moorsel CH, Ruven HJ, van den Bosch JM. Toll-like receptor (TLR) 4 polymorphism Asp299Gly is not associated with disease course in Dutch sarcoidosis patients. Clin Exp Immunol 2006;145(2):215–8. doi:10.1111/j.1365-2249.2006.03127.x.
Veltkamp M, Wijnen PA, van Moorsel CH, Rijkers GT, Ruven HJ, Heron M, et al. Linkage between Toll-like receptor (TLR) 2 promotor and intron polymorphisms: functional effects and relevance to sarcoidosis. Clin Exp Immunol 2007;149(3):453–62.
Schurmann M, Kwiatkowski R, Albrecht M, Fischer A, Hampe J, Muller-Quernheim J, et al. Study of Toll-like receptor gene loci in sarcoidosis. Clin Exp Immunol 2008;152(3):423–31.
Lauener RP, Birchler T, Adamski J, Braun-Fahrlander C, Bufe A, Herz U, et al. Expression of CD14 and Toll-like receptor 2 in farmers’ and non-farmers’ children. Lancet 2002;360(9331):465–6. doi:10.1016/S0140-6736(02)09641-1.
Droemann D, Goldmann T, Tiedje T, Zabel P, Dalhoff K, Schaaf B. Toll-like receptor 2 expression is decreased on alveolar macrophages in cigarette smokers and COPD patients. Respir Res 2005;6:68. doi:10.1186/1465-9921-6-68.
Valeyre D, Soler P, Clerici C, Pre J, Battesti JP, Georges R, et al. Smoking and pulmonary sarcoidosis: effect of cigarette smoking on prevalence, clinical manifestations, alveolitis, and evolution of the disease. Thorax 1988;43(7):516–24.
Homma T, Kato A, Hashimoto N, Batchelor J, Yoshikawa M, Imai S, et al. Corticosteroid and cytokines synergistically enhance toll-like receptor 2 expression in respiratory epithelial cells. Am J Respir Cell Mol Biol 2004;31(4):463–9. doi:10.1165/rcmb.2004-0161OC.
Bosisio D, Polentarutti N, Sironi M, Bernasconi S, Miyake K, Webb GR, et al. Stimulation of toll-like receptor 4 expression in human mononuclear phagocytes by interferon-gamma: a molecular basis for priming and synergism with bacterial lipopolysaccharide. Blood 2002;99(9):3427–31. doi:10.1182/blood.V99.9.3427.
Iwahashi M, Yamamura M, Aita T, Okamoto A, Ueno A, Ogawa N, et al. Expression of Toll-like receptor 2 on CD16+ blood monocytes and synovial tissue macrophages in rheumatoid arthritis. Arthritis Rheum 2004;50(5):1457–67. doi:10.1002/art.20219.
Jendraschak E, Kaminski WE, Kiefl R, von Schacky C. IGF-1, PDGF and CD18 are adherence-responsive genes: regulation during monocyte differentiation. Biochim Biophys Acta 1998;1396(3):320–35.
Krause SW, Kreutz M, Andreesen R. Differential effects of cell adherence on LPS-stimulated cytokine production by human monocytes and macrophages. Immunobiology 1996;196(5):522–34.
Martin TM, Doyle TM, Smith JR, Dinulescu D, Rust K, Rosenbaum JT. Uveitis in patients with sarcoidosis is not associated with mutations in NOD2 (CARD15). Am J Ophthalmol 2003;136(5):933–5. doi:10.1016/S0002-9394(03)00892-4.
Gazouli M, Koundourakis A, Ikonomopoulos J, Gialafos EJ, Rapti A, Gorgoulis VG, et al. CARD15/NOD2, CD14, and toll-like receptor 4 gene polymorphisms in Greek patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2006;23(1):23–9.
Iannuzzi MC. Advances in the genetics of sarcoidosis. Proc Am Thorac Soc 2007;4(5):457–60. doi:10.1513/pats.200606-136MS.
Strausz J, Mannel DN, Pfeifer S, Borkowski A, Ferlinz R, Muller-Quernheim J. Spontaneous monokine release by alveolar macrophages in chronic sarcoidosis. Int Arch Allergy Appl Immunol 1991;96(1):68–75.
Idali F, Wiken M, Wahlstrom J, Mellstedt H, Eklund A, Rabbani H, et al. Reduced Th1 response in the lungs of HLA-DRB1*0301 patients with pulmonary sarcoidosis. Eur Respir J 2006;27(3):451–9. doi:10.1183/09031936.06.00067105.
Berlin M, Fogdell-Hahn A, Olerup O, Eklund A, Grunewald J. HLA-DR predicts the prognosis in Scandinavian patients with pulmonary sarcoidosis. Am J Respir Crit Care Med 1997;156:1601–5.
Uehara A, Yang S, Fujimoto Y, Fukase K, Kusumoto S, Shibata K, et al. Muramyldipeptide and diaminopimelic acid-containing desmuramylpeptides in combination with chemically synthesized Toll-like receptor agonists synergistically induced production of interleukin-8 in a NOD2- and NOD1-dependent manner, respectively, in human monocytic cells in culture. Cell Microbiol 2005;7(1):53–61. doi:10.1111/j.1462-5822.2004.00433.x.
Netea MGFG, de Jong DJ, Jansen T, Jacobs L, Kramer M, Naber TH, et al. Nucleotide-binding oligomerization domain-2 modulates specific TLR pathways for the induction of cytokine release. J Immunol 2005;174(10):6518–23.
Watanabe T, Kitani A, Murray PJ, Strober W. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol 2004;5(8):800–8. doi:10.1038/ni1092.
Kramer M, Netea MG, de Jong DJ, Kullberg BJ, Adema GJ. Impaired dendritic cell function in Crohn’s disease patients with NOD2 3020insC mutation. J Leukoc Biol 2006;79(4):860–6. doi:10.1189/jlb.0805484.
Werts C, Girardin SE, Philpott DJ. TIR, CARD and PYRIN: three domains for an antimicrobial triad. Cell Death Differ 2006;13(5):798–815. doi:10.1038/sj.cdd.4401890.
Acknowledgements
The authors thank Margitha Dahl, Gunnel de Forest, Heléne Blomqvist, and Berit Olsson for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by the Swedish Heart–Lung Foundation, King Oscar II Jubilee Foundation, the Swedish Research Council, the U.S. National Institutes of Health (Grant No. 1 R21 HL077579-01), the Stockholm County Council and Karolinska Institutet.
Electronic supplementary material
Below is the link to the electronic supplementary material
Table EI
Characterization of Patient Subgroups (DOC 52.0 KB)
Figure E1
TLR2 (A) and TLR4 (B) expression on peripheral blood monocytes of sarcoidosis patients with Löfgren’s syndrome (white boxes) and non-Löfgren’s syndrome (grey boxes) after in vitro stimulation with ligands indicated on the x-axis. Boxes show median and interquartile range, whiskers show range. Numbers below the boxes state the number of patients and controls in each analysis. Numbers within the parenthesis state the number of Löfgren’s syndrome patients and non-Löfgren’s syndrome patients, respectively, in each analysis (DOC 32.0 KB).
Figure E2
Secretion of TNFα (A), IL-1β (B), IL-6 (C), IL-8 (D) and IL-10 (E) from human mononuclear cells of sarcoidosis patients with Löfgren’s syndrome (white boxes) and non-Löfgren’s syndrome (grey boxes) after stimulation with ligands indicated on the x-axis. Boxes show median and interquartile range, whiskers show range. Numbers below the boxes state the number of patients and controls in each analysis. Numbers within the parenthesis state the number of Löfgren’s syndrome patients and non-Löfgren’s syndrome patients, respectively, in each analysis (DOC 38.0 KB).
Figure E3
Representative histograms of flow cytometric analysis of TLR2 (a) and TLR4 (b), after stimulation with various ligands (DOC 375 KB).
Rights and permissions
About this article
Cite this article
Wikén, M., Grunewald, J., Eklund, A. et al. Higher Monocyte Expression of TLR2 and TLR4, and Enhanced Pro-inflammatory Synergy of TLR2 with NOD2 Stimulation in Sarcoidosis. J Clin Immunol 29, 78–89 (2009). https://doi.org/10.1007/s10875-008-9225-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-008-9225-0